Abstract.
This study was performed to examine whether prenatal lipid-based nutrient supplementation is an effective means of improving birth outcomes compared with other types of supplementation including iron folic acid (IFA), United Nations multiple micronutrient preparation (UNIMAP), other multiple micronutrients (MMN), and fortified corn–soy blend (CSB). A meta-analysis was performed to determine the relative risks and mean differences in birth outcomes between prenatal lipid-based nutrients versus prenatal IFA, UNIMAP, other MMN, and CSB in randomized controlled trials. Eleven databases, including PubMed (MEDLINE), were searched. Study quality was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias. Fifty-eight overall good-quality studies extracted from 11 eligible articles with 101,553 mother–baby pairs were included. Lipid-based nutrient supplementation significantly reduced the risks of low birthweight, small for gestational age, and stunting (n = 5, 5, and 4, respectively) and significantly increased the means of birthweight, birth length, arm circumference, and weight-for-age z-score (n = 5, 5, 4, and 3, respectively). Lipid-based nutrient supplementation did not significantly reduce the risk of preterm birth, stillbirth, abortion, perinatal death, or underweight (n = 5, 5, 5, 3, or 3, respectively) or significantly increase the mean of head circumference or height-for-age z-score (n = 4 or 2, respectively). In conclusion, the results supported the efficacy of prenatal lipid-based nutrient supplementation compared with IFA, UNIMAP, other MMN, and CSB for reducing the risk of small birth size.
INTRODUCTION
Adverse birth outcomes, such as low birthweight, small for gestational age (SGA), preterm birth, stillbirth, and abortion, contribute to mortality and morbidity in the neonatal and infantile periods as well as later stages of life.1 Supplementation has been attempted with various types of nutrients, including iron folic acid (IFA), United Nations multiple micronutrient preparation (UNIMAP), other multiple micronutrients (MMN), fortified corn–soy blend (CSB), and lipid-based nutrients,2–12 to improve the health status of mothers and/or their babies to reduce adverse birth outcomes in developing countries. A lipid-based nutrient supplement is composed of vitamins, minerals, protein, and lipid, with most of its energy content being derived from lipid. Therefore, lipid-based nutrients may be nutritionally rich compared with IFA, UNIMAP, other MMN, and CSB. On the other hand, there is evidence supporting the efficacy of UNIMAP and other MMN compared with IFA in improving some birth outcomes.13 Therefore, lipid-based nutrients provided to pregnant women would be most useful as a low-cost solution to reduce adverse birth outcomes.
Here, a meta-analysis was performed to evaluate whether prenatal lipid-based nutrients are effective for improving birth outcomes compared with other supplements, including IFA, UNIMAP, other MMN, and CSB.
METHODS
Inclusion criteria and primary outcomes.
The inclusion criteria were English language studies of randomized control trials involving pregnant women to provide the primary outcomes mentioned as follows between lipid-based nutrients and other supplements, including IFA, UNIMAP, other MMN, and CSB. The primary outcomes were divided into relative risks for dichotomous outcomes and mean differences for continuous outcomes between pregnant women provided with lipid-based nutrients (i.e., lipid-based nutrient groups) and those given other supplements, including IFA, UNIMAP, other MMN, and CSB (i.e., control groups). The former were low birthweight (i.e., birthweight < 2,500 g), SGA (i.e., birthweight < the 10th percentile for gestational age), preterm birth (i.e., gestational age at birth < 37 weeks), stillbirth, abortion, perinatal death (i.e., stillbirth and death in the perinatal period commencing at 22 weeks of gestation and ending in the first week of life), underweight (i.e., weight-for-age z-score [WAZ] at birth < 2), and stunting (i.e., height-for-age z-score [HAZ] at birth < 2). The latter were birthweight, birth length, head circumference, chest circumference, arm circumference, ponderal index (i.e., weight [kg] divided by height [m]3), WAZ, HAZ, and weight-for-height z-score (WHZ).
Source for searches, study selection, and data extraction.
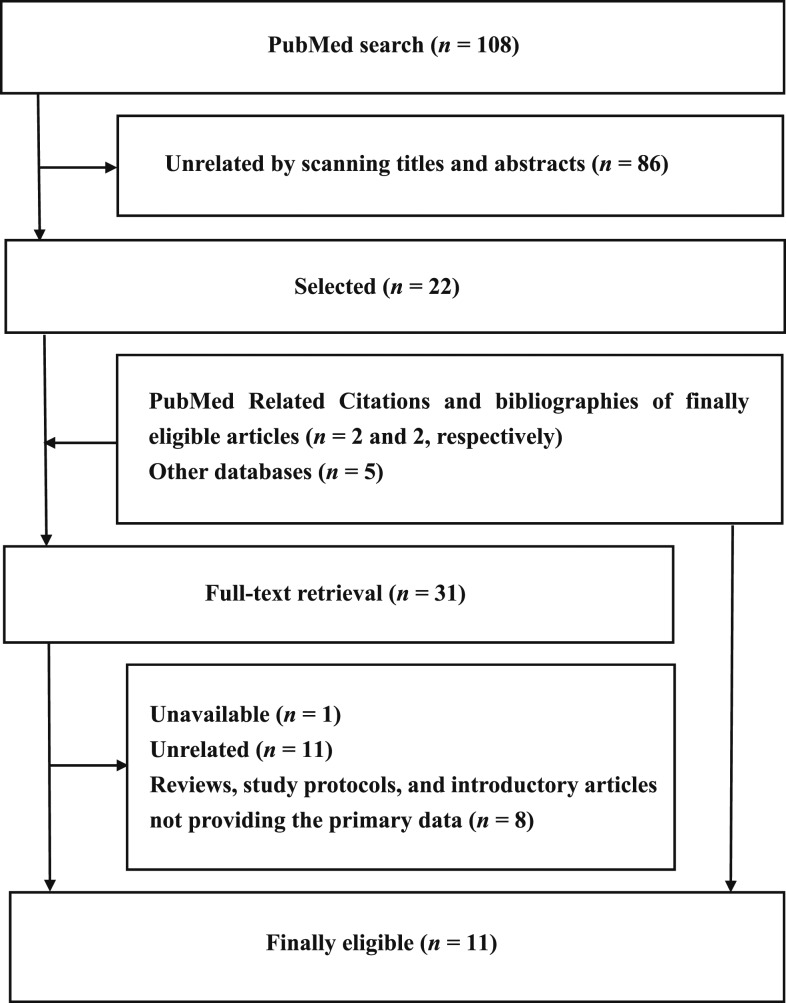
PubMed (MEDLINE) was first searched using the terms described in supplemental methods (see Supplemental Material). There were no restrictions regarding publication date. Article titles and abstracts were scanned, and those that were determined to be unrelated were excluded. Full texts of the remaining articles were retrieved. Articles determined to be unrelated by retrieving the full texts were excluded. Reviews, study protocols, and introductory articles that did not provide the primary data were also excluded. The remaining articles were finally eligible for inclusion in the analysis. The following strategies were added to locate additional articles, the full texts of which had to be retrieved. First, the PubMed Related Citations shown by clicking the “See all…” tab at the right side of the screen displaying each finally eligible article and the bibliographic references of each finally eligible article were investigated. Second, 10 other databases, that is, ClinicalTrials.gov, CINAHL, PsycINFO, Wiley Online Library, ProQuest Central (e.g., ProQuest Health and Medical Complete and ProQuest Nursing & Allied Health Source), ProQuest Dissertations & Theses Global, the entire Cochrane Library (e.g., CENTRAL), Web of Knowledge, Google Scholar, and Sage Publication Online, were searched. The literature search was repeated five times between June 2017 and March 2018. Duplicated articles were integrated. The numbers of mother–baby pairs with and without the dichotomous outcomes in the lipid-based nutrient groups and control groups, merging IFA, UNIMAP, other MMN, and CSB groups, and the mean differences in the continuous outcomes between the two groups and their standard deviations were extracted from the studies reported in the finally eligible articles.
Study quality assessment.
Study quality was assessed using the Cochrane Collaboration’s tool for assessing risk of bias that addressed random sequence generation and allocation concealment (i.e., selection bias), blinding of participants and personnel (i.e., performance bias), blinding of outcome assessment (i.e., detection bias), incomplete outcome data (i.e., attrition bias), selective outcome reporting (i.e., reporting bias), and other sources of bias (i.e., other bias).14 The judgments were categorized as having “low risk,” “unclear risk,” or “high risk” of bias. Study quality was assessed five times at intervals, and the most frequent responses were regarded as the final responses. “Low risk” was assigned a value of 1, whereas ”unclear risk” and “high risk” were assigned a value of 0.
Analysis.
Qualitative analyses, that is, systematic reviews, were performed to elucidate the number of studies and mother–baby pairs for each of the primary outcomes that were extracted from finally eligible articles and study regions to evaluate the generalizability of the findings of the meta-analyses (external validity). They also elucidated the results of study quality assessment to evaluate the influence of bias on the findings (internal validity). Stata/MP 13.1 (StataCorp LP, College Station, TX) was used for quantitative and statistical analyses, that is, meta-analyses. Heterogeneity was assessed using I2. Attempts were made to achieve homogeneity (i.e., I2 < 50%) from heterogeneous data (i.e., I2 ≥ 50%) by selecting the studies (investigation of heterogeneity sources). The selection was dependent on 1) study region, that is, individual countries versus other countries; Africa, Asia, Europe, Latin America, the Middle East, North America, and Oceania versus other regions; and developing versus developed countries; 2) energy content of lipid-based nutrients, that is, 118 kcal versus 373–920 kcal; 3) supplements used as controls, that is, the inclusion versus exclusion of IFA, UNIMAP, other MMN, 2-fold UNIMAP, and CSB; and 4) study quality, that is, “low risk” versus “unclear risk” and “high risk” of bias in responses to seven items in the study quality assessment.
The relative risks of low birthweight, SGA, preterm birth, stillbirth, abortion, perinatal death, underweight, and stunting and the mean differences in birthweight, birth length, head circumference, chest circumference, arm circumference, ponderal index, WAZ, HAZ, and WHZ at birth between the lipid-based nutrient and control groups were summarized (meta-analysis). A fixed-effects model (i.e., inverse variance method) and a random-effects model (i.e., the DerSimonian and Laird method) were used to summarize homogenous data and heterogeneous data, respectively.
The data were also summarized by selecting studies in the same way as described for investigation of heterogeneity sources (subgroup analysis). Whether there were statistically significant differences in the log-transformed relative risks of the dichotomous outcomes and the mean differences of the continuous outcomes between lipid-based nutrient groups and control groups was examined by changing from the categories in subgroup analysis to their counterparts (meta-regression analysis). The variability in the results by excluding each one of the studies from the meta-analysis was also evaluated (sensitivity analysis). Publication bias was assessed using Egger’s test (publication bias assessment).15
RESULTS
Eleven articles were finally eligible for the analysis (Table 1, Figure 1).2–12 The following articles including duplicated mother–baby pairs were integrated into single data sources: that is, 1) two articles by Ashorn et al.3,4; 2) two articles by Huybregts et al.,6,7 one article by Lanou et al.,9 and one article by Toe et al.12; and 3) two articles by Mridha et al.10,11 Therefore, six data sources were included in the meta-analysis. All of the six data sources evaluated from four to 11 birth outcomes (Table 1). The same number of studies as these birth outcomes were extracted from each of the data sources, and consequently a total of 58 studies with 101,553 mother–baby pairs were included in the meta-analyses to evaluate the relative risks of low birthweight, SGA, preterm birth, stillbirth, abortion, perinatal death, underweight, and stunting (Figure 1, Supplemental Table 1) and the mean differences of birthweight, birth length, head circumference, arm circumference, WAZ, and HAZ (Figure 1, Supplemental Table 2).2–12 Whereas articles with duplicated data were integrated into single data sources,6,7,9,12 one study to evaluate the mean difference of chest circumference,6,7,9,12 ponderal index,6,7,9,12 or WHZ5 was excluded because of the infeasibility of the meta-analysis.
Table 1.
Characteristics of the included studies
| Author (year) | Country | Outcome | Interventions | Controls | ||
|---|---|---|---|---|---|---|
| Energy (kcal) | Nutrient contents added to controls | Energy (kcal) | Composition | |||
| Adu-Afarwuah (2015) | Ghana | LBW, SGA, PB, SB, AB, PD, BW, BL,HC, WAZ, and HAZ | 118 | Mg, P, K, Ca, protein, and lipid | 0 | Other MMN; IFA |
| Ashorn (2015) | Malawi | LBW, SGA, PB, SB, AB, PD, UW, ST, BW, BL, and AC | 118 | Mg, P, K, Ca, protein, and lipid | 0 | Other MMN; IFA |
| Callaghan-Gillespie (2017) | Malawi | SB, AB, UW, ST, BW, BL, HC, AC, WAZ, and HAZ | 920 | Lipid | 893 | UNIMAP and CSB; IFA and CSB |
| Huybregts (2009, 2013)* | Burkina Faso | LBW, SGA, PB, SB, AB, PD, ST, BW, BL, HC, and AC | 373 | Ca, protein, lipid, carbohydrates, and DF | 0 | UNIMAP |
| Johnson (2017) | Gambia | LBW, SGA, PB, and WAZ | 746 | Protein and lipid | 0 | Nearly 2-fold UNIMAP; IFA |
| Mridha (2015, 2017) | Bangladesh | LBW, SGA, PB, SB, AB, UW, ST, BW, BL, HC, and AC | 118 | Mg, P, K, Ca, Cu, Zn, Se, I, protein, and lipid | 0 | IFA |
AB = abortion; AC = arm circumference; BL = birth length; BW = birthweight; CSB = corn–soy blend; DF = dietary fiber; HAZ = height-for-age z-score; HC = head circumference; IFA = iron folic acid; LBW = low birthweight; MMN = multiple micronutrients; PB = preterm birth; PD = perinatal death; SB = stillbirth; SGA = small for gestational age; ST = stunting; UNIMAP = United Nations multiple micronutrient preparation; UW = underweight; WAZ = weight-for-age z-score.
* Sources into which those of Huybregts (2009, 2013), of Lanou (2014), and of Toe (2015) were integrated.
Figure 1.
Flow diagram of the meta-analysis. Fifty-eight overall good-quality studies with 101,553 mother–baby pairs in five developing countries in Africa and Asia were extracted from 11 finally eligible articles.
Studies were conducted in four developing countries in Africa and one developing country in Asia (Table 1). Whereas three data sources described the gestational ages at enrollment, that is, < 20 weeks2–4 and ≥ 20 weeks,8 not all of the included studies described clearly the period of interventions. In addition, studies by Mridha et al. were subjected to interruption of delivery of lipid-based nutrients for a few months because of quality control.10,11 Study quality assessment indicating more studies with low and unclear risk of bias versus fewer studies with high risk of bias, that is, longer black and gray bars versus shorter white bars in Supplemental Figure 1, suggested the overall good quality of the studies included in the analysis.
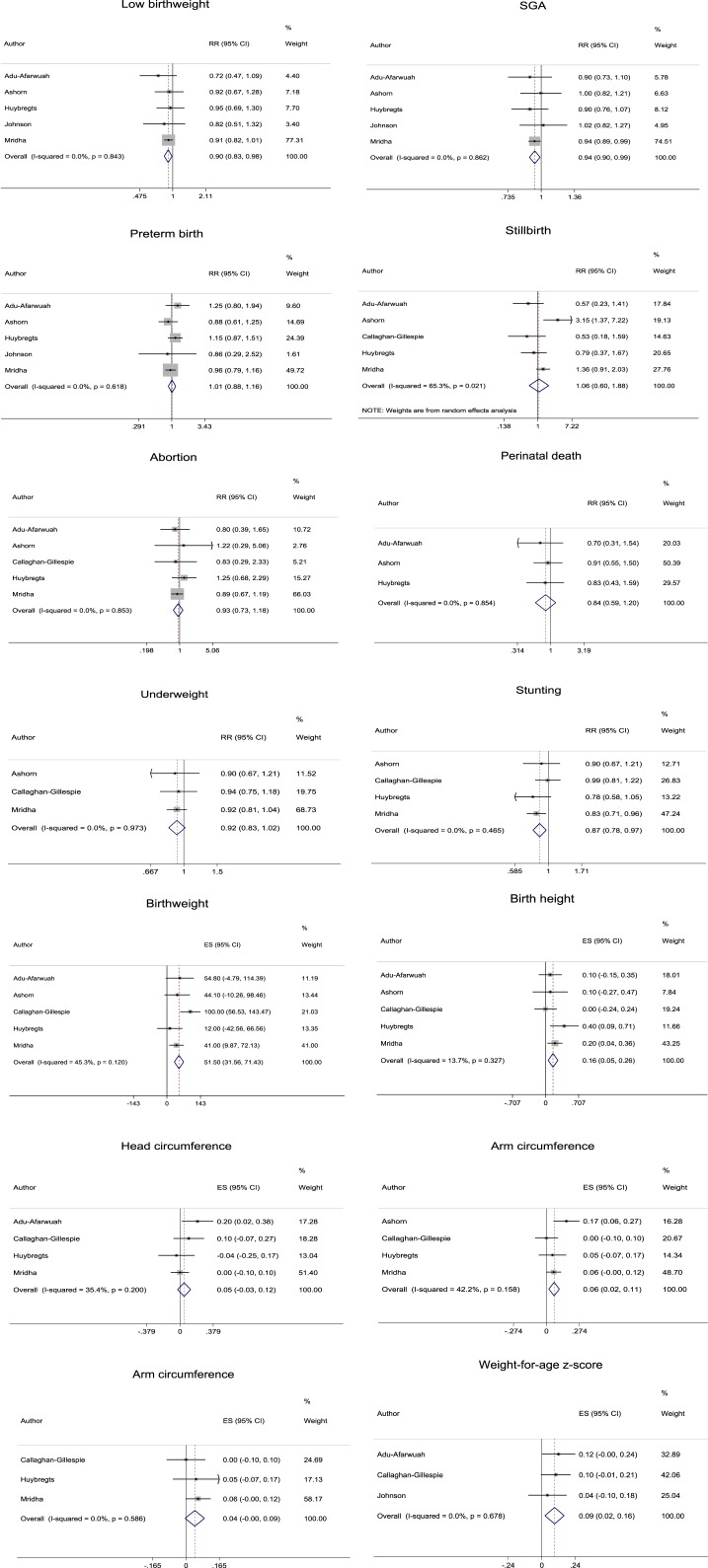
The data to evaluate the relative risks of low birthweight, SGA, preterm birth, abortion, perinatal death, underweight, and stunting and the mean differences in birthweight, birth length, head circumference, arm circumference, WAZ, and HAZ in the total population were summarized using a fixed-effects model because of homogeneity (I2 = 0.0–45.3%) (Figure 2, Supplemental Tables 1 and 2). The data to evaluate the relative risks of stillbirth were summarized using a random-effects model because of heterogeneity (I2 = 65.3%). Although homogeneity in the data to evaluate the relative risk of stillbirth was achieved with selection of studies limiting energy content of lipid-based nutrients to 373–920 kcal (I2 = 0.0%), there was no alteration of the reductions in risk of stillbirth by supplementation with lipid-based nutrients compared with IFA, UNIMAP, other MMN, and CSB (i.e., controls) from nonsignificant to significant.
Figure 2.
Forest plots of meta-analysis. RR = relative risk; SGA = small for gestational age. This figure appears in color at www.ajtmh.org.
In the total population, the risks of low birthweight, SGA, and stunting were significantly reduced (n = 5, 5, and 4, respectively) and the means of birthweight, birth length, arm circumference, and WAZ were significantly increased (n = 5, 5, 4, and 3, respectively) by providing lipid-based nutrient supplementation compared with controls (Figure 2, Supplemental Tables 1 and 2). The numbers needed to treat (NNTs) for low birthweight, SGA, and stunting were 45.45 (95% CI: 25.00–250.00), 37.04 (95% CI: 19.61–333.33), and 33.33 (95% CI: 19.23–125.00), respectively. In the total population, the risks of preterm birth, stillbirth, abortion, perinatal death, and underweight were not significantly reduced (n = 5, 5, 5, 3, and 3, respectively), and the means of head circumference and HAZ were not significantly increased (n = 4 and 2, respectively) by providing lipid-based nutrient supplementation compared with controls.
The significant reductions in risks of low birthweight, SGA, and stunting due to lipid-based nutrient supplementation compared with controls became nonsignificant with selection of the following categories in subgroup analysis: that is, Africa, lipid-based nutrient limited to 373–920 kcal, and inclusion of UNIMAP and other MMN (low birthweight, SGA, or stunting) (Supplemental Table 1). The significant increases in the means of birthweight, birth length, and arm circumference due to lipid-based nutrient supplementation compared with controls became nonsignificant with the selection of the following categories in the subgroup analysis: that is, lipid-based nutrient limited to 373–920 kcal (birthweight), Malawi, Africa; lipid-based nutrient limited to 373–920 kcal; and inclusion of UNIMAP and other MMN (birth length or arm circumference) (Supplemental Table 2). No other significant reductions in risks of low birthweight, SGA, preterm birth, stillbirth, abortion, perinatal death, underweight, and stunting and no other significant increases in means of birthweight, birth length, head circumference, arm circumference, WAZ, and HAZ became nonsignificant by selection of any category in the subgroup analysis and vice versa.
Within the limits of availability of P-values, no covariates were considered to be confounders of any outcome (P = 0.091–0.976) (Supplemental Tables 1 and 2). The significant reductions in risks of low birthweight, SGA, and stunting and the means of birth length and arm circumference due to lipid-based nutrient supplementation compared with controls became nonsignificant with exclusion of the study by Mridha et al. (Supplemental Tables 3 and 4).10,11 Egger’s test detected no publication bias in the data used to evaluate any outcome by including more than two studies (P = 0.160–0.965).
DISCUSSION
The results of the present study indicated that prenatal lipid-based nutrient supplementation is effective compared with controls for reducing some adverse birth outcomes (Figure 2, Supplemental Tables 1 and 2). The findings reported here were based on a total of 58 studies with 101,553 mother–baby pairs2–12 and were, therefore, generalizable (external validity). Studies conducted in Africa were extracted from five data sources. On the other hand, studies conducted in Asia were extracted from one data source but were the most influential, as discussed in the following paragraphs. This may have supported the generalizability of these findings both to African and Asian contexts. They were also based on the inclusion of overall good-quality studies, as indicated by the greater number of studies with low or unclear risk of bias versus fewer studies with high risk of bias (Supplemental Figure 1), and were, therefore, unlikely to have been affected by serious bias (internal validity). Based on NNTs for low birthweight, SGA, and stunting and mean differences of birthweight, birth length, arm circumference, and WAZ, the impact on birth outcomes was not great but cannot be ignored because lipid-based nutrient supplementation is relatively cheap and can be provided easily to large communities.
Interpretation of the results was not seriously affected by heterogeneity sources, confounders or publication bias. Sensitivity analysis indicated alternation of the reductions in risks of low birthweight, SGA, and stunting and the means of birth length and arm circumference by supplementation with lipid-based nutrients compared with controls from significant to nonsignificant by exclusion of the study by Mridha et al. (Supplemental Tables 3 and 4).10,11 The forest plot showed that their study was not an outlier, but instead was the most influential, that is, the largest weighted, among all of the studies included in the analysis (Figure 2) at least in part because of the greater number of mother–baby pairs than in other included studies, that is, 3,449 versus 620–1,467, respectively. Theirs was also the only study to show significant reduction in the risk of SGA or stunting by supplementation with lipid-based nutrient compared with controls at least in part because of the use of FA alone as a control (Table 1).
The study by Mridha et al.10,11 was the most influential, but the interpretation of the results was supported by the findings of other studies included in the analysis. That is, the mean values of the relative risks of low birthweight, SGA, and stunting by supplementation with lipid-based nutrient compared with controls were < 1 in all, all but one, and all of the studies included in the analysis, respectively (Figure 2). The forest plot showed that the relative risk of SGA had a mean value of 1.00 in the study by Ashorn et al.,3,4 but the exact value was 0.996. The means of birth length, arm circumference, and WAZ were also ≥ 0 in all of the studies included in the analysis. Furthermore, there was no alternation from significant to nonsignificant for the increases in mean values of birthweight due to lipid-based nutrient supplementation compared with controls by exclusion of the study by Mridha et al. (Supplemental Table 4).10,11 A significant mean difference of WAZ due to lipid-based nutrient supplementation compared with controls was also observed by summarizing the data in the studies other than those by Mridha et al. (Figure 2).10,11
Subgroup analysis revealed further alternation of the reductions in risks of low birthweight, SGA, and stunting and of the increases in means of birthweight, birth length, and arm circumference by supplementation with lipid-based nutrients compared with controls from significant to nonsignificant by selecting several categories (Supplemental Tables 1 and 2). All of these categories excluded the most influential study, that is, the study by Mridha et al. (Figure 2).10,11 Based on the findings of sensitivity analysis, this was probably due at least in part to the exclusion of the study by Mridha et al.10,11 rather than due just to the selection of these categories per se. That is, it may be inappropriate to conclude that prenatal lipid-based nutrients confer no benefit with regard to reducing the risks of low birthweight, SGA, and stunting or for increasing birthweight, birth length, and arm circumference specifically in these categories.
The recently published Cochrane review excluded the article by Johnson et al.8 that was included in the present study because the intervention did not involve the use of lipid-based nutrients only but it also involved a mix of lipid-based nutrient plus protein energy (PE).16 However, the nutrient contents that the intervention added to the control in other included studies included those reported in the article by Johnson et al. (i.e., protein and lipid)16 (Table 1). In addition, the study objective of their article was to test the associations of lipid-based nutrients with fetal growth. Their conclusions were regarding the effectiveness of lipid-based nutrients. Therefore, the present study included the studies extracted from the article by Johnson et al.
The Cochrane review also concluded that lipid-based nutrient supplementation has positive effects on birth size and stunting compared with IFA.16 However, the Cochrane review did not conclude the effects of lipid-based nutrients compared with MMN. This was at least in part because the Cochrane review excluded the article by Callaghan-Gillespie et al. using MMN plus CSB as a control,5 according to its inclusion criteria that limited controls to no regimen or a single regimen. When the data in the article by Callaghan-Gillespie et al. were added to the data in studies included in the Cochrane review, synthetic evidence suggested statistically significant differences in the mean values of birthweight (g) and head and arm circumferences (cm) between lipid-based nutrients and MMN (plus CSB) (45.288, 95% CI: 3.631–86.946; 0.124, 95% CI: 0.006–0.242; and 0.084, 95% CI: 0.016–0.152, respectively). Whether differences were statistically significant was also independent of the addition of the data using lipid-based nutrient plus PE as an intervention in the article by Johnson et al.8 Therefore, the findings of the present study that lipid-based nutrients may be effective in improving birth size compared with MMN were more acceptable.
This study had a number of strengths, including consistency of the procedures with the guidance to conduct meta-analysis, that is, formulating a research question, setting primary outcomes and inclusion criteria, searching the literature, selecting studies, extracting data, performing statistical analyses, interpreting the results, and drawing conclusions.17 The second strength was the internal and external validity based on the inclusion of 58 overall good-quality studies (Supplemental Figure 1) with 101,553 mother–baby pairs. The third strength was the lack of serious effects on interpretation of the results by heterogeneity sources, confounders, and publication bias. However, the present meta-analysis also had some limitations, including the exclusion of non-English language studies and only a single person selected and reviewed the studies. The second limitation was the inapplicability of the results to groups not subjected to subgroup analysis. The third limitation was the alternations of some estimates from significant in the total population to nonsignificant in certain categories on subgroup analysis (Supplemental Tables 1 and 2) probably due at least in part to the exclusion of the most influential study10,11 rather than due to being related to these categories per se.
Supplemental material, tables and figure
Acknowledgments:
I thank the staff of the Medical Library, the Japan Medical Association (Tokyo, Japan), for their help in retrieving the full texts of the articles included in the analysis. A native English language check was performed by Dolphin Corporation (1005 Kichijyoji Nagatami City Plaza 1-20-1 Kichijyojihonmachi, Musashino, 180-0004, Japan, trust@dolphin-tr.com).
Note: Supplemental material, tables and figure appear at www.ajtmh.org.
REFERENCES
- 1.Woods N, Gilliland J, Seabrook JA, 2017. The influence of the built environment on adverse birth outcomes. J Neonatal Perinatal Med 10: 233–248. [DOI] [PubMed] [Google Scholar]
- 2.Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, Arimond M, Vosti S, Dewey KG, 2015. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr 101: 835–846. [DOI] [PubMed] [Google Scholar]
- 3.Ashorn P, et al. 2015. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. Am J Clin Nutr 101: 387–397. [DOI] [PubMed] [Google Scholar]
- 4.Ashorn P, et al. 2015. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. J Nutr 145: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan-Gillespie M, et al. 2017. Trial of ready-to-use supplemental food and corn-soy blend in pregnant Malawian women with moderate malnutrition: a randomized controlled clinical trial. Am J Clin Nutr 106: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Van Camp J, Kolsteren P, 2009. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. Am J Clin Nutr 90: 1593–1600. [DOI] [PubMed] [Google Scholar]
- 7.Huybregts L, Roberfroid D, Lanou H, Meda N, Taes Y, Valea I, D’Alessandro U, Kolsteren P, Van Camp J, 2013. Prenatal lipid-based nutrient supplements increase cord leptin concentration in pregnant women from rural Burkina Faso. J Nutr 143: 576–583. [DOI] [PubMed] [Google Scholar]
- 8.Johnson W, Darboe MK, Sosseh F, Nshe P, Prentice AM, Moore SE, 2017. Association of prenatal lipid-based nutritional supplementation with fetal growth in rural Gambia. Matern Child Nutr 13: e12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanou H, Huybregts L, Roberfroid D, Nikièma L, Kouanda S, Van Camp J, Kolsteren P, 2014. Prenatal nutrient supplementation and postnatal growth in a developing nation: an RCT. Pediatrics 133: e1001–e1008. [DOI] [PubMed] [Google Scholar]
- 10.Mridha MK, et al. 2016. Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am J Clin Nutr 103: 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mridha MK, Matias SL, Paul RR, Hussain S, Sarker M, Hossain M, Peerson JM, Vosti SA, Dewey KG, 2017. Prenatal lipid-based nutrient supplements do not affect pregnancy or childbirth complications or cesarean delivery in Bangladesh: a cluster-randomized controlled effectiveness trial. J Nutr 147: 1776–1784. [DOI] [PubMed] [Google Scholar]
- 12.Toe LC, Bouckaert KP, De Beuf K, Roberfroid D, Meda N, Thas O, Van Camp J, Kolsteren PW, Huybregts LF, 2015. Seasonality modifies the effect of a lipid-based nutrient supplement for pregnant rural women on birth length. J Nutr 145: 634–639. [DOI] [PubMed] [Google Scholar]
- 13.Haider BA, Bhutta ZA, 2017. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 4: CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S, 2011. Introduction to sources of bias in clinical trials. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. Available at: http://handbook-5-1.cochrane.org/chapter_8/8_4_introduction_to_sources_of_bias_in_clinical_trials.htm. Accessed April 15, 2019. [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Z, Bhutta ZA, 2018. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst Rev 8: CD012610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Altman DG, 2007. Systematic Reviews in Healthcare: Meta-Analysis in Context, 2nd edition London, United Kingdom: BMJ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.