Abstract.
Coinfections with malaria and soil-transmitted helminths (STHs) has been common among school-aged children in Tanzania. However, after a countrywide scaling up of interventions for malaria and STHs, there are limited data on the prevalence of malaria–STH coinfections and its effect on anemia in schoolchildren in Tanzania. We assessed the distribution and risk factors for malaria, STHs, and malaria–STH coinfections, and its relation to anemia among 445 primary schoolchildren in Muheza district. A semi-structured questionnaire was used to collect demographic characteristics of the children. Malaria rapid diagnostic test (mRDT) was used to diagnose malaria infection. Soil-transmitted helminths were diagnosed using the Kato–Katz technique. Primary outcome was anemia, defined as hemoglobin concentration < 11 g/dL. Chi-square (χ2) or Fisher’s exact tests, Kruskal–Wallis or t-test, and logistic models were used as appropriate. Overall, the prevalence of malaria, STHs, malaria–STH coinfection, and anemia were 18.4%, 6.1%, 1.6%, and 19.8%, respectively. Anemic children were more likely to have malaria (adjusted odds ratio [aOR] = 4.538, 95% CI: 2.189–9.409), whereas frequent use of bed nets was associated with reduced risk of malaria (aOR = 0.234, 95% CI: 0.130–0.42). On the other hand, not always using latrines and eating raw uncooked food increased the risk of STH infection. The prevalence of anemia was high and was associated with both malaria and malaria–STH infections, therefore calling for more integrated malaria–STH control approaches to target school-aged children.
INTRODUCTION
Malaria is still a public health problem in sub-Saharan Africa, despite the recent substantial gains in reduction of the infection as a result of scaled-up interventions.1 The change in malaria epidemiology has led to a shift of malaria burden from under-fives to older children.2,3 This change has consequences on malaria immunity, which is now acquired much slowly compared with the past when transmission was high.4
The African continent on the other hand continues to bare a heavy burden of helminth infections, and schoolchildren are experiencing the heaviest burden of the disease.5,6 The most common helminth infections are the soil-transmitted helminths (STHs): Ascaris lumbricoides, Trichuris trichiura, and hookworms, which were previously estimated to infect more than one-third of the continent’s population.5,7,8 Previous studies using predictive models have shown that in areas of stable malaria transmission, school-aged children are at high risk of both STH and malaria infections.9,10 The occurrence of coinfections is largely dependent on the prevalence of individual species, environmental factors such as temperature and humidity, and lack of effective control/preventive measures.10 The consequences of coinfections include impaired growth, anemia, immunity, low school attendance, and educational performance.2,10–12
In recent years, the epidemiology of malaria and helminth infections have shifted from high to moderate/low prevalence rates because of the scale-up of malaria control and deworming campaigns in sub-Saharan Africa.1,6 For malaria, the interventions include mainly the use of artemisinin-based combination therapy (ACT) to manage clinical cases and long-lasting insecticide-treated nets (LLINs) to control transmission.1 The school-based deworming program has also been implemented to control STHs.6,13 This program provides 400 mg single dose of albendazole to schoolchildren annually.14 These children also receive albendazole and ivermectin through community-directed distribution campaigns in areas targeted for lymphatic filariasis (LF) and onchocerciasis elimination.15 However, there are limited data on the prevalence of malaria–helminth coinfections and its effect on anemia in schoolchildren following the scale-up of these interventions in Tanzania. This study was therefore conducted to assess the distribution and risk factors for malaria, STHs, and malaria–STH coinfections, and its relation to anemia among schoolchildren in Muheza district, and guide policy-makers in deciding the best mechanisms to implement delivery of malaria and helminth interventions targeting school-aged children.
MATERIALS AND METHODS
Study area.
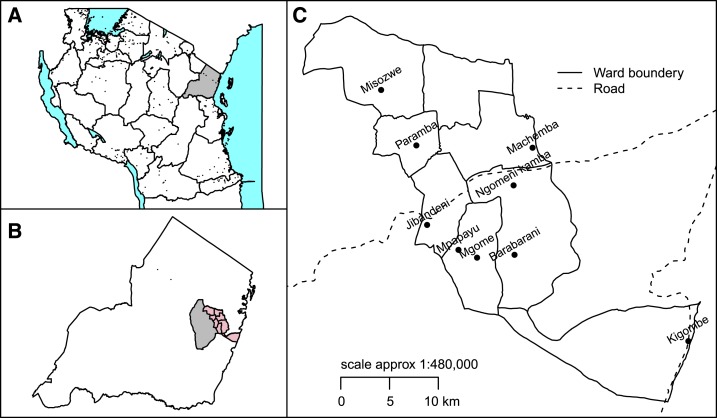
The study was conducted in Ngomeni division, one of the four administrative divisions in Muheza district, Tanga Region, Tanzania (Figure 1), between October and December 2014. Muheza district has tropical climate, with annual rainfall averaging 1,280 mm, which facilitates transmission for malaria and STH infections. Malaria control largely depends on case management using ACTs and transmission control using LLINs. School deworming program targeting STHs has also been implemented in this district since 2005 through the National Schistosomiasis and STH Control Program. Muheza district is also among the LF-endemic districts in Tanzania and has been receiving mass drug administration (MDA) of ivermectin and albendazole for the control/elimination of LF since 2004.15 The community coverage of MDA using ivermectin and albendazole for LF has decreased from 82% in 2004 when the program was initiated to around 68% in 2010, whereas that of albendazole for STH is estimated to be > 90%.16 Following years of albendazole plus ivermectin MDA, Muheza district was confirmed to have interrupted the transmission of LF in the year 2014, and MDA was stopped in 2015. Most Ngomeni residents depend on subsistence farming where the main crops are rain-fed rice, maize, oranges, and vegetables.
Figure 1.
Map of Tanzania (A) showing Tanga Region (gray), Muheza district (B) together with wards of Ngomeni Division. (C) Locations of primary schools in Ngomeni division. This figure appears in color at www.ajtmh.org.
Study design and study population.
This was a school-based cross-sectional study conducted among primary school pupils attending nine randomly selected primary schools (of 31 schools). Pupils from class one to six who attended the school on the day of the survey were invited to participate. The number of pupils per school was determined using proportion to sample size. Children were selected randomly from each school using a random number list which was generated corresponding to the number of pupils during the survey.
PROCEDURES
Sociodemographic data collection.
A semi-structured questionnaire was used to collect participants’ demographic information, and practices toward malaria and STHs. The demographic information collected included age, gender, and grade, whereas the information on practices included the use of insecticide-treated nets (ITNs), participation on deworming campaign, presence of latrines, latrine use, eating habit, hand-washing practices, source of water for domestic use, and wearing of shoes.
Blood sample collection and analysis.
Finger-prick blood samples were collected for diagnosis of malaria using malaria rapid diagnostic test (mRDT) (SD Bioline Malaria Ag P. f/Pan test, Abbot Laboratories, Lake Forest, IL), thick blood smears for microscopy, and for measurement of hemoglobin concentration using HemoCue machine (hemoglobin [Hb] 201 + (HemoCue AB, Ängelholm Sweden). When testing for malaria using mRDT, the test was considered invalid in case the control line did not appear, and the test was repeated immediately. Blood smears were stained using 10% Giemsa solution for 30 minutes and examined under a light microscopy at a magnification of 100 to detect parasite and determine its density. Malaria parasites were counted against 200 white blood cells (WBCs) for parasites ≥ 100 rings and against 500 WBCs if malaria parasites were less than 100 rings.17 The obtained parasite density value per WBC was converted into parasites per microliter, assuming 8,000 WBCs per microliter of blood. A blood slide was declared negative if no malaria parasites were seen in 100 consecutive fields. Two qualified microscopists read the blood slides. The microscopists were blinded to the mRDT results. In case of discordant results between the two readers or when parasite density difference between the two readers was two-folds or more, then the reading was subjected to a third independent reader. The average of the two close results was deemed the correct result. Children diagnosed with malaria based on mRDT test were treated with artemether–lumefantrine (20 mg/120 mg), with exception of children with a recent history of treatment of malaria in the past 2 weeks.
Stool sample collection and analysis.
Stool samples were collected from the participants, and the presence and egg counts of STHs were determined using the Kato–Katz technique. One slide per stool sample was prepared using a fixed quantity of sieved 41.7 mg of stool on a punched template. The sieved stool was mounted on a slide and covered with a malachite green–impregnated coverslip and left to clear for 30 minutes before examining under a microscopy. The parasite egg concentration was expressed as egg per gram (EPG) of stool. The number of eggs counted in the Kato–Katz thick smear was multiplied by a factor of 24 to obtain a standard infection intensity measure, which is expressed in EPG of stool. The intensity of STH infection was classified into three categories, that is, light, moderate, and heavy infection, according to the WHO guideline.18 Children diagnosed with STHs were treated with albendazole (400 mg) in accordance with the national treatment guidelines.
Ethical considerations.
The Ethics Committee of Muhimbili University of Health and Allied Sciences approved the study. Parents/guardians were invited at the schools and provided written consents for their children to participate in the study. Verbal assent was also obtained from pupils who agreed to participate, following the informed consent signed by their parents.
Sample size and statistical analysis.
The sample size was estimated using sample for population estimate, where prevalence was assumed to be 50%, which provides the maximum sample size. The margin of error was set at 5%, an alpha of 0.05. The proportion of 50% was chosen to ensure sufficient power for the three health conditions (malaria, geo-helminths, and anemia). An attrition rate of 10% was assumed, and a sample size of 423 was deemed sufficient.
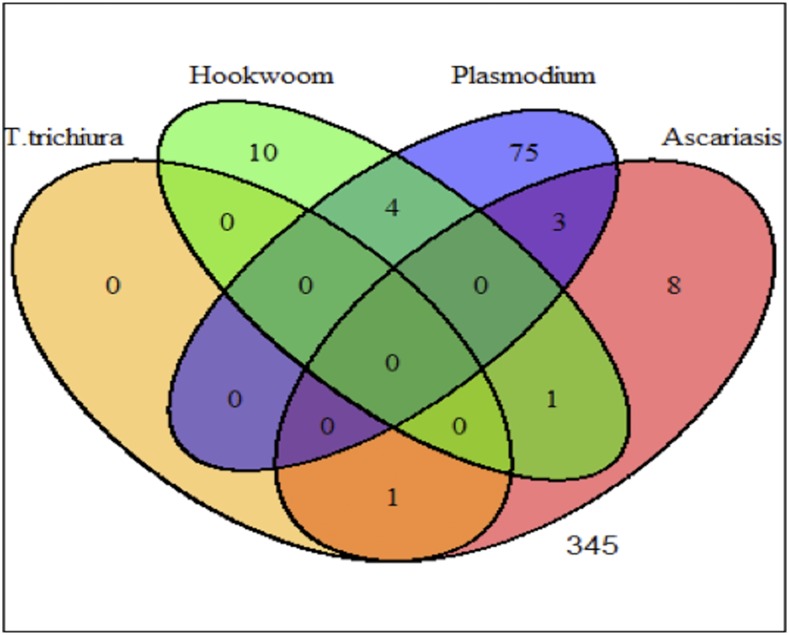
Collected data using paper-based questionnaires were entered in the Statistical Package for Social Sciences (SPSS) database and analyzed using STATA version 13 and R statistical packages. Schools’ geographical positions were collected using tablets in Open Data Kit application. Frequencies of sociodemographic data of participants, and presence of malaria infection by mRDT or blood slide, and malaria–STH coinfections were determined. Regardless of age and gender, anemia was classified as hemoglobin concentration below 11 g/dL. Because of few numbers of individuals coinfected with malaria and STHs, three groups of coinfections were defined: 1) malaria (Plasmodium) alone, 2) malaria (Plasmodium) + any STH, and 3) any combination of STH (Figure 2). Children were also stratified into two age groups, children aged ≤ 10 years and those aged > 10 years. Geographical position (coordinates) was exported to R and linked to base maps of Tanzania at ward levels to indicate the location of the school. The distance of the school to the nearby point (point to point) on the main road network (trunk and primary roads) was calculated as proxy for accessibility.
Figure 2.
Venn diagram showing distribution of malaria (Plasmodium) and soil-transmitted helminths. This figure appears in color at www.ajtmh.org.
Continuous variables were summarized using medians with interquartile ranges (IQRs) for non-normal distributions, whereas for normally distributed variables, a mean ± SD was used. Frequencies were compared using χ2 or Fisher’s exact tests, whereas continuous variables were compared using Kruskal–Wallis or t-test. Unadjusted odds ratio (OR) with 95% CI was used to assess the association between response variable (the presence of malaria [Plasmodium] infection according to mRDT, helminths, or anemia) against an explanatory variable. Multiple logistic regressions were performed to estimate the adjusted odds ratio (aOR) between the response variable and set of explanatory variables to control for confounding. Associations were deemed significant if P-values were less than 0.05.
RESULTS
General findings.
A total of 445 pupils (median age 11 years, 52.8% females) from nine primary schools were involved in the survey. About 35.1% (156/445) of the pupils were in the grades 1 and 2. More than 60% of children reported to have participated in the deworming campaign 1 year before this survey. The overall reported use of ITNs was 71.7%, with Jibangeni and Kigombe pupils reporting the highest use above 92%, whereas Mpapayu pupils reported the lowest at 32.7% (Table 1). Most schools were situated within 5 km from the main roads, with Misozwe and Barabarani been located at the furthest (Table 1).
Table 1.
Characteristics of the study participants
| School name | Number of children | Distance to road (km) | Altitude (metres above sea level) | Median age (interquartile range) | Bed net use (%) |
|---|---|---|---|---|---|
| Barabarani | 25 | 8.1 | 103.3 | 11 (10–13) | 72 |
| Jibandeni | 51 | 0.7 | 209.7 | 11 (9–12) | 96.1 |
| Kigombe | 50 | 0.1 | 4.4 | 13 (11–13) | 92.0 |
| Machemba | 20 | 2.3 | 88.1 | 11 (10–13) | 65.0 |
| Mgome | 42 | 6.1 | 210 | 10 (9–12) | 61.9 |
| Misozwe | 54 | 12.9 | 235.6 | 11 (9–13) | 61.1 |
| Mpapayu | 55 | 4.2 | 260 | 10 (8–13) | 32.7 |
| Ngomeni Kamba | 96 | 1.7 | 132.9 | 10 (9–12) | 83.3 |
| Paramba | 42 | 6.5 | 194 | 11 (9–13) | 69.2 |
| Total*/average | 445* | 4.4 | 166.2 | 11 (9–13) | 71.7 |
Represent total number of children assessed.
Prevalence of malaria, STHs, and malaria–STH coinfections.
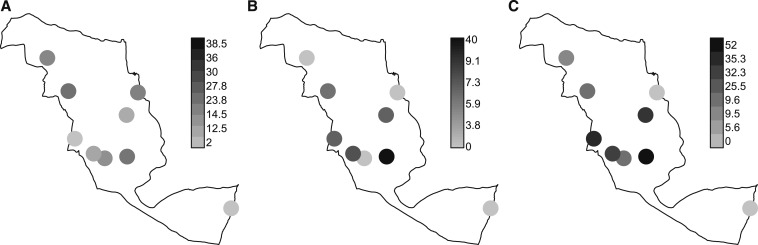
Overall, the prevalence of malaria infection based on mRDT was 18.4% (82/445) (95% CI: 14.8–22.0). Barabarani, Paramba, and Machemba primary schools had the highest prevalence of malaria, whereas Kigombe and Jibandeni schools had the lowest (Figure 3A). Microscopy results were not available for pupils from three schools (Barabarani, Jibandeni, and Ngomeni Kamba). Of the pupils with blood slide results, 17.6% (48/273) (95% CI: 13.0–22.1) had malaria infection, with a geometric mean parasite density of 620.4 (95% CI: 397.3–968.8) parasites per microliter of blood. The corresponding mRDT positivity from schools with blood slide results was 22.0% (60/273) (95% CI: 17.0–26.9), with only 56.7% (34/60) of the pupils who were positive by mRDT being positive by blood slides. Further analysis was based on mRDT results.
Figure 3.
Distribution of malaria infection based on malaria rapid diagnostic test (A), any soil-transmitted helminth (B), and anemia (C) by school.
The overall prevalence of STHs was 6.1% (27/445) (95% CI: 3.8–8.3), and most these infections were single infections: hookworm (51.9%, 14/27) and A. lumbricoides (40.7%, 11/27) infections. All the pupils with STHs had light-intensity infections (range: 200–1,000 EPG). The prevalence of malaria–STH coinfections was 1.6% (7/445) (95% CI: 0.4–2.7), and 57.1% (4/7) of them were malaria–hookworm coinfections. The prevalence of mixed STH infections was each 0.2% (1/445) for Ascaris–hookworm or Ascaris–Trichuris (Figure 2).
The STH (all STHs combined) prevalence was highest in Barabarani primary school (40%) and was followed by Mpapayu (9.1%) (Figure 3B). Similarly, Barabarani had the highest prevalence of anemia, whereas Kigombe and Machamba had no case of anemia. Figure 3C shows high prevalence of anemia to occur in schools with either high prevalence of malaria infection and/or STH and vice versa.
Risk factors for malaria infection.
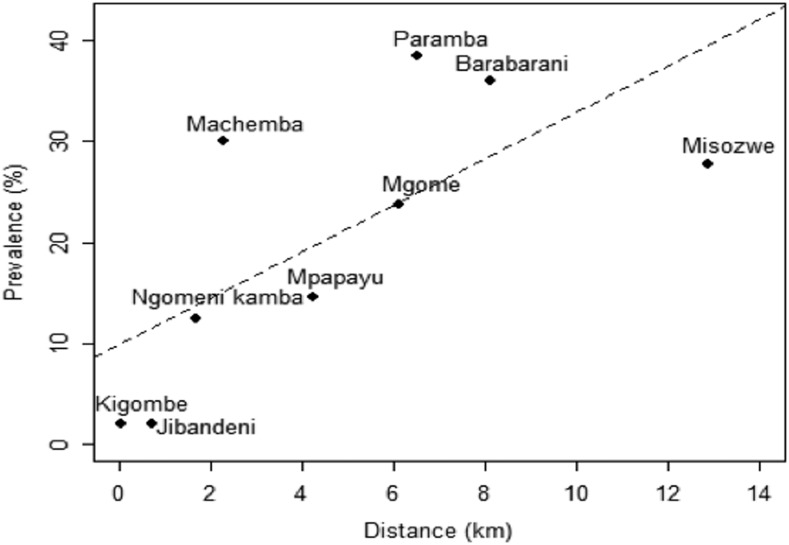
In univariate analysis, the risk of malaria infection was low in children who were frequently using bed nets (OR = 0.236, 95% CI: 0.143–0.389, P < 0.001) compared with those with infrequent use of nets. The risk of malaria infection was higher in anemic children (OR = 2.254, 95% CI: 1.314–3.866). Schools far from the main roads had a higher risk of malaria infection, where the risk increased by 15.1% (95% CI: 8.7–21.9%, P < 0.001) for increase in distance by 1 km from the main road (Figure 4).
Figure 4.
Relationship between malaria rapid diagnostic test prevalence and distance of the study school (dots) with line of best-fit superimposed (dashed line).
The multivariate logistic regression model showed that frequent use of ITNs was associated with reduced risk of acquiring malaria infection compared with nonuse of ITNs (aOR = 0.234, 95% CI: 0.130–0.42, P = 0.001). Pupils with anemia were more likely to have malaria (aOR = 4.538, 95% CI: 2.189–9.409) (Table 2).
Table 2.
Risk factors associated with malaria infection in a logistic regression model
| Variable name | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | Adjusted odds ratio | 95% CI | P-value | |
| Age-group | 0.927 | 0.572–1.501 | 0.758 | – | – | – |
| Gender (male) | 1.219 | 0.754–1.969 | 0.419 | – | – | – |
| Anemia | 2.254 | 1.314–3.866 | 0.003 | 4.538 | 2.189–9.409 | < 0.001 |
| Distance | 1.151 | 1.087–1.219 | < 0.001 | – | – | – |
| Altitude | 1.003 | 1.000–1.007 | 0.050 | – | – | – |
| Bed net | 0.236 | 0.143–0.389 | < 0.001 | 0.234 | 0.130–0.420 | < 0.001 |
| Latrine use | 3.310 | 1.641–6.673 | 0.001 | – | – | – |
| Barabarani | 1 | – | – | 1 | – | – |
| Jibandeni | 0.036 | 0.004–0.303 | 0.002 | 0.052 | 0.006–0.469 | 0.008 |
| Kigombe | 0.036 | 0.004–0.309 | 0.002 | 0.108 | 0.012–1.010 | 0.051 |
| Machemba | 0.762 | 0.217–2.68 | 0.672 | 1.564 | 0.374–6.546 | 0.541 |
| Mgome | 0.556 | 0.188–1.64 | 0.287 | 0.869 | 0.251–3.011 | 0.825 |
| Misozwe | 0.684 | 0.249–1.879 | 0.461 | 1.173 | 0.359–3.838 | 0.792 |
| Mpapayu | 0.303 | 0.100–0.917 | 0.035 | 0.223 | 0.064–0.773 | 0.018 |
| Ngomeni Moja | 0.254 | 0.092–0.702 | 0.008 | 0.352 | 0.116–1.066 | 0.065 |
| Paramba | 1.111 | 0.413–2.989 | 0.835 | 2.204 | 0.693–7.010 | 0.181 |
Altitude and distance were excluded from multivariate model because of high multicollinearity with school.
Risk factors for helminth infection.
A logistic regression model presented in Table 3 shows that the factors that were associated with the helminth infections in the univariate model were also significant in the multivariate model. Not always using latrines, eating raw unwashed food, and lack of use of antihelminthic drugs in the year of the study were the factors associated with increased risk of helminth infection. Male pupils had twice the risk of helminth infection although the association was marginally significant (P = 0.084). Age and other social demographic factors were not associated with the risk of having helminth infections (Table 3).
Table 3.
Risk factors associated with helminth infection in a logistic regression model
| Variable | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | Adjusted odds ratio | 95% CI | P-value | ||
| Age (years) | 1.014 | 0.852–1.208) | 0.875 | – | – | – | |
| Age-group (≤ 10 years) | 0.964 | 0.44–2.11 | 0.927 | – | – | – | |
| Gender (male) | 1.982 | 0.887–4.43 | 0.096 | 2.095 | 0.904–4.855 | 0.084 | |
| Source of water (tap) | 0.427 | 0.153–1.194 | 0.105 | 0.530 | 0.179–1.564 | 0.250 | |
| Latrine present (yes) | 0.498 | 0.108–2.285 | 0.369 | – | – | – | |
| Not always use latrines | 4.369 | 1.72–11.13 | 0.002 | 1.853 | 1.1–3.122 | 0.020 | |
| Wash hands before meals | 0.628 | 0.139–2.840 | 0.546 | – | – | – | |
| Not eating raw unwashed food | 0.310 | 0.117–0.820 | 0.018 | 0.303 | 0.099–0.933 | 0.037 | |
| Wear shoes | 0.804 | 0.369–1.753 | 0.584 | – | – | – | |
| Use of antihelminths | Not used recently | 1 | |||||
| Current year | 0.335 | 0.109–1.031 | 0.057 | 0.289 | 0.086–0.964 | 0.043 | |
| 1–2 years ago | 0.786 | 0.323–1.901 | 0.594 | 0.986 | 0.39–2.493 | 0.976 | |
Prevalence and risk factors for anemia.
Overall, the prevalence of anemia among pupils was 19.8% g/dL (95% CI: 16.1–23.5 g/dL), with no pupil having severe anemia (Hb < 8 g/dL). The median hemoglobin concentration was higher (12.1 g/dL; IQR, 12.0–12.3 g/dL) among children with mRDT negative than among those with positive mRDT tests (11.8 g/dL; IQR, 11.5–12.2; P = 0.052). The univariate and multivariate analyses showed that age > 10 years, positivity for malaria infection, and malaria–STH coinfections were factors associated with anemia (Table 4). Malaria infection was associated with more than 3-fold increase in the risk of anemia (P = 0.001), whereas the coinfections of malaria and STHs had a huge impact on anemia, with risk being 24 times higher (95% CI: 2.315–242.6; P = 0.008) than that in uninfected children. The wider CI can be explained by the small sample size of children with coinfections of malaria and STHs.
Table 4.
Risk factors for anemia
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | Adjusted odds ratio | 95% CI | P-value | |
| Age-group (≤ 10 years) | 1 | – | – | – | – | – |
| Age-group (> 10 years) | 0.457 | 0.284–0.735 | 0.001 | 0.431 | 0.249–0.749 | 0.003 |
| Gender (male) | 1.087 | 0.682–1.734 | 0.726 | – | – | – |
| Altitude (mASL) | 1.002 | 999–1.005 | 0.231 | 0.973 | 0.958–0.988 | < 0.001 |
| No parasite | 1 | – | – | – | – | – |
| Any STHs | 1.496 | 0.303–7.393 | 0.621 | 1.542 | 0.532–4.470 | 0.425 |
| Malaria (malaria rapid diagnostic test + ve) alone | 1.904 | 1.058–3.426 | 0.032 | 3.640 | 1.715–7.728 | 0.001 |
| Malaria + any STH | 31.42 | 3.709–266.12 | 0.002 | 23.70 | 2.315–242.6 | 0.008 |
| Not always use latrine | 2.010 | 0.971–4.163 | 0.060 | – | – | – |
| Not eating raw unwashed food | 0.366 | 0.184–0.729 | 0.004 | – | – | – |
| Not wearing shoe | 2.836 | 1.698–4.737 | < 0.001 | 1.710 | 0.899–3.252 | 0.102 |
STH = soil-transmitted helminth.
DISCUSSION
We conducted this study to assess the prevalence of malaria and STH infections and their impact on anemia among primary school pupils in Muheza district after several rounds of MDAs for STH and LF and scale-up of malaria interventions. Our findings show that there was high prevalence of malaria infection (> 18%) and anemia (> 19%) among pupils in Ngomeni division, Muheza district. The prevalence of STHs was low (about 6%), contributing to low (< 2%) prevalence of malaria–STH coinfections among pupils. The high prevalence of malaria infection observed among pupils is similar to the recent nationwide school malaria prevalence of 21%, marked by high variation across transmission and administrative zones in Tanzania.19 The nationwide survey showed that the northeastern zone (Tanga Region) had malaria prevalence ranging from 10% to 25%.19 However, the malaria prevalence in this study is much higher than that reported recently in Morogoro and elsewhere in Tanzania.20–23 Malaria infection was more common in children with infrequent use of bed nets and in schools located far away from the main road. Our findings are similar to the findings of the nationwide malaria survey and those of other previous studies, which showed a low prevalence of malaria in semi-urban areas compared with rural areas.19,21
We did not observe any association between age and malaria prevalence similar to the nationwide malaria school survey.19 Previous studies in Tanzania and other parts of Africa have shown an age shift in malaria infection with prevalence being higher in age-group > 10 years than in lower age-groups.20,21,24,25 This might probably suggest the influence of other factors on malaria transmission, including environmental and climatic factors.21,25,26
There was marked low prevalence of STHs infection among schoolchildren, and, similar to other previous studies, this was associated with frequent use of latrines and recent use of antihelminthic drugs.27 Frequent use of improved latrines reduces the rates of reinfections after antihelminthic treatment.28,29 However, similar to a study in Kenya,29 other water, sanitation, and hygiene conditions such as handwashing and wearing of shoes were not significantly associated with STH infections. The relatively low prevalence of STH in our study area may have contributed to the observed very low prevalence of malaria–STH coinfections. The low prevalence of STH infection could be attributed to the effect of several rounds of school-based antihelminthic MDA with albendazole against STHs and LF MDA with ivermectin/albendazole.15,30,31 The MDA program for control and elimination of LF was introduced in 2004 and that for STHs in schoolchildren started in 2009.15 Antihelminthic drugs reduce transmission of parasites by decreasing worm load and shedding of eggs.14,28 High prevalence of malaria–STH coinfections have been reported in areas with high prevalence of both malaria and helminth infections and with no or few rounds of MDAs.32–34
Anemia, on the other hand, is a public health problem among schoolchildren in malaria-endemic areas. Effects of anemia include impaired physical growth, cognition, and school performance.9,33,35 Although causes of anemia are multifactorial in the tropics including helminth infections, hemoglobinopathies, and malnutrition, scale-up of malaria interventions including use of LLINs, ACT, and indoor residual spraying, and antihelminthic MDA led to the decline of prevalence of anemia among schoolchildren.1,31 Our findings showed that there was a significant association between anemia and malaria or malaria–STH coinfections. The high increase (more than 24-folds) in risk of anemia among children with malaria–STH coinfections is alarming. Our findings are similar to those of previous studies, which have shown malaria and helminth (mainly hookworm) coinfections to have profound effect on anemia in school-aged children.9,36–38 Contrary to our findings, a recent review by Degarege et al. has shown lower risk of anemia in children with malaria–STH coinfections than in children with malaria alone.35 However, sub-analysis of the reviewed studies showed that the risk of anemia was higher in children with malaria–hookworm coinfections than in those with other STHs alone.35 This calls for an integrated approach in malaria and helminth control. Presently, the National Malaria Control Program and Neglected Tropical Disease Control Program work independently in the delivery and evaluation of the impact of the interventions. It is now becoming clear that distribution of LLINS and MDA with ivermectin and albendazole have positive effects on STHs and malaria.39,40 The same community health workers involved in LLINs distribution can be employed in STHs MDA campaign; this will lead to an increase in the uptake of antihelminthic drugs and LLINs.
We recognize a number of limitations in our study, including the use of parasitological tests mRDT and microscopy, which cannot detect asymptomatic infections at the submicroscopic level. In addition to mRDT, only microscopy thick smear was used to detect asexual malaria parasites; thus, we were not able to determine the species of the infecting malaria parasites. Furthermore, some schools had mRDT results only, and the test is known to yield false results mainly because of persistent histidine-rich protein II, following antimalarial treatment. However, recent studies have shown mRDT to be more reliable than microscopy.41,42 In addition, collection of single slides for STHs might have biased the findings considering day-to-day variability of STH eggs excretion as well as in children with low intensity. Inclusion of schoolchildren present on the day of the survey may introduce minimal selection bias; however, our study team did community sensitization through school, ward, and village meetings with leaders who communicated with the parents. We anticipated this approach reduced the effect of selection bias. We also acknowledge that sample size in some schools was small and this could adversely affect generalization of our findings. We did not collect some household information that could have provided more insight on sanitation and hygiene practices related to helminth control and ownership, and use of LLINs.
CONCLUSION
The prevalence of anemia was high and was associated with both malaria and malaria–STH infection, therefore calling for more integrated malaria–STH control approaches to target school-aged children.
Acknowledgments:
We would like to extend our profound gratitude to the primary schoolchildren from selected schools for participating in the study, as well as schools’ teachers and parents/guardians for their immense support.
REFERENCES
- 1.World Health Organisation , 2018. World Malaria Report. Geneva, Switzerland: World Health Organization; Available at: https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/. Accessed February 24, 2019. [Google Scholar]
- 2.Nankabirwa J, Brooker SJ, Clarke SE, Fernando D, Gitonga CW, Schellenberg D, Greenwood B, 2014. Malaria in school–age children in Africa: an increasingly important challenge. Trop Med Int Health 19: 1294–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nkumama IN, O’Meara WP, Osier FHA, 2017. Changes in Malaria epidemiology in Africa and new challenges for elimination. Trends Parasitol 33: 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pemberton-Ross P, Smith TA, Hodel EM, Kay K, Penny MA, 2015. Age-shifting in malaria incidence as a result of induced immunological deficit: a simulation study. Malar J 14: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, et al. 2006. Helminth infections: soil-transmitted Helminth infections and schistosomiasis. Disease Control Priorities in Developing Countries. Washington, DC. [Google Scholar]
- 6.Okoyo C, et al. 2016. Monitoring the impact of a national school based deworming programme on soil-transmitted helminths in Kenya: the first three years, 2012–2014. Parasite & Vectors 9: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooker S, Hotez PJ, Bundy DA, 2010. The global atlas of helminth infection: mapping the way forward in neglected tropical disease control. PLoS Negl Trop Dis 4: e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ, 2014. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez PJ, 2007. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg 77: 88–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, Kabatereine NB, Snow RW, 2012. Plasmodium-helminth coinfection and its sources of heterogeneity across east Africa. J Infect Dis 205: 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardim-Botelho A, Brooker S, Geiger SM, Fleming F, Souza Lopes AC, Diemert DJ, Correa-Oliveira R, Bethony JM, 2008. Age patterns in undernutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Trop Med Int Health 13: 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mwangi TW, Bethony JM, Brooker S, 2006. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol 100: 551–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization , 2011. Soil-transmitted helminthiases: estimates of the number of children needing preventive chemotherapy and number treated, 2009 [article in English, French]. Wkly Epidemiol Rec 86: 257–267. [PubMed] [Google Scholar]
- 14.World Health Organization , 2006. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization; Available at: https://apps.who.int./iris/bitstream/handle/10665/43545/9241547103_eng.pdf. [Google Scholar]
- 15.Simonsen PE, Pedersen EM, Rwegoshora RT, Malecela MN, Derua YA, Magesa SM, 2010. Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLoS Negl Trop Dis 4: e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neglected Tropical Diseases Control Program–NTDCP , 2017/2018. Annual Program Report. Ministry of Health, Gender, Elderly and Children. [Google Scholar]
- 17.World Health Organization , 2000. Bench Aids for the Diagnosis of Malaria Infections, 2nd edition Geneva, Switzerland: WHO. [Google Scholar]
- 18.World Health Organisation , 2002. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. Report of a WHO Expert Committee, Technical Report Series, No. 912. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 19.Chacky F, et al. 2018. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar J 17: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishengoma DS, Mmbando BP, Segeja MD, Alifrangis M, Lemnge MM, Bygbjerg IC, 2013. Declining burden of malaria over two decades in a rural community of Muheza district, north-eastern Tanzania. Malar J 12: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mmbando BP, Vestergaard LS, Kitua AY, Lemnge MM, Theander TG, Lusingu JP, 2010. A progressive declining in the burden of malaria in north-eastern Tanzania. Malar J 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nzobo BJ, Ngasala BE, Kihamia CM, 2015. Prevalence of asymptomatic malaria infection and use of different malaria control measures among primary school children in Morogoro Municipality, Tanzania. Malar J 14: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF International , 2013. Tanzania HIV/AIDS and Malaria Indicator Survey 2011–12 (Preliminary Results). Dar es Salaam, Tanzania: Available at: https://dhsprogram.com/pubs/pdf/AIS11//AIS11.pdf. Accessed December 14, 2018. [Google Scholar]
- 24.Griffin JT, Ferguson NM, Ghani AC, 2014. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun 5: 3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, Greenwood B, Schellenberg D, 2010. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One 5: e8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doolan DL, Dobano C, Baird JK, 2009. Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidlin T, et al. 2013. Effects of hygiene and defecation behavior on helminths and intestinal protozoa infections in Taabo, Cote d’Ivoire. PLoS One 8: e65722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organisation 2011. Helminth Control in School Age Children: A Guide for Managers of Control Programmes, 2nd edition Geneva, Switzerland: World Health Organization; Available at: http://www.who.int. Accessed December 5, 2018. [Google Scholar]
- 29.Freeman MC, Chard AN, Nikolay B, Garn JV, Okoyo C, Kihara J, Njenga SM, Pullan RL, Brooker SJ, Mwandawiro CS, 2015. Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children. Parasit Vectors 8: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ, 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt HL, Brooker S, Kihamia CM, Hall A, Bundy DA, 2001. Evaluation of efficacy of school-based anthelmintic treatments against anaemia in children in the United Republic of Tanzania. Bull World Health Organ 79: 695–703. [PMC free article] [PubMed] [Google Scholar]
- 32.Mazigo HD, Kidenya BR, Ambrose EE, Zinga M, Waihenya R, 2010. Association of intestinal helminths and Plasmodium falciparum infections in co-infected school children in northwest Tanzania. Tanzan J Health Res 12: 299–301. [DOI] [PubMed] [Google Scholar]
- 33.Kepha S, Nuwaha F, Nikolay B, Gichuki P, Edwards T, Allen E, Njenga SM, Mwandawiro CS, Brooker SJ, 2015. Epidemiology of coinfection with soil transmitted helminths and Plasmodium falciparum among school children in Bumula district in western Kenya. Parasite & Vectors 8: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geus D, et al. 2019. Co-infections with Plasmodium, Ascaris and Giardia among Rwandan schoolchildren. Trop Med Int Health 24: 409–420. [DOI] [PubMed] [Google Scholar]
- 35.Degarege A, Veledar E, Degarege D, Erko B, Nacher M, Madhivanan P, 2016. Plasmodium falciparum and soil-transmitted helminth co-infections among children in sub-Saharan Africa: a systematic review and meta-analysis. Parasit Vectors 9: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naing C, Whittaker MA, Nyunt-Wai V, Reid SA, Wong SF, Mak JW, Tanner M, 2013. Malaria and soil-transmitted intestinal helminth co-infection and its effect on anemia: a meta-analysis. Trans R Soc Trop Med Hyg 107: 672–683. [DOI] [PubMed] [Google Scholar]
- 37.Sumbele IU, Nkemnji GB, Kimbi HK, 2017. Soil-transmitted helminths and Plasmodium falciparum malaria among individuals living in different agroecosystems in two rural communities in the mount Cameroon area: a cross-sectional study. Infect Dis Poverty 6: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matangila JR, Doua JY, Linsuke S, Madinga J, Inocencio da Luz R, Van Geertruyden JP, Lutumba P, 2014. Malaria, schistosomiasis and soil transmitted helminth burden and their correlation with anemia in children attending primary schools in Kinshasa, Democratic Republic of Congo. PLoS One 9: e110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaccour CJ, et al. 2015. Establishment of the ivermectin research for malaria elimination network: updating the research agenda. Malar J 14: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organisation , 2015. Assessing the Epidemiology of Soil-Transmitted Helminths during a Transmission Assessment Survey in the Global Programme for the Elimination of Lymphatic Filariasis. Geneva, Switzerland: World Health Organization; Available at: www.int. Accessed January 22, 2019. [Google Scholar]
- 41.Gitonga CW, Kihara JH, Njenga SM, Awuondo K, Noor AM, Snow RW, Brooker SJ, 2012. Use of rapid diagnostic tests in malaria school surveys in Kenya: does their under-performance matter for planning malaria control? Am J Trop Med Hyg 87: 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdalla ZA, Rahma NA, Hassan EE, Abdallah TM, Hamad HE, Omer SA, Adam I, 2019. The diagnostic performance of rapid diagnostic tests and microscopy for malaria diagnosis in eastern Sudan using a nested polymerase chain reaction assay as a reference standard. Trans R Soc Trop Med Hyg trz069. [DOI] [PubMed] [Google Scholar]