Abstract.
Tick-borne diseases (TBDs) are a growing public health threat and are increasingly identified as the cause of undifferentiated febrile illness. There is a significant gap in our understanding of ticks and their associated pathogens in Ecuador. An arboviral surveillance study allowed us to explore potential exposure to TBDs in febrile subjects. We tested plasma samples from 222 febrile subjects for spotted fever group rickettsial (SFGR) antibodies from southern coastal Ecuador in 2014–2015 via ELISA. Fifty-five (25%) subjects had evidence of anti-SFRG IgG or IgM antibodies. Although attempts to detect Rickettsia species in plasma by polymerase chain reaction were unsuccessful, these preliminary data suggest the possibility of endemic SFGR transmission in Ecuador. To better understand the burden and entomological risk for TBDs in Ecuador, future studies should expand TBD surveillance in humans, document common human-biting ticks, and measure pathogen carriage rates in questing ticks.
INTRODUCTION
Ticks transmit debilitating and sometimes fatal diseases to people around the world. The incidence of tick-borne diseases (TBDs) is increasing globally because of several factors, including climate change, weak TBD surveillance, and shortages of prevention and education programs.1–4
The spotted fever group Rickettsiae (SFGR) include several pathogens spread by ticks, fleas, or mites. Clinical manifestations of SFGR infection include fever, headache, rash, and myalgia. When left untreated, some SFGR infections have case fatality rates as high as 10% in North America; however, recent epidemiologic evidence from South America reports case fatality rates as high as 55%.2,5 Diagnosis of SFRG infection is challenging, especially in low- and middle-income countries (LMICs), where undifferentiated febrile illness (UFI) is one of the most common reasons for seeking health care and little to nothing is known regarding the eco-epidemiology of ticks and their pathogens. The current gold standard for SFGR infection is a ≥ 4-fold rise in antirickettsial antibodies in paired serum samples.2 Without paired serum samples, SFGR diagnosis may be achieved through signs and symptoms with single serology, molecular evidence, and entomological risks (when known). In areas with constrained public health resources, such as Ecuador, diagnostic testing of TBDs is often unavailable because of the large number of potential pathogens that cause UFI and the complexity and cost of available diagnostic assays.6 This leads to unknown TBD burdens and undertreatment or mistreatment of underlying infections. In this study, banked human plasma samples from an ongoing arboviral disease surveillance study in Machala, a tropical coastal city in Ecuador, were analyzed for antibodies to SFGR using an indirect enzyme immunoassay.
METHODS
Machala (population 286,120) is a city in southern coastal Ecuador and is the capital of El Oro Province (Figure 1A). Samples used in this study were collected as part of a previously described arboviral febrile surveillance study from January 1, 2014 to December 31, 2015.7 Briefly, participants in that study were older than 6 months, presented to one of our clinical sites, and were clinically diagnosed with dengue fever by a Ministry of Health physician.7 Clinical sites were operated by the Ministry of Health in Machala and consisted of primarily four sentinel health clinics and the reference hospital for the province (Hospital Teófilo Dávila, Figure 1B). Clinical staff collected subject information via interviews, including questions regarding subject demographics, clinical signs and symptoms, inpatient/outpatient status, self-reported contact with animals, and self-reported travel outside of the city within the last month. At the time of clinical evaluation, temperature was recorded and symptoms within the last 7 days were documented as reported by the participant. On the day of presentation to the clinic, a 20-mL blood specimen was obtained by venipuncture in an ethylenediaminetetraacetic acid (EDTA) vacutainer from each study participant. Plasma was separated via centrifugation and aliquoted in multiple tubes and stored at −80°C. Subjects with more than one aliquot of plasma were selected for use in the present study (Supplemental Table 1). The study protocol for sample collection was reviewed and approved by Institutional Review Boards at SUNY Upstate Medical University, the Human Research Protection Office of the US Department of Defense, the Luis Vernaza Hospital in Guayaquil, Ecuador, and the Ecuadorean Ministry of Health.
Figure 1.
Location of study site. The location of Ecuador (A) relative to other countries in South America, with El Oro Province highlighted in red. Machala’s location in El Oro Province (B) is highlighted in pink, with the location of each recruiting health center used in this study marked with a black point. The box in A indicates the relative position of B. This figure appears in color at www.ajtmh.org.
Subject samples (n = 222) meeting the aforementioned criteria were screened for the presence of IgG and IgM antibodies to SFGR via a commercially available ELISA (Fuller Laboratories, Fullerton, CA). The Rickettsia ELISA plates are coated with lipopolysaccharide (IgG) antigens or outer membrane protein B (IgM). In an attempt to identify rickettsial species responsible for antibody response in study subjects, DNA was extracted from IgM-positive plasma samples via commercial kit (Omega Bio-Tek, Norcross, GA), and gDNA was tested for the presence of rickettsial DNA by PCR targeting three genes, 17-kDa antigen (htrA), citrate synthase (gltA), and ompA, as previously described.8 Samples were also previously tested for dengue infections using commercial NS1 rapid test, dengue IgM/IgG, and NS1 ELISA (PanBio, Abbott Park, IL) and for dengue, chikungunya, and Zika viruses via real-time reverse transcription PCR (rRT-PCR) as previously described.7 We defined an acute or recent dengue infection as those subjects with positive results for NS1 rapid test, NS1 ELISA, IgM ELISA, or rRT-PCR. Acute chikungunya or Zika infections were defined as those subjects positive by rRT-PCR.7
Seropositivity for anti-SFGR IgG and IgM was calculated among 10-year age groups. Relationships between subject characteristics and anti-SFGR IgM positivity were assessed using binomial generalized linear models. Subjects with missing data were excluded from the final analyses. Data cleaning, analysis, and visualization were performed using R version 3.2.2 (Vienna, Austria).
RESULTS
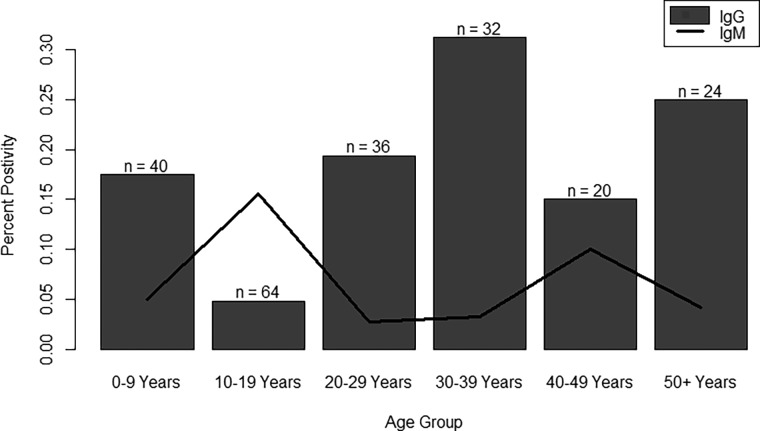
Overall study subject characteristics are presented in Supplemental Table 1. The mean age of study subjects was 25 years, 53% were female and 28% were enrolled through the hospital. A total of 55 (25%) subjects had evidence of anti-SFGR antibodies. Thirty-nine (18%) subjects were positive for anti-SFGR IgG and 17 (8%) subjects were positive for IgM. One (< 0.5%) subject was positive for both anti-SFGR IgG and IgM. Anti-SFGR seropositivity by age group is presented in Figure 2. IgG positivity was highest among 30- to 39-year olds and IgM positivity was highest among 10- to 19-year olds. We identified acute infections for dengue, chikungunya, and Zika virus among subjects with anti-SFGR IgM seropositivity and report the prevalence of possible SFGR-arbovirus coinfections (Supplemental Table 2). Among subjects with a suspected acute TBD (positive for anti-SFGR IgM and negative for anti-SFGR IgG, n = 16), 12 (of 17 tested) also had an acute or recent dengue infection and two (of five tested) had an acute chikungunya infection (Supplemental Table 3). No subjects were positive for both SFGR and Zika virus, which is consistent with reported detection of the first Zika cases in Machala in 2016.
Figure 2.
Seropositivity by age group. Positivity for anti–spotted fever group rickettsial (SFGR) IgG or IgM response was calculated for 10-year age groups. Anti-SFGR IgG response was most common among ages 30–39. Anti-SFGR IgM response was most common among ages 10–19.
Two hundred twelve samples were used for further statistical analyses; 10 samples were excluded because of missing subject data. Results of this analysis are presented in Table 1. Subject demographics, travel history, and animal contact were not associated with anti-SFGR IgM positivity. Subjects reporting a rash were more likely to be positive for anti-SFGR IgM (Odds ratio [OR]: 6.5, 95% CI: 1.7–26.0); other symptoms were not notably associated with anti-SFGR IgM positivity. Subjects with an acute or recent dengue infection were also more likely to be positive for anti-SFGR IgM (OR: 7.7, 95% CI: 2.2–35.0).
Table 1.
Results of association between study subject factors and anti–spotted fever group rickettsial IgM response
| Factor | Odds ratio (95% CI) | P-value | |
|---|---|---|---|
| Age | 1.00 (0.95–1.04) | 0.91 | |
| Gender | Male | 0.82 (0.24–2.76) | 0.75 |
| Female | Ref | ||
| History of travel in the last month | 1.04 (0.30–3.35) | 0.95 | |
| History of animal contact | 0.54 (0.02–4.00) | 0.61 | |
| Hospitalization status | Hospitalized | 0.19 (0.01–1.54) | 0.19 |
| Status unknown | 2.46 (0.07–33.27) | 0.55 | |
| Not hospitalized | Ref | ||
| Signs and symptoms (positive for presence) | Temperature (°C) | 1.74 (0.90–3.39) | 0.10 |
| Headache | 2.36 (0.32–50.36) | 0.47 | |
| Rash | 6.54 (1.72–25.99) | 0.01 | |
| Nausea | 0.97 (0.23–4.32) | 0.96 | |
| Vomit | 2.58 (0.67–10.56) | 0.17 | |
| Drowsiness | 1.48 (0.24–15.96) | 0.71 | |
| Abdominal pain | 0.49 (0.15–1.60) | 0.25 | |
| Acute or recent dengue virus infection | 7.67 (2.27–34.97) | < 0.01 | |
DISCUSSION
Results from this retrospective analysis of banked febrile subject samples suggest that TBDs may be present in Ecuador. Although antibodies only indicate past exposure to a microbe, the presence of IgM response specific to SFGR in febrile subjects suggests recent exposure. This is further supported with the association between rash symptoms and a positive IgM response, as rash is commonly reported in SFGR infections.2 Anti-SFGR IgG response can be present for ≥ 12 months post exposure; therefore, without paired samples demonstrating seroconversion or a 4-fold rise in IgG titers, a positive anti-SFGR IgG response can only be interpreted as past exposure to an SFGR.9 One significant limitation with SFGR serology is its inability to differentiate between the rickettsial species. Unfortunately, PCR targeting three different rickettsial genes produced no positive results from our samples; however, this was not surprising, considering the low sensitivity of this method on blood samples.10 Despite this, the IgG antibody data, coupled with findings that IgM-positive febrile subjects were 6.5 times more likely to have had a rash, provide convincing evidence warranting expanded TBD surveillance in humans, vectors, and animal hosts in Ecuador. Indeed in the United States, many of these subjects would fulfill the CDC’s current surveillance definition for a probable case of spotted fever rickettsiosis.11 In addition, ongoing vector surveillance confirms the widespread presence of competent SFGR vectors, including Amblyomma cajennense, Amblyomma ovale, and Rhipicephalus sanguineus in the province (S. Enríquez and B. Leydet, unpublished). Interestingly, subjects in our study with an acute or recent dengue infection were also more likely to be positive for anti-SFGR IgM than those with an equivocal or negative result. This result may be explained by some common exposure to ticks and mosquitos, such as outdoor activity or location. Indeed, dengue-SFGR coinfections have been reported elsewhere in South America.12 Finally, our IgM results are limited by a small number of positive subjects (n = 17), leading to wide CIs on some effect size estimates.
Importantly, untreated SFGR diseases have reported case fatality rates as high as 55% in areas of South America.5 Spotted fever group rickettsial infections respond well to certain antibiotics (e.g., doxycycline) if treated early,2 but local physicians have indicated that SFGRs are not typically considered during differential diagnosis in this region of Ecuador. Our findings suggest there is potentially a significant burden of TBDs in this region that may go undiagnosed and untreated, despite suitable treatments being readily available and affordable in Ecuador. Limited access to diagnostic testing is a significant barrier to TBD diagnoses in Ecuador and other LMIC countries. Currently, no TBD is included in Ecuador’s national disease surveillance program.13 This study adds to the limited literature on TBDs in South America.12–17 Further work is needed to determine carriage of specific TBD agents in tick vectors in Ecuador. Importantly, these findings highlight the need for surveillance studies to detect TBDs and their vectors, as well as the development of novel low-cost diagnostics for various TBDs.
Supplemental tables
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Adams DA, Thomas KR, Jajosky RA, Foster L, Sharp P, Onweh DH, Schley AW, Anderson WJ; Nationally Notifiable Infectious Conditions Group , 2016. Summary of notifiable infectious diseases and conditions–United States, 2014. MMWR Morb Mortal Wkly Rep 63: 1–152. [DOI] [PubMed] [Google Scholar]
- 2.Biggs HM, et al. 2016. Diagnosis and management of tickborne rickettsial diseases: rocky mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis–United States. MMWR Recomm Rep 65: 1–44. [DOI] [PubMed] [Google Scholar]
- 3.Eisen RJ, Kugeler KJ, Eisen L, Beard CB, Paddock CD, 2017. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J 58: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randolph SE, 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129 (Suppl): S37–S65. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira SV, et al. 2016. An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 22: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouqui P, et al. Escmid Study Group on Coxiella AR, Bartonella, European Network for Surveillance of Tick-Borne D , 2004. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect 10: 1108–1132. [DOI] [PubMed] [Google Scholar]
- 7.Stewart-Ibarra AM, et al. 2018. The burden of dengue fever and chikungunya in southern coastal Ecuador: epidemiology, clinical presentation, and phylogenetics from the first two years of a prospective study. Am J Trop Med Hygeine 98: 1444–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noda H, Munderloh UG, Kurtti TJ, 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol 63: 3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilfert CM, MacCormack JN, Kleeman K, Philip RN, Austin E, Dickinson V, Turner L, 1985. The prevalence of antibodies to Rickettsia rickettsii in an area endemic for Rocky Mountain spotted fever. J Infect Dis 151: 823–831. [DOI] [PubMed] [Google Scholar]
- 10.Paris DH, Dumler JS, 2016. State of the art of diagnosis of rickettsial diseases: the use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Curr Opin Infect Dis 29: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Council of State and Territorial Epidemiologists , 2009. Public Health Reporting and National Notification for Spotted Fever Rickettsioses (Including Rocky Mountain Spotted Fever) Position Statement. Buffalo, NY: Council of State and Territorial Epidemiologists Annual Conference. [Google Scholar]
- 12.Faccini-Martinez AA, et al. 2017. Epidemiology of spotted fever group rickettsioses and acute undifferentiated febrile illness in Villeta, Colombia. Am J Trop Med Hyg 97: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faccini-Martinez AA, Munoz-Leal S, Acosta ICL, de Oliveira SV, de Lima Dure AI, Cerutti CJ, Labruna MB, 2018. Confirming Rickettsia rickettsii as the etiological agent of lethal spotted fever group rickettsiosis in human patients from Espirito Santo state, Brazil. Ticks Tick Borne Dis 9: 496–499. [DOI] [PubMed] [Google Scholar]
- 14.Quintero VJ, Paternina TL, Uribe YA, Muskus C, Hidalgo M, Gil J, Cienfuegos GA, Osorio QL, Rojas AC, 2017. Eco-epidemiological analysis of rickettsial seropositivity in rural areas of Colombia: a multilevel approach. PLoS Negl Trop Dis 11: e0005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocher C, et al. 2016. Rickettsial disease in the Peruvian Amazon basin. PLoS Negl Trop Dis 10: e0004843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faccini-Martinez AA, Felix ML, Armua-Fernandez MT, Venzal JM, 2018. An autochthonous confirmed case of Rickettsia parkeri rickettsiosis in Uruguay. Ticks Tick Borne Dis 9: 718–719. [DOI] [PubMed] [Google Scholar]
- 17.Manock SR, et al. 2009. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg 81: 146–151. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.