Abstract.
The discovery and characterization of novel parasite antigens to improve the diagnosis of Trypanosoma cruzi by serological methods and for accurate and rapid follow-up of treatment efficiency are still needed. TcTASV is a T. cruzi–specific multigene family, whose products are expressed on the parasite stages present in the vertebrate host. In a previous work, a mix of antigens from subfamilies TcTASV-A and TcTASV-C (Mix A + C) was sensitive and specific to identify dogs with active infection of high epidemiological relevance. Here, TcTASV-A and TcTASV-C were assayed separately as well as together (Mix A + C) in an ELISA format on human samples. The Mix A + C presented moderate sensitivity (78%) but high diagnostic accuracy with a 100% of specificity, evaluated on healthy, leishmaniasic, and Strongyloides stercoralis infected patients. Moreover, antibody levels of pediatric patients showed—2 years posttreatment—diminished reactivity against the Mix A + C (P < 0.0001), pointing TcTASV antigens as promising tools for treatment follow-up.
Chagas disease, caused by Trypanosoma cruzi, is a major public health problem in Latin America, where the disease is endemic and 5.7 million people are estimated to be infected.1 The WHO highlighted the need to improve the diagnostic methods currently available both for initial diagnosis and follow-up after treatment.2 Conventional serology (usually, ELISA and indirect hemagglutination assay (IHA) using parasite crude extracts) is often used to follow up the response to etiological treatment, being the negativization the accepted criteria of cure. However, antibody levels against epitopes of parasite antigens present in complex mixtures can persist for long periods of time, even after the parasite has been eliminated (or is believed eliminated) (children: ∼5 years and adults: > 20 years after treatment).3 Other alternatives to measure treatment effectiveness include polymerase chain reaction (PCR)-based techniques (more useful to confirm therapeutic failure or drug resistance than to evaluate an accurate response to treatment) and the analysis of biochemical and immunological biomarkers (antibodies against host or parasite antigens, cytokines).4,5 Particularly, some trypomastigote-specific antigens (i.e., TSSA (ACY54510), Tc13Tul (AF091620), trans-sialidase, KMP11, and SAPA) have been evaluated for follow-up treatment effectiveness, with variable outcomes.6–10 The use of T. cruzi–specific antigens would avoid, in addition, potential serum cross-reactivities with shared epitopes between related kinetoplastid parasites with overlapping endemic areas. However, the heterogeneity in methodologies and scarce data evaluating specificity and sensitivity of assays using these biomarkers prevents their implementation in clinical practice.4 Further studies are necessary to identify novel markers for conclusive and early follow-up of treated patients, and to improve existing ones. Ideally, these novel biomarkers should be indicative of active infection and its signal should disappear or suddenly drop, shortly after etiological treatment.
The TcTASV protein family of T. cruzi, discovered a decade ago, has no orthologues in related trypanosomatids and is mainly expressed in the trypomastigote stage.11,12 According to the length and sequence of the central region, four TcTASV subfamilies can be defined, TcTASV-A and TcTASV-C being the most abundant.12 TcTASV-C subfamily is expressed at the surface, secreted and highly abundant on bloodstream trypomastigotes.13,14 On the other hand, TcTASV-A has an intracellular localization and is expressed both in trypomastigotes and amastigotes.12 In a previous work, we demonstrated that an ELISA using an antigenic mixture of TcTASV‐A and TcTASV-C (Mix A + C) resulted in a promising tool to detect infection in dogs, the most epidemiologically relevant domestic reservoir in endemic areas.15 The Mix A + C resulted in high sensitivity (94%) and specificity (100%) in dogs with evidence of infection by xenodiagnosis and/or PCR (in addition to serology), therefore probably coursing an active infection.15 Here, we describe the performance of these antigens to detect T. cruzi infection in humans and its utility for the follow-up of pediatric patients after treatment.
ELISA assays were carried out with recombinant TcTASV-A (CBI68031.1 [GenBank]; amino acids 37–173) and/or TcTASV-C (Tcruzi_1863-4-1211-93 [ORF at tritrypdb.org]; amino acids 65–330)13 genes that were cloned as glutathione S-transferase fusion proteins, and expressed and purified as described previously.13,15
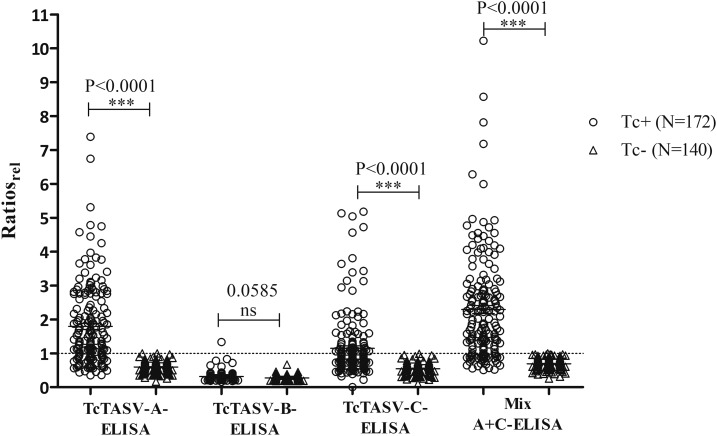
We first evaluated the performance of TcTASV-A, B, and C antigens separately and the Mix A + C, on an ELISA format, on 312 human samples (T. cruzi infected: Tc+, N = 172; T. cruzi negative: Tc−, N = 140) (Table 1). TcTASV-B was unsuitable to discriminate between infected and non-infected humans, as previously observed in dogs.15 ELISAs with TcTASV-A and TcTASV-C obtained sensitivities of 66% and 39%, respectively. Considering that most of the evaluated sera proceeded from chronically infected individuals and that TcTASV-A is expressed in amastigotes (stage of the parasite preponderant in the chronic phase) a higher reactivity against TcTASV-A than against TcTASV-C is reasonable. The combination of both subfamilies in the antigenic Mix A + C gave the highest sensitivity (78%), with an area under the curve receiver operating characteristic (AUC ROC) of 0.94, which indicates a high diagnostic accuracy to discriminate infected from healthy individuals (Table 1, Figure 1). As observed for other proteins,8,16 the sensitivity is improved when TcTASV-A and TcTASV-C are combined in an antigenic mixture versus its individual use. The specificity was maximum, considering that it was evaluated not only in healthy controls but also in leishmaniasic patients and in patients with strongyloidiosis, coendemic affections with Chagas in northern Argentina and Latin America.
Table 1.
Diagnostic performance of TcTASV–ELISA
| ELISA | Sensitivity, positive/total [95% CI] | Specificity, positive/total [95% CI] | AUC ROC,* cutoff [95% CI] |
|---|---|---|---|
| TcTASV-A | 66%, 114/172 [57–73] | 100%, 0/140 [97–100] | 0.89, 1.768 [0.86–0.93] |
| TcTASV-B | 2%, 3/172 [0–8] | 100%, 0/140 [94–100] | 0.62, 3.839 [0.53–0.72] |
| TcTASV-C | 39%, 67/172 [32–47] | 100%, 0/140 [97–100] | 0.83, 1.643 [0.79–0.88] |
| Mix A + C | 78%, 134/172 [71–84] | 100%, 0/140 [97–100] | 0.94, 1.425 [0.91–0.97] |
The cutoff was selected through ROC curves to allow maximum sensitivity for a 100% specificity.15 Sera from infected patients (Tc+, N = 172) were positive at least with two commercial serological tests (lysate/recombinant-ELISA and/or IHA). Sera from patients without Trypanosoma cruzi infection (Tc−, N = 140) included healthy (N = 47), leishmaniasic (N = 73), and Strongyloides stercoralis–infected (N = 20) individuals. Origin of Tc+ samples: endemic areas in Argentina (N = 82, Salta Province; N = 80, Chaco Province) and Bolivia (N = 10). Sera from Salta Province and Bolivia were gently provided by Asociación para el Desarrollo Sanitario Regional (Buenos Aires Province) Foundation. Sera from Chaco Province were collected during cross-sectional studies in different rural settlements.17 Sera from patients without T. cruzi infection were from the collection of serum samples of the “Cátedra de Química Biológica” and/or “Instituto de Investigaciones de Enfermedades Tropicales,” National University of Salta. The protocol was approved by the Bioethics Committee; Faculty of Health Sciences, National University of Salta, Argentina.
* AUC ROC interpretation: values between 0.5 and 0.7 indicate low accuracy, between 0.7 and 0.9 indicate moderate accuracy and may be useful for some purposes, and greater than 0.9 indicate high accuracy.
Figure 1.
Reactivity of sera from Trypanosoma cruzi–infected and control patients against TcTASV antigens. Sera were assayed in duplicate and the ratio optical density (OD)TcTASV/ODGST was calculated.15 Then the ratio of each serum was relativized to the cutoff value as: Ratiorel = Ratio/cutoff. Samples were considered positive when the value of the Ratiorel was equal to or greater than 1, and negative when the value of the Ratiorel was less than 1. Normal distribution of data was tested using the Shapiro–Wilk normality test (P < 0.05). Differences between the groups of positive and negative control sera were evaluated through the Mann–Whitney U test (P < 0.05). GST = glutathione-S-transferase.
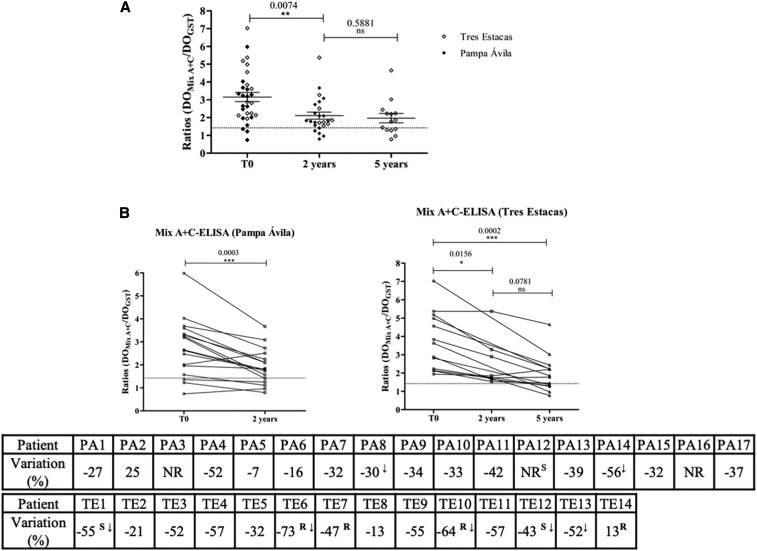
Next, the response to treatment in children was followed up by measuring the antibody response against the Mix A + C in serum samples from children (aged 3–15 years old) from two settlements in Chaco Province (Pampa Avila and Tres Estacas), Argentina. These samples came from a previous study, where patients treated with a standard scheme of benznidazole were followed up serologically by recombinant ELISA and IHA (Wiener Labs).17 Thirty-one of these 57 sera were available at the collection of the “Cátedra de Química Biológica,” National University of Salta to be tested in our study. Of these, 28/31 (90%) were TcTASV positive at T0 (pretreatment) and 75% (21/28) showed some degree of variation in antibody levels, which indicates treatment impact (decrease greater than 30%)9 (Figure 2). Importantly, seven of these patients showed seroconversion. Considering that the effectiveness of treatment in most of pediatric patients is well documented,18 diminished reactivity against the Mix A + C was the expected scenario for a good biomarker antigen. In both settlements, the decrease in antibody reactivity against the Mix A + C was evidenced from 2 years after treatment (T0 versus T1; Figure 2B). In Tres Estacas, antibody levels continued decreasing 5 years after treatment (T2). Although our results do not completely agree with those reported by Monje Rumi et al. (two patients seroconverted and four with significant decrease of antibody titers at T2 [5 years after treatment]),17 it is worth mentioning that those assays were carried out with a commercial kit that includes several T. cruzi–recombinant antigens. Indeed, all but one of the patients that previously showed treatment impact (TE1, TE6, TE7, TE10, TE12, and TE14) also presented diminished reactivity against TcTASV antigens after treatment (Table in Figure 2B).
Figure 2.
Follow-up of treated children using Mix A + C–ELISA. Patients from rural settlements “Tres Estacas” and “Pampa Ávila,” Chacabuco Department, province of Chaco, were included in this study. In “Pampa Ávila” blood samples were collected before treatment (T0; 2007) and 2 years after treatment (T1; 2009); in Tres Estacas, blood samples were taken before treatment (T0; 2004) and 2 and 5 years after treatment (T1; 2006 and T2; 2009). (A) Pre- and posttreatment reactivity against Mix A + C–ELISA of all treated children. (B) Individual follow-up of anti-TcTASV antibody levels after treatment in each settlement. The degree of variation (%) was calculated as: ([Final Abs-Initial Abs]/Initial Abs) × 100.19 A negative sign in the percentage indicates a decrease in posttreatment antibody levels, whereas a positive sign indicated an increase. ↓ = patients who seroconverted. A decrease greater than 30% with respect to T0 is considered a positive therapy impact9; a decrease greater than 75% in antibody levels is indicative of a tendency to seroconversion.19 Differences between pretreatment and posttreatment reactivity were evaluated by the paired comparison Wilcoxon test (P < 0.05). NR = indicates sera that were not reactive by Mix A + C–ELISA at T0. R = sera that showed a significative reduction in titers or S = seroconverted by ELISA, as previously evaluated by Monje-Rumi et al.17
Previous studies have also evidenced a faster decrease in antibody titers against recombinant antigens of the trypomastigote stage compared with conventional serology,18,19 although not every individual antigen was appropriate as a serological biomarker. Indeed, Sanchez Negrette et al.20 reported that some trypomastigote-specific antigens may cause a persistent humoral response, whereas certain others showed faster decrease of antibody titers (i.e., antigen 13 and SAPA versus antigens 2 and 30) in adult patients. Here, we focused on TcTASV antigens that, in light of the promising results observed 2 years after treatment with the Mix A + C, seem to belong to the first group (rapid decrease). As mentioned, TcTASV-C is an exclusive protein of the surface of trypomastigotes, whereas TcTASV-A has an intracellular localization, expressed both in trypomastigotes and amastigotes.12 In addition, as TcTASV proteins have no repetitive sequences, we hypothesize that they produce lower activation of B lymphocytes than the classical antigens of the parasite’ surface that can act as mitogens or superantigens causing hypergammaglobulinemia (e.g., the repetitive antigen, SAPA). Although numerous biomarkers of treatment effectiveness have been described, none of them has been validated yet in replacement of standard serological techniques. The decrease of anti-TcTASV antibodies posttreatment could reflect either the true elimination of the parasite or a significant decrease of parasitic load and parasitemia and, therefore, be a short-term indicator of treatment success. In summary, the results presented here strongly suggest that the mixture of TcTASV-A and TcTASV-C should be considered among the outstanding candidate antigens for monitoring treatment in children with chronic infections. Despite the Mix A + C presented a moderate sensitivity as diagnostic antigens, its strength lies in its excellent performance as a biomarker for treatment follow-up.
Acknowledgments:
This work was funded by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina, PICT-2014-1151 to V. T.) and by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). V. T. and D. O. S. are career investigators from CONICET. N. F. Y. is a fellow of CONICET. The support of the “Instituto de Investigaciones de Enfermedades Tropicales” staff for the handling of the samples from infected patients with Leishmania spp. and S. stercoralis is appreciated.
REFERENCES
- 1.World Health Organization , 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 6: 33–44. [PubMed] [Google Scholar]
- 2.World Health Organization , 2012. Research priorities for Chagas disease, human African trypanosomiasis and leishmaniasis. World Health Organ Tech Rep Ser 975: 1–100. [PubMed] [Google Scholar]
- 3.Sguassero Y, Cuesta CB, Roberts KN, Hicks E, Comandé D, Ciapponi A, Sosa-Estani S, 2015. Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One 10: e0139363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinazo MJ, et al. 2014. Biological markers for evaluating therapeutic efficacy in Chagas disease, a systematic review. Expert Rev Anti Infect Ther 12: 479–496. [DOI] [PubMed] [Google Scholar]
- 5.Albareda M, et al. 2018. Distinct treatment outcomes of antiparasitic therapy in Trypanosoma cruzi-infected children is associated with early changes in cytokines, chemokines, and T-cell phenotypes. Front Immunol 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santamaría AL, De Rissio AM, Riarte A, Garavaglia PA, Bruballa AC, Rodríguez MA, Irazu LE, Ruiz AM, García GA, 2013. Use of an enzyme-linked immunosorbent assay that utilizes the Tc13Tul antigen of Trypanosoma cruzi to monitor patients after treatment with benznidazole. Diagn Microbiol Infect Dis 76: 197–205. [DOI] [PubMed] [Google Scholar]
- 7.Zrein M, et al. 2018. A novel antibody surrogate biomarker to monitor parasite persistence in Trypanosoma cruzi-infected patients. PLoS Negl Trop Dis 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granjon E, Dichtel-Danjoy ML, Saba E, Sabino E, Campos de Oliveira L, Zrein M, 2016. Development of a novel multiplex immunoassay multi-cruzi for the serological confirmation of Chagas disease. PLoS Negl Trop Dis 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viotti R, et al. 2011. Impact of aetiological treatment on conventional and multiplex serology in chronic Chagas disease. PLoS Negl Trop Dis 5: e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balouz V, Melli LJ, Volcovich R, Moscatelli G, Moroni S, Ballering G, Bisio M, Buscaglia CA, Altcheh J, 2017. The trypomastigote small surface antigen from Trypanosoma cruzi improves treatment evaluation and diagnosis in pediatric Chagas disease. J Clin Microbiol 55: 3444–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tekiel V, Alba-Soto CD, González Cappa SM, Postan M, Sánchez DO, 2009. Identification of novel vaccine candidates for Chagas’ disease by immunization with sequential fractions of a trypomastigote cDNA expression library. Vaccine 27: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 12.García EA, Ziliani M, Agüero F, Bernabó G, Sánchez DO, Tekiel V, 2010. TcTASV: a novel protein family in Trypanosoma cruzi identified from a subtractive trypomastigote cDNA library. PLoS Negl Trop Dis 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernabó G, Levy G, Ziliani M, Caeiro LD, Sánchez DO, Tekiel V, 2013. TcTASV-C, a protein family in Trypanosoma cruzi that is predominantly trypomastigote-stage specific and secreted to the medium. PLoS One 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caeiro LD, Alba-Soto CD, Rizzi M, Solana ME, Rodriguez G, Chidichimo AM, Rodriguez ME, Sánchez DO, Levy GV, Tekiel V, 2018. The protein family TcTASV-C is a novel Trypanosoma cruzi virulence factor secreted in extracellular vesicles by trypomastigotes and highly expressed in bloodstream forms. PLoS Negl Trop Dis 12: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floridia-Yapur N, et al. 2016. The TcTASV proteins are novel promising antigens to detect active Trypanosoma cruzi infection in dogs. Parasitology 143: 1382–1389. [DOI] [PubMed] [Google Scholar]
- 16.Camussone C, Gonzalez V, Belluzo MS, Pujato N, Ribone ME, Lagier CM, Marcipar IS, 2009. Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin Vaccine Immunol 16: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monje-Rumi M, et al. 2013. Benznidazole treatment in chronic children infected with Trypanosoma cruzi: serological and molecular follow-up of patients and identification of discrete typing units. Acta Trop 128: 130–136. [DOI] [PubMed] [Google Scholar]
- 18.Sosa-Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C, 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. Am J Trop Med Hyg 59: 526–529. [DOI] [PubMed] [Google Scholar]
- 19.Escribà JM, Ponce E, Romero AdeD, Viñas PA, Marchiol A, Bassets G, Palma PP, Lima MA, Zúniga C, Ponce C, 2009. Treatment and seroconversion in a cohort of children suffering from recent chronic Chagas infection in Yoro, Honduras. Mem Inst Oswaldo Cruz 104: 986–991. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez Negrette O, Sánchez Valdéz FJ, Lacunza CD, García Bustos MF, Mora MC, Uncos AD, Basombrío MA, 2008. Serological evaluation of specific-antibody levels in patients treated for chronic Chagas’ disease. Clin Vaccine Immunol 15: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]