Abstract.
Disseminated nontuberculous mycobacterial (NTM) infections usually occur in severely immunosuppressed patients. These infections may also occur in previously immunocompetent patients with acquired anti–interferon-gamma antibodies (anti–IFN-γ Abs). A previously healthy 33-year-old man presented with a 3-week history of cough and fever. Chest computed tomography showed air-space consolidation in the middle lobe of the right lung and enlargement of the supraclavicular, mediastinal, and hilar lymph nodes. Tissue samples obtained via mediastinoscopy showed granuloma formation with acid-fast bacteria; cultures from the tissue revealed Mycobacterium kansasii. Accordingly, a diagnosis of disseminated M. kansasii disease was made. The positive control tested negative in the QuantiFERON-TB Gold In-tube test, suggesting the presence of anti–IFN-γ Abs. The ELISA test for anti–IFN-γ Abs demonstrated an increased titer. Antimycobacterial drug treatments were initiated after diagnosis. His symptoms improved over 2 months, and he remains well on outpatient management. Disseminated M. kansasii disease is a very rare condition suggestive of immunosuppression. Testing for anti–IFN-γ antibodies might be important in all cases of disseminated M. kansasii disease.
INTRODUCTION
Disseminated nontuberculous mycobacterial (NTM) infections are rare, usually affecting immunosuppressed individuals. Recent reports suggest that acquired immunodeficiency caused by anti–interferon-gamma antibodies (anti–IFN-γ Abs) is associated with severe disseminated mycobacterial infections.1–5 Disseminated nontuberculous mycobacterial infections are often difficult to diagnose because they involve the lung, bone, lymph nodes, and bone marrow and mimic other inflammatory diseases or malignant lymphoma; thus, the presence of anti–IFN-γ Abs should be suspected in patients with no clinically evident immunodeficiency who present with such manifestations.
Mycobacterium kansasii has some distinct clinical features compared with other NTM. Mycobacterium kansasii causes lung disease, which resembles Mycobacterium tuberculosis infection, both clinically and radiologically.6 In addition to M. tuberculosis antigens, M. kansasii encodes CFP-10 and ESAT-6, two antigens targeted by IFN-γ release assays; thus, these tests often show positive results in patients with M. kansasii infections.7 Moreover, in HIV-negative patients, M. kansasii infections typically respond well to antimicrobial therapy,8 and patients are expected to recover completely, which is different from other NTM infections.
Lymphadenitis and other extrapulmonary occurrences of M. kansasii disease, such as those in the musculoskeletal and genitourinary systems, are infrequent8 and are suggestive of disseminated disease. Compared with other NTM infections, disseminated M. kansasii infections with anti–IFN-γ Abs are very rare.9
Here, we report a case of disseminated M. kansasii disease with lymphadenitis accompanied with anti–IFN-γ Abs in a patient without clinically evident immunodeficiency.
CASE REPORT
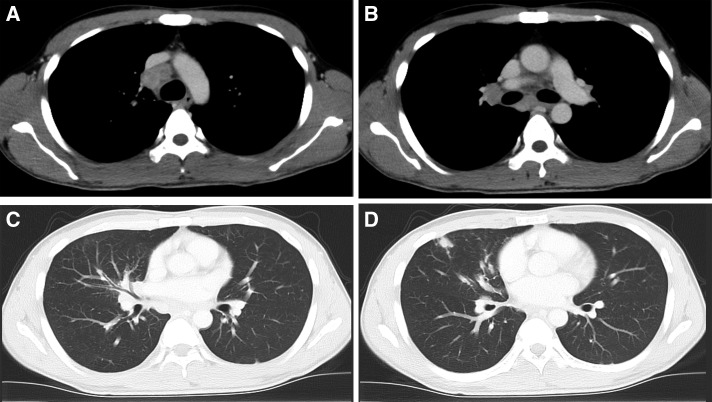
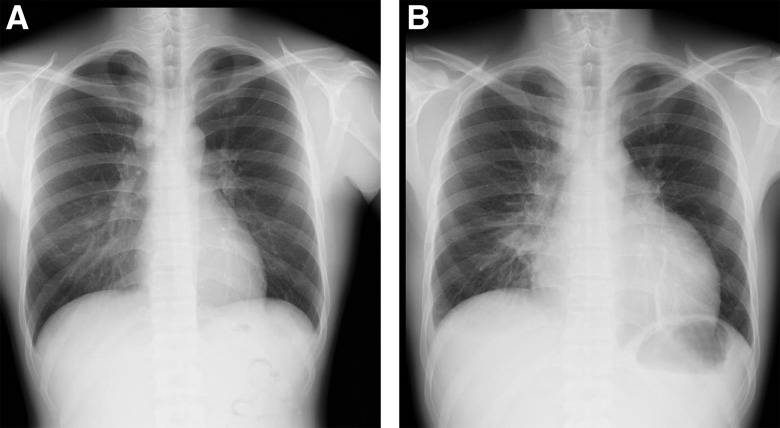
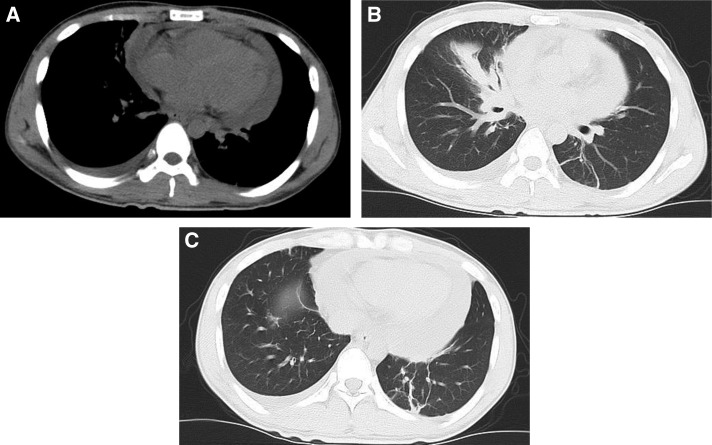
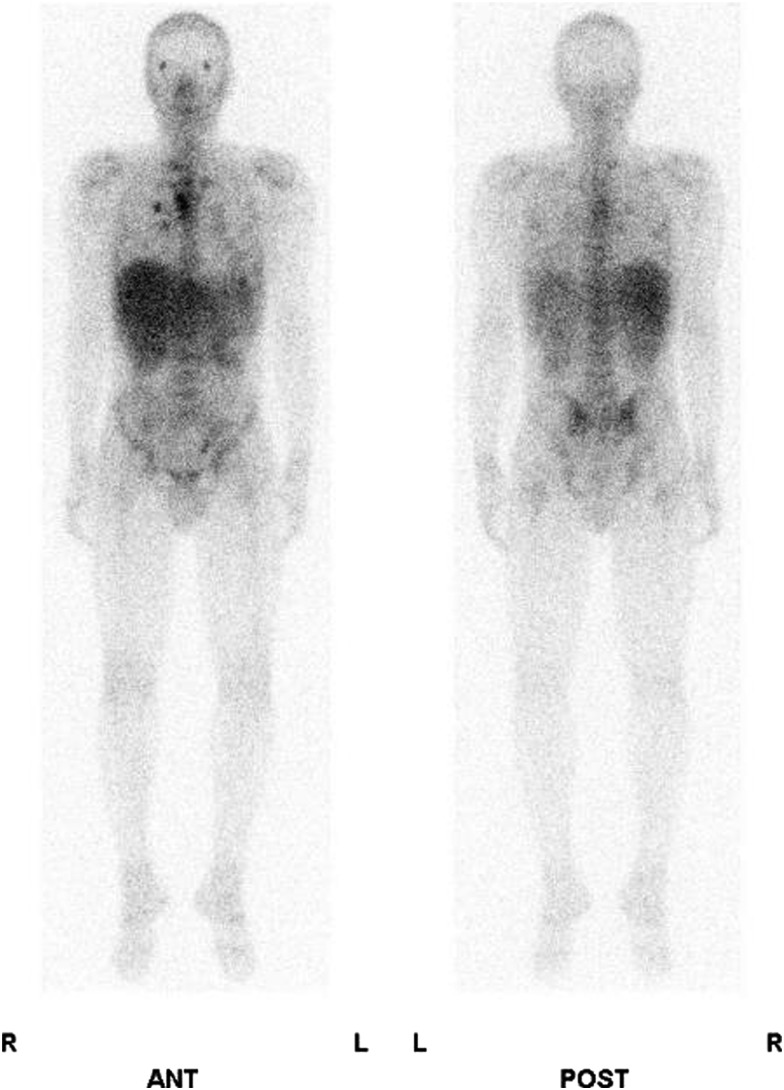
A previously healthy 33-year-old man presented with a 3-week history of cough and fever, which persisted after antibiotic treatment. He had never smoked and had worked for the building industry. On physical examination, he had high fever (38.5°C) and enlarged right supraclavicular lymph nodes. The initial laboratory investigations revealed a white blood cell count of 19.6 × 103/mm3 (80.6% neutrophils) and a C-reactive protein level of 11.17 mg/dL. Chest contrast-enhanced computed tomography (CT) revealed enlargement of the supraclavicular, mediastinal, and bilateral hilar lymph nodes (Figure 1A and B); multiple small nodules; and bronchovascular septal thickening in the middle lobe of the right lung (Figure 1C and D). He was admitted in the Division of General Medicine. Investigations were then performed for assessing the cause for immunodeficiency. He tested negative for HIV antibodies and for markers of vasculitis: proteinase 3 antineutrophil cytoplasmic antibody and myeloperoxidase antineutrophil cytoplasmic antibody. The angiotensin converting enzyme level was 5.9 IU/L. However, the T-SPOT test showed positive results; tissue samples obtained on mediastinoscopy showed granuloma formation, with acid-fast bacteria, suspicious of tuberculous lymphadenitis. Combination therapy was initiated with isoniazid, ethambutol, rifampin, and pyrazinamide; he was discharged from the hospital the next day. However, he experienced high fever (temperature > 39°C), cough, and right-sided chest pain for 10 more days. On the subsequent visit, chest radiography revealed an enlarged cardiac shadow (Figure 2), and CT revealed pericardial and pleural effusions (Figure 3A) with enlargement of the pulmonary lesion (Figure 3B and C). This was suspected to be a paradoxical reaction to antitubercular treatment. He was hospitalized in the Department of Respiratory Medicine, and adjunctive corticosteroid therapy was initiated. We administered prednisolone 1 mg/kg (60 mg) for 3 days and 40 mg for 4 days. Mycobacterium kansasii was isolated from cultures of the tissue previously obtained by mediastinoscopy and from the material obtained on bronchoscopy after readmission. Mycobacterium kansasii was identified using real-time polymerase chain reaction analysis and the DNA–DNA hybridization method. Therefore, he was diagnosed with disseminated M. kansasii infection. Technetium 99m (Tc-99m) skeletal scintigraphy (Figure 4) was performed to locate the site of chest pain. Technetium 99m accumulated in the right costal region, suggestive of a skeletal lesion owing to disseminated M. kansasii infection. As he had disseminated NTM infection, we made investigations regarding associated immunodeficiency, but he was found to be previously healthy. The positive control tested negative on the QuantiFERON-TB Gold In-tube (QFT-GIT) test, which suggested the presence of anti–IFN-γ Abs. After the diagnosis of M. kansasii infection, we stopped administering pyrazinamide. His symptoms, mediastinal lymphadenopathy, and pericardial effusion improved after 2 months of treatment, and he remained well on outpatient therapy. During treatment, an increasing serum anti–IFN-γ Ab titer was confirmed using the ELISA test (Human anti–IFN-γ autoantibody assay kit; U-Cy Tech company, Utrecht, The Netherlands) (Figure 5).
Figure 1.
Chest contrast-enhanced computed tomography showing enlarged supraclavicular, mediastinal, and bilateral hilar lymph nodes (A and B) and multiple small nodules with bronchovascular septal thickening in the right middle lobe (C and D).
Figure 2.
Chest radiography on the subsequent visit showing enlarged cardiac shadow (B) compared with that performed on the first visit (A).
Figure 3.
Chest computed tomography showing pericardial and pleural effusion (A) and enlargement of the pulmonary lesion (B and C).
Figure 4.
Technetium 99m (Tc-99m) skeletal scintigraphy showing right costal Tc-99m accumulation.
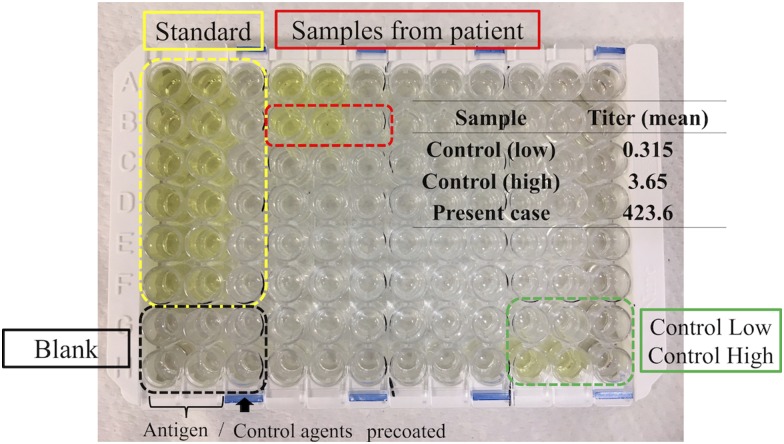
Figure 5.
ELISA test (U-Cy Tech company) showing an increased IFN-γ Ab titer in the serum samples obtained from the patient.
DISCUSSION
We reported a case of disseminated M. kansasii disease in a patient without any previous evidence of immunodeficiency. Although several cases of disseminated NTM infection have been reported, disseminated M. kansasii infections with associated anti–IFN-γ Abs are rarely reported.
Recent studies have reported that disseminated NTM infections are associated with anti–IFN-γ Abs.1–5 In the innate immune system, the interleukin-12–mediated IFN-γ pathway plays a critical role in the biological defense against NTM infections.10 In their study cohort, Brown et al.1 reported that 81% of patients with disseminated NTM infections and normal CD4 lymphocyte counts retained anti–IFN-γ neutralizing Ab positivity. Aoki et al.9 reported that anti–IFN-γ neutralizing Abs were present in most patients with disseminated NTM infections in the absence of clinically evident immunodeficiency. Although long-term antimicrobial therapy is required, the prognosis of patients with disseminated NTM with anti–IFN-γ Abs is more favorable than that of patients with HIV infections or disseminated NTM infections without anti–IFN-γ Abs.9 Because disseminated NTM infections may be misdiagnosed as inflammatory or malignant diseases, it is important to estimate anti–IFN-γ Ab titers in suspicious cases.
In addition, in the present case, the patient showing anti–IFN-γ Abs probably developed a paradoxical reaction, which may indicate that IFN-γ is important for control of NTM infections but is not necessary for the development for paradoxical reactions, as reported previously.11,12
In the present case, biopsies of the lymph nodes and lung nodules aided in the diagnosis of disseminated M. kansasii infection. Mycobacterium kansasii has been reported to be the sixth most commonly isolated NTM in a recent study that analyzed data from 62 laboratories across 30 countries.13 Mycobacterium kansasii has certain distinct clinical features compared with other NTM.6 Mycobacterium kansasii has been infrequently isolated from natural water or soil.8 In Japan, M. kansasii infections have been reported to be associated with occupational exposure to the iron and steel industry and with a history of smoking.14 The clinical symptoms and radiological findings of M. kansasii infections are mostly indistinguishable from those caused by M. tuberculosis infections.6 However, some clinical features differ between M. kansasii and M. tuberculosis infections. Unlike M. tuberculosis infections, lymphadenitis is infrequent in M. kansasii infections; lymphadenitis suggests immunodeficiencies, including the presence of anti–IFN-γ Abs.15 In addition to lymphadenitis, other extrapulmonary manifestations, including involvement of the musculoskeletal and genitourinary systems, are suggestive of disseminated infections. These infections are very rare and usually occur in immunosuppressed individuals, in contrast to infections with other NTM. In HIV-negative patients, M. kansasii infections typically respond well to antimicrobial therapy,8 and patients are expected to recover completely. Therefore, the duration of therapy may be shorter for disseminated M. kansasii infections than that for other disseminated NTM infections. In patients presenting with lymphadenitis or other extrapulmonary manifestations, the possibility of disseminated disease should be considered for precise diagnosis and prompt treatment.
In the present case, indeterminate results of the positive control in the QFT-GIT test suggested the presence of anti–IFN-γ Abs. In addition to M. tuberculosis antigens, M. kansasii encodes CFP-10 and ESAT-6, two antigens targeted by IFN-γ release assays.7 However, QFT-GIT tests, which measure the concentration of IFN-γ, may provide an indeterminate result if active anti–IFN-γ Abs in the patient’s blood substantially neutralize the IFN-γ released in the mitogen tube.16 Conversely, patients test positive for the T-SPOT test because it detects whether cells can produce IFN-γ. In the present case, the positive T-SPOT result and the indeterminate QFT-GIT result demonstrated the absence of IFN-γ, although IFN-γ–producing cells were present; this indicated the presence of anti–IFN-γ Abs. In the diagnosis of disseminated NTM, indeterminate QFT-GIT results may prevent delays by suggesting the presence of neutralizing anti–IFN-γ Abs. Furthermore, because lymphadenitis is infrequent in M. kansasii infections, it can also indicate the presence of anti–IFN-γ Abs in such cases.
The diagnosis of the present case had a limitation that needs to be addressed in future cases. The serum-neutralizing capacity against exogenous IFN-γ was not estimated in this case because we could not obtain the appropriate materials. In accordance with previous reports, a basic experimental procedure using Jurkat cell lines with signal transducer and activator of transcription one antibody is required to evaluate the presence of neutralizing capacity against IFN-γ.9 It is difficult to perform this technique in a community hospital such as our institution. Therefore, it is not known whether the anti–IFN-γ Abs in this patient actually had neutralizing capacity. Knowledge of neutralizing capacity is important in determining predisposition to the disease. However, in a community hospital setting, estimation of anti–IFN-γ Ab titers is the most that can be achieved.
In conclusion, the possibility of the presence of anti–IFN-γ Abs should be considered whenever a diagnosis of disseminated M. kansasii infection is made, even in the absence of clinically evident predisposing immunodeficiencies.
REFERENCES
- 1.Brown SK, et al. 2012. Adult-onset immunodeficiency in Thailand and Taiwan. N Eng J Med 367: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kampmann B, et al. 2005. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest 115: 2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampitak T, Suwanpimolkul G, Brown S, Suankratay C, 2011. Anti-interferon-γ autoantibody and opportunistic infections: case series and review of the literature. Infection 39: 65–71. [DOI] [PubMed] [Google Scholar]
- 4.Hase I, Morimoto K, Sakagami T, Kazumi Y, van Ingen J, 2015. Disseminated Mycobacterium gordonae and Mycobacterium mantenii infection with elevated anti-IFN-γ neutralizing autoantibodies. J Infect Chemother 21: 468–472. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura T, Fujita-Suzuki Y, Yonemaru M, Ohkusu K, Sakagami T, Carpenter SM, Otsuka Y, Namkoong H, Yano I, Hasegawa N, 2015. Recurrence of disseminated Mycobacterium avium complex disease in a patient with anti-gamma interferon autoantibodies be reinfection. J Clin Microbiol 53: 1436–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matveychuk A, Fuks L, Priess R, Hahim I, Shitrit D, 2012. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Respir Med 106: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 7.Sato R, et al. 2016. Interferon-gamma release assays in patients with Mycobacterium kansasii pulmonary infection: a retrospective survey. J Infect 72: 706–712. [DOI] [PubMed] [Google Scholar]
- 8.Johnston JC, Chiang L, Elwood K, 2017. Mycobacterium Kansasii Microbiol Spectr 5. 5: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki A, et al. 2018. Clinical significance of interferon-γ neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis 66: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 10.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanai O, van Dissel JT, 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet 32: 97–105. [DOI] [PubMed] [Google Scholar]
- 11.Xie YL, Rosen LB, Sereti I, Barder DL, Chen RY, Hsu DC, Qasba SS, Zerbe CS, Holland SM, Browne SK, 2016. Severe paradoxical reaction during treatment of disseminated tuberculosis in a patient with neutralizing anti-IFN-γ autoantibodies. Clin Infect Dis 62: 770–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber DL, Andrade BB, McBerry C, Sereti I, Sher A, 2014. Role of IL-6 in Mycobacterium avium associated immune reconstitution inflammatory syndrome. J Immunol 192: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoefsloot W, et al. 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 14.Shimode H, Fului T, Anzai E, Mizutani S, 1991. Studies on epidemiology of nontuberculous mycobacteriosis on the regional difference of the incidence of pulmonary diseases due to M. kansasii and M. avium complex in Tokyo area. Kekkaku 66: 671–677. [PubMed] [Google Scholar]
- 15.Lovell JP, Zerbe CS, Olivier KN, Claypool RJ, Frein C, Anderson VL, Freeman AF, Holland SM, 2016. Mediastinal and disseminated Mycobacterium kansasii disease in GATA2 deficiency. Ann Am Thorac Soc 13: 2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu UI, Chuang YC, Sheng WH, Sun HY, Jhong YT, Wang JY, Chang SC, Wang JT, Chen YC, 2018. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon γ autoantibodies in patents with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect 24: 159–165. [DOI] [PubMed] [Google Scholar]