Abstract.
Microscopy-determined Plasmodium falciparum positivity rates exceeding 10% on day 3 after initiation of artemisinin-based combination therapy (ACT) is an important indicator of artemisinin resistance. However, microscopy does not detect low-density parasitemia, contrary to molecular tools such as loop-mediated isothermal amplification (LAMP) and polymerase chain reaction (PCR). We compared microscopy, LAMP, and PCR for detection of P. falciparum on day 3 after ACT in 256 patients with uncomplicated malaria in Bagamoyo District, Tanzania. Day 3 positivity rates were 0%, 84.8%, and 84.4% for each method, respectively. The sensitivity and specificity of LAMP against PCR was 100% (95% CI, 96.1–100) and 77.4% (95% CI, 58.9–90.4) when quantitative PCR-determined parasite densities were ≥ two parasites/µL. Loop-mediated isothermal amplification had comparable diagnostic accuracy to PCR and could potentially represent a field-friendly tool for determining day 3 positivity rates. However, what day 3 P. falciparum positivity determined using molecular methods represents needs to be further elucidated.
Artemisinin resistance in Plasmodium falciparum malaria was first reported from Southeast Asia in 20091; today, it constitutes a major threat to global malaria control.2 Artemisinin resistance is associated with polymorphisms in the propeller domain of the kelch 13 gene and is phenotypically characterized by delayed parasite clearance after artemisinin-based combination therapy (ACT).2 Day 3 microscopy positivity rates exceeding 10% is considered an alert for artemisinin resistance.3
Despite case reports of delayed microscopy-determined parasite clearance, artemisinin resistance has not yet been documented in Africa.4 Recently reported polymerase chain reaction (PCR)–determined P. falciparum positivity rates ranged between 27.6 and 74.2% on day 3 after initiation of artemether–lumefantrine treatment during 2006–2014 in Bagamoyo district, Tanzania.5 We believe these findings require careful consideration, especially because other reports on persistent submicroscopic parasitemia after ACT have shown association with treatment failure, longer gametocyte carriage, and subsequently higher transmission potential.6,7
The WHO recommends therapeutic efficacy studies (TES) to be conducted with regular intervals as part of programmatic drug resistance surveillance in malaria-endemic countries.3 In TES, finger-prick blood samples are collected and examined by light microscopy on a daily basis for determining parasite clearance up to day 3 after initiation of ACT treatment. Microscopy has a detection limit of 50–200 parasites/µL of blood in endemic field settings, compared with ∼ one parasite/µL for molecular methods such as PCR, potentially resulting in missed detection of low-density parasitemia. Polymerase chain reaction is, however, time-consuming and requires access to a quality-assured molecular laboratory, the reason why PCR results often are not available until several months after the completion of a TES. Loop-mediated isothermal amplification (LAMP) is a field-friendly alternative to PCR, with time-to-result from blood sampling of 1–2 hours.8 Loop-mediated isothermal amplification has a detection limit of 2–5 parasites/µL and has comparable sensitivity and specificity to PCR for detection of low-density P. falciparum infections.9,10
The availability of a field-friendly molecular system could improve parasite detection and determination of positivity rates after initiated treatment in TES. The aim of this study was, therefore, to evaluate LAMP as a field-friendly molecular surveillance tool in comparison with microscopy and PCR for P. falciparum detection on day 3 after ACT.
Finger-prick blood samples for microscopy, LAMP, and PCR were collected from a cohort of 265 patients with uncomplicated P. falciparum malaria on day 3 after initiation of directly observed standard weight-based artemether–lumefantrine treatment. The study was conducted in Bagamoyo District, Tanzania, where malaria transmission is moderate and P. falciparum is the predominant malaria species.11 Patients were enrolled between July 2017 and February 2018. Inclusion criteria were fever (axillary temperature ≥ 37.5°C) or history of fever in the last 24 hours, age 1–65 years, microscopy-confirmed uncomplicated P. falciparum mono-infection irrespective of parasite density, and written informed consent. The study (Identifier: NCT03241901) received ethical clearance from the National Institute for Medical Research and Muhimbili University of Health and Allied Sciences, Tanzania.
Giemsa-stained blood slides were read by two professional, certified independent technicians in the field. Parasite densities (per µL) were calculated assuming a white blood cell count of 8,000/µL. A blood slide was considered negative when examination of 1,000 white blood cells or 100 fields containing at least 10 white blood cells per field revealed no asexual parasites. Discordant results and/or blood slides from patients with day 3 quantitative PCR (qPCR)–determined densities > 20 parasites/µL were reviewed by a third independent expert microscopist. For LAMP, 40 µL of whole blood was collected in Eppendorf tubes containing extraction solution (400 mM NaCl, 40 mM Tris, pH 6.5, and 0.4% sodium dodecyl sulfate) and stored at −80°C. Loop-mediated isothermal amplification analyses were conducted in Bagamoyo Research and Training Center in March 2018, after patient enrollment was completed. DNA was extracted by the boil and spin method with minor modifications,12 followed by LAMP detection using Loopamp™ Malaria Pan Detection Kit (Eiken, Japan) according to the manufacturer’s instructions.13,14 Loop-mediated isothermal amplification results were interpreted visually under ultra violet light for fluorescence. Negative and positive controls were included in every batch of 46 samples. Dried blood spots for PCR were collected on a PerkinElmer 226 filter paper (PerkinElmer, Waltham, MA), allowed to air-dry for 2–3 hours and stored in individual ziplock bags with desiccants at room temperature until shipment. Polymerase chain reaction analyses were conducted at Karolinska Institutet, Stockholm, Sweden, in April 2018. DNA was extracted by the Chelex®-100 (Biorad Laboratory, Hercules, CA) boiling method.15 Two PCR methods were used: a single Cytochrome B PCR assay followed by gel electrophoresis15 and a qPCR targeting the 18S ribosomal RNA gene conducted in triplicate for parasite quantification.15,16 A sample was defined as PCR positive if at least two of the four PCRs had a positive result, that is, either having a positive band of correct size in the gel electrophoresis and/or cycle quantification values below 40. The limit of quantification was set at one parasite/µL.
Analyses were conducted in Stata 15.0 IC (StataCorp, College Station, TX). Proportions were calculated with 95% CIs and compared by chi-squared test, whereas medians were compared by the Mann–Whitney U test. Sensitivity, specificity, positive and negative predictive values, McNemar statistics, and kappa values were calculated for LAMP with PCR as reference standard. Analyses were repeated after excluding samples with qPCR-determined parasite densities below the detection limit of LAMP, that is, < two parasites/µL. Statistical significance was defined as P < 0.05.
Microscopy, LAMP, and PCR positivity rates on day 3 were 0/265 (0%), 217/256 (84.8%), and 224/265 (84.5%), respectively. Patients who were LAMP and/or PCR positive on day 3 had higher microscopy-determined baseline parasitemia (P < 0.001) and higher body temperature (P = 0.001) than those who were LAMP and/or PCR negative (Table 1). Day 3 LAMP-positive patients had lower hemoglobin levels at baseline (P = 0.03). Other pretreatment characteristics were similar.
Table 1.
Baseline characteristics of patients in relation to parasitological day 3 results
| Characteristics | Microscopy (N = 265) | LAMP (N = 256) | PCR (N = 265) | ||||
|---|---|---|---|---|---|---|---|
| Negative (n = 265) | Positive (n = 217) | Negative (n = 39) | P-value | Positive (n = 224) | Negative (n = 41) | P-value | |
| Gender, n (%) Male | 148 (55.8) | 120 (55.3) | 22 (56.4) | 0.90* | 122 (54.5) | 26 (63.4) | 0.29* |
| Age (years.), median (IQR) | 10 (5–16) | 10 (5-15) | 12 (6–27) | 0.22† | 10 (5–15) | 14 (5–35) | 0.08† |
| Age group ≤ 5 years, n (%) | 76 (28.7) | 64 (29.5) | 9 (23.1) | 0.41* | 66 (25.5) | 10 (24.4) | 0.5* |
| Age group > 5 years, n (%) | 189 (71.3) | 153 (70.5) | 30 (76.9) | 0.41* | 158 (70.5) | 31 (75.6) | 0.5* |
| Weight (Kg), median (IQR) | 27 (18–47) | 27 (17–45) | 27 (18–57) | 0.23† | 27 (17–44) | 27 (18–56) | 0.19† |
| Day 0–parasitemia by microscopy (/µL), median (IQR) | 16,800 (4,720–63,360) | 24,600 (7,280–65,920) | 3,360 (1,040–6,680) | < 0.001† | 23,160 (7,390–66,400) | 3,120 (820–7,080) | < 0.001† |
| Hemoglobin g/dL, median (IQR) | 12.2 (10.7–13.2) | 12.0 (10.6–13.1) | 12.7 (11.5–14.1) | 0.03† | 12.0 (10.7–13.3) | 12.5 (11–14) | 0.09† |
| Body temperature (°C), median (IQR) | 38.4 (37.7–39.1) | 38.5 (37.7–39.1) | 37.9 (37.2–38.9) | 0.05† | 38.5 (37.7–39.1) | 37.7 (37.2–38.7) | 0.001† |
* Chi-squared tests.
† Mann–Whitney U test.
Significant P-values are highlighted in bold.
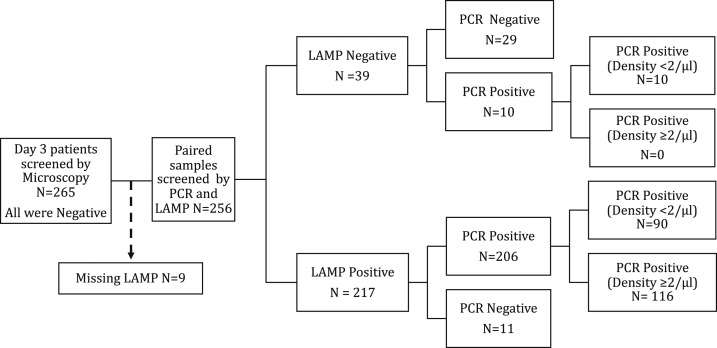
Paired day 3 blood samples for LAMP and PCR were available for 256 patients (Figure 1). Day 3 qPCR-determined geometric mean parasite density was two parasites/µL (range < 1–1,051/µL) in both LAMP- and PCR-positive samples (Table 2). Among LAMP and PCR-positive samples, 36.2% (76/210) and 37.5% (84/224) had detectable parasitemia below the limit of quantification by qPCR (i.e., < one parasite/µL), respectively. Sensitivity, specificity, and positive and negative predictive values of LAMP compared with PCR as well as McNemar statistics and Kappa values are presented in Table 2.
Figure 1.
Flowchart of screening by microscopy, loop-mediated isothermal amplification (LAMP), and polymerase chain reaction (PCR). Reference standard: cytochrome B + 18 seconds PCR.
Table 2.
Loop-mediated isothermal amplification sensitivity, specificity, positive and negative predictive value, McNemar statistics, and Kappa value against as reference standard
| All paired samples (N = 256) | ≥ 2/µL* (N = 147) | |||
|---|---|---|---|---|
| LAMP | PCR | LAMP | PCR | |
| Positive, n (%) | 217 (84.8) | 216 (84.4) | 123 (83.7) | 116 (78.9) |
| Parasite density/µL, mean (range) | 2 (< 1–1,050) | 2 (< 1–1,050) | 6 (2–1,051) | 6 (2–1,051) |
| Sensitivity % (95% CI) | 95.4 (91.7–97.8) | Ref | 100 (96.9–100) | Ref |
| Specificity % (95% CI) | 72.5 (56.1–85.4) | Ref | 77.4 (58.8–90.4) | Ref |
| Positive predictive value % (95% CI) | 94.9 (91.1–97.4) | Ref | 94.3 (88.6–97.7) | Ref |
| Negative predictive value% (95% CI) | 74.4 (57.9–87.0) | Ref | 100 (85.5–100) | Ref |
| McNemar | 1.0 | Ref | 0.02 | Ref |
| Kappa (agreement %) | 0.69 (91.8) | Ref | 0.84 (95.2) | Ref |
LAMP = loop-mediated isothermal amplification; PCR = polymerase chain reaction; Ref = reference.
* Analyses repeated after excluding samples with quantitative PCR-determined parasite densities below the LAMP detection limit, that is, < 2 parasites/µL.
Lack of P. falciparum detection by microscopy on day 3, after 12 years of wide-scale use of artemether–lumefantrine in Bagamoyo District, Tanzania, is encouraging. However, day 3 positivity rates by both LAMP (84.8%) and PCR (84.4%) were higher than previously reported PCR-determined day 3 positivity rates after ACT in the same study area, that is, 28% (14/50) in 2006 (before initiation of ACT treatment policy in Bagamoyo District), 74.2% (132/178) in 2007–2008, and 27.6% (60/217) in 2014.5 Similar to previous findings,5 day 3 P. falciparum LAMP- and PCR-determined positivity rates were associated with pretreatment characteristics (Table 1).
The sensitivity and specificity of LAMP versus PCR are in agreement with previous studies for the detection of submicroscopic malaria parasitemia.10,17 As demonstrated by our results, the negative predictive value of LAMP improves from 74.5% to 100% when analysis is performed for samples above the detection limit; however, the positive predictive value remains similar at 94%. Day 3 positivity rates may vary depending on which molecular method has been used.5,18 In addition, the repeatability of results for molecular methods is low when parasite densities approach the limit of detection.19 This may contribute to the lower specificity of LAMP compared with PCR, although DNA contamination could also be a potential issue with sensitive molecular methods.17
Other studies conducted in Kenya6 and Uganda20 reported up to 62% and 76% P. falciparum positivity by qPCR on day 3 after ACT. In the Ugandan study, day 3 P. falciparum PCR positivity was associated with detection of persisting asexual ring stage parasites and mature gametocytes using specific molecular markers, but without increased risk to treatment failure within the 28-day follow-up.20 Furthermore, molecular techniques are sensitive enough to detect small amounts of circulating DNA in cellular debris after an antimalarial treatment, and the positivity may, therefore, not represent viable parasites.20 Further works are required to determine what day 3 P. falciparum positivity determined using molecular methods represents. Whether these are viable persisting asexual parasites, gametocytes, or DNA debris. Parasite culture and improved assessment of clonal clearance beyond day 3 may provide opportunity to further characterize persisting infections and broaden our understanding of whether molecular tools for parasite detection on day 3 after treatment will be useful in artemisinin resistance surveillance.
In conclusion, the positivity rates of P. falciparum by LAMP and PCR on day 3 after initiation of ACT treatment in this study were similarly high. Importantly, LAMP had comparable diagnostic accuracy to PCR and may, therefore, represent a potential field-friendly tool for determination of positivity rates on day 3 after initiated treatment in TES. However, before LAMP can be considered as such a tool, it needs to be further elucidated what day 3 P. falciparum positivity determined using molecular methods represents.
Acknowledgments:
We would like to extend our heartfelt appreciation to the patients and parents/guardians who participated in the study and to the health-care workers at Fukayosi and Yombo dispensaries. We would also like to extend our gratitude to Anders Bjorkman and Andreas Mårtensson for their invaluable support.
REFERENCES
- 1.Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wwarn K, Group GS, 2019. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments-a WWARN individual patient data meta-analysis. BMC Med 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization , 2018. Status Report on Artemisinin Resistance and ACT Efficacy. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.Lu F, et al. 2017. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med 376: 991–993. [DOI] [PubMed] [Google Scholar]
- 5.Mwaiswelo R, Ngasala B, Jovel I, Xu W, Larsson E, Malmberg M, Gil JP, Premji Z, Mmbando BP, Mårtensson A, 2019. Prevalence of and risk factors associated with polymerase chain reaction-determined Plasmodium falciparum positivity on day 3 after initiation of artemether–lumefantrine treatment for uncomplicated malaria in Bagamoyo District, Tanzania. Am J Trop Med Hyg 100: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth JM, Omweri G, de Jong MD, Osoti V, Mens PF, Sawa P, Makio N, Schallig HDFH, 2018. Molecular detection of residual parasitemia after pyronaridine–artesunate or artemether–lumefantrine treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. Am J Trop Med Hyg 99: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beshir KB, et al. 2013. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis 208: 2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins H, Gonzalez IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, Agaba B, Kyabayinze DJ, Sutherland CJ, Perkins MD, Bell D, 2013. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 208: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pöschl B, Waneesorn J, Thekisoe O, Chutipongvivate S, Panagiotis K, 2010. Comparative diagnosis of malaria infections by microscopy, nested PCR, and LAMP in northern Thailand. Am J Trop Med Hyg 83: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aydin-Schmidt B, Xu W, González IJ, Polley SD, Bell D, Shakely D, Msellem MI, Björkman A, Mårtensson A, 2014. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS One 9: e103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Malaria Control Programme, Mainland Tanzania , 2018. Supplementary Malaria Midterm Strategic Plan (2018–2020); Malaria Control Series 43. Dar es Dalaam, Tanzania: Ministry of Health, Community Development, Gender, Elderly & Children, p. 73. [Google Scholar]
- 12.Foundation for innovative new diagnostics FIND , 2012. Manual of Standard Operating Procedures for Malaria LAMP Available at: https://www.finddx.org/wp-content/uploads/2016/04/SOP-LAMP-Malaria-Aug2012.pdf. Accessed February 3, 2019. [Google Scholar]
- 13.Polley SD, Mori Y, Watson J, Perkins MD, González IJ, Notomi T, Chiodini PL, Sutherland CJ, 2010. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J Clin Microbiol 48: 2866–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T, 2000. Loop-Mediated Isothermal Amplification of DNA, Vol. 28 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC102748/pdf/gnd064.pdf. Accessed February 9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Morris U, Aydin-Schmidt B, Msellem MI, Shakely D, Petzold M, Björkman A, Mårtensson A, 2015. SYBR green real-time PCR-RFLP assay targeting the Plasmodium cytochrome B gene–a highly sensitive molecular tool for malaria parasite detection and species determination. PLoS One 10: e0120210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, Muringo L, Ogutu B, Waitumbi JN, Ockenhouse CF, 2011. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of Plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol 49: 2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris U, et al. 2015. Field deployment of loop-mediated isothermal amplification for centralized mass-screening of asymptomatic malaria in Zanzibar: a pre-elimination setting. Malar J 14: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katrak S, et al. 2017. Performance of loop-mediated isothermal amplification for the identification of submicroscopic Plasmodium falciparum infection in Uganda. Am J Trop Med Hyg 97: 1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paris DH, Imwong M, Faiz AM, Hasan M, Yunus E Bin, Silamut K, Lee SJ, Day NPJ, Dondorp AM, 2007. Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. Am J Trop Med Hyg 77: 972–976. [PubMed] [Google Scholar]
- 20.Chang H-H, et al. 2016. Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci Rep 6: 26330. [DOI] [PMC free article] [PubMed] [Google Scholar]