Abstract.
Rocky Mountain spotted fever (RMSF), caused by Rickettsia rickettsii, is a severe tick-borne infection endemic to the Americas. Oral doxycycline is effective, but during severe life-threatening disease, intravenous therapy is recommended. Unfortunately, intravenous formulations of doxycycline are not always available. Therefore, we aimed to determine the susceptibility of R. rickettsii to an alternative parenteral agent, tigecycline, in vitro and in vivo. To determine the minimum inhibitory concentration of tigecycline, R. rickettsii–inoculated Vero cells were incubated with medium containing tigecycline. At various time points, monolayers were collected and R. rickettsii was quantified via real-time polymerase chain reaction (PCR). The growth of R. rickettsii was inhibited in the presence of ≥ 0.5 µg/mL of tigecycline. To determine the effectiveness of tigecycline in vivo, guinea pigs were inoculated with R. rickettsii. Five days after inoculation, they were treated twice daily with subcutaneous tigecycline 3.75 mg/kg or subcutaneous doxycycline 5 mg/kg. Treated animals improved, whereas untreated controls remained ill. Tissues were collected for quantitative PCR–determined bacterial loads on day 8. Median bacterial loads in the tigecycline group were less than those in untreated animals: liver (0 versus 2.9 × 104 copies/mg), lung (0 versus 8.3 × 103 copies/mg), skin (2.6 × 102 versus 2.2 × 105 copies/mg), spleen (0 versus 1.3 × 104 copies/mg), and testes (0 versus 1.0 × 105 copies/mg, respectively). There were no significant differences in the bacterial loads between doxycycline-treated versus tigecycline-treated guinea pigs. These data indicate that tigecycline is effective against R. rickettsii in cell culture and in an animal model of RMSF.
INTRODUCTION
Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever (RMSF), is an obligately intracellular Gram-negative bacterium and a member of the spotted fever group (SFG)—a phylogenetic clustering of tick-borne rickettsiae.1 Rocky Mountain spotted fever is endemic to the Americas and is the most severe tick-borne rickettsial infection.2 The untreated case fatality rate in the United States is 23%,3 but in Mexico and Brazil, the current case fatality rate has been reported to be as high as 40%.4,5 Fortunately, effective antimicrobial therapy is available. Antibiotics in the tetracycline class are highly effective, and doxycycline, with its tolerability, affordability, and ease of its twice daily administration, is the specific drug of choice.6 Although doxycycline is highly bioavailable through the oral route,7 pathophysiologic changes associated with critical illness may decrease the absorption of enterally administered medications, thus, necessitating the use of parenteral formulations.8 Nausea and vomiting, which frequently accompany RMSF, may also prevent oral treatment. In cases of severe life-threatening RMSF, intravenous doxycycline is recommended.6 During meetings with physicians at the Universidad de Antioquia during the 2017 Latin American Meeting for Rickettsial Diseases in Medellin, Colombia, we were surprised to learn that parenteral doxycycline is not available in their country. Interestingly, tigecycline, a newer generation tetracycline-like antibiotic in the glycylcycline class, is available in Colombia. Tigecycline has extended antimicrobial coverage because of its ability to overcome tetracycline-specific efflux pumps and ribosomal protection, mechanisms that confer bacterial resistance to its predecessor tetracyclines (e.g., doxycycline and minocycline).9 It is indicated for use in community-acquired pneumonia, complicated intraabdominal infections, and complicated skin and soft tissue infections.10–12 In addition to broad Gram-positive and Gram-negative coverage, the drug is effective against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila.13 As a derivative of minocycline, it is natural to presume that tigecycline would have activity against Rickettsia spp. We were, thus, posed with the question of its effectiveness against R. rickettsii when intravenous doxycycline is unavailable. In this study, we aimed to determine the susceptibility of R. rickettsii to tigecycline in a cell culture assay and a lethal animal model of RMSF.
MATERIALS AND METHODS
Antibiotic.
For in vivo experiments, tigecycline (Pfizer, New York, NY) was reconstituted with the addition of sterile water as per the manufacturer’s instructions to make a 10 mg/mL stock. The stock was aliquoted and stored at −20°C until further use. To prepare medium, a tube of tigecycline stock was thawed and added to cell culture medium (Dulbecco’s modified Eagle media [DMEM] [Thermo Fisher Scientific, Waltham, MA] with 3% fetal bovine serum [FBS] [GE Healthcare Life Sciences, Pittsburg, PA] and 1% HEPES buffer [Corning, Corning, NY]) to make a concentration of 4 µg/mL tigecycline. Serial 2-fold dilutions (down to 0.06 µg/mL) were performed using antibiotic-free cell culture medium. The aforementioned medium was made fresh immediately before the application to the cell culture assay as described in the following paragraphs. For in vivo experiments, tigecycline was reconstituted and diluted in sterile phosphate-buffered saline (PBS) to achieve a concentration of 3.75 mg/mL. Doxycycline was reconstituted and diluted in sterile PBS to achieve a concentration of 5 mg/mL. These solutions were prepared fresh daily for use in the animal model as described in the following paragraphs.
Cell culture assay.
Six-well plates were prepared by seeding with 1 × 106 Vero cells per well with DMEM containing 10% FBS and 1% HEPES buffer and incubated overnight at 37°C with 5% CO2 to achieve confluence. A frozen vial of Vero cell–passaged R. rickettsii Sheila Smith strain (stock previously quantified using methods as previously described)14 was thawed, diluted in antibiotic-free cell culture medium, and inoculated on each well (estimated to inoculate approximately 3 × 103 viable organisms per well). Plates were centrifuged for 5 minutes at 800 × g at 22°C and then incubated at 37°C for 1 hour. The medium from each well was aspirated, each well was washed with PBS three times, and fresh cell culture medium with tigecycline was placed on each monolayer at 2-fold decreasing concentrations (4–0.06 µg/mL) as well as antibiotic-free controls. The plates were then incubated at 34°C with 5% CO2. Each antibiotic concentration was tested in duplicate at each time point. Medium with tigecycline was prepared fresh and changed in each well every 48 hours. At time 0 (before the addition of tigecycline), 72, and 120 hours after the inoculation of R. rickettsii, the medium from the wells was removed and 200 µL of PBS and 200 µL of AL cell lysis buffer (Qiagen, Germantown, MD) were added. The resultant lysates were transferred to microcentrifuge tubes for DNA extraction. To ensure that a uniform amount of the lysate was collected, the plates were placed at a slight incline for 1 minute to aggregate the lysate contents before removal of 400 µL. These experiments were performed three times.
Animal model.
The following experiments were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee. Male Hartley strain guinea pigs (≥ 500 g) (Charles River Laboratories, Wilmington, MA) were inoculated intraperitoneally with a previously determined lethal dose (3 × 103 infectious organisms) of R. rickettsii, Sheila Smith strain, contained in 1 mL of 50% R. rickettsii–infected guinea pig spleen blood suspension diluted in PBS. The animals were monitored twice daily for signs of illness. Rectal temperatures, weights, and clinical observations were recorded daily. On day 5 following initial infection, which corresponds to the time when animals had been febrile for 48 to 72 hours, treatment was initiated. Animals were separated in three groups to receive tigecycline 3.75 mg/kg subcutaneous every 12 hours, doxycycline 5 mg/kg subcutaneous every 12 hours, or PBS 1 mL/kg subcutaneous every 12 hours. The dose of tigecycline was based on studies to determine the effectiveness of tigecycline in a guinea pig model of L. pneumophila pneumonia.15 At experimental day 8, the animals were sacrificed by carbon dioxide narcosis and cardiac exsanguination. Aliquots of blood were placed in ethylenediaminetetraacetic acid-coated microtubes before DNA extraction. The animals were necropsied for the collection of tissues (liver, lung, skin, spleen, and testis) for real-time PCR analysis of bacterial loads. The experiment was performed twice with a total of five guinea pigs in the tigecycline, doxycycline, and control arms. In addition, five animals in each treatment group completed 7 days of treatment and were monitored until day 18 post R. rickettsii inoculation.
Molecular analysis of bacterial loads.
DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen). Cell culture monolayer lysates, guinea pig blood (100 µL), and guinea pig tissues were processed using the manufacturer’s instructions for cultured cells, blood, and tissues, respectively. Quantitative PCR was performed using a Bio-Rad CFX96 real-time PCR detection system and oligonucleotides (Bio-Rad, Hercules, CA). The latter of which consisted of 10 pmol forward primer CS5 (5′-GAGAGAAAATTATATCCAAATGTTGAT-3′), 10 pmol reverse primer CS6 (5′-AGGGTCTTCGTGCATTTCTT-3′), and 4 pmol FAM-labeled probe (5′-CATTGTGCCATCCAGCCTACGGT-3′) to detect and quantify rickettsial gltA from these specimens. Thermal cycling consisted of a melting cycle of 95°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds, 50°C for 30 seconds, and 60°C for 30 seconds. Detected gene copies correspond to individual rickettsiae in a 1:1 ratio.
Analysis.
We defined the minimum inhibitory concentration (MIC) as the lowest concentration of tigecycline which inhibited the growth of R. rickettsii as demonstrated by the quantity of organisms being ≤ to the quantity on day 0. The bacterial loads from guinea pig tissues collected at day 8 were compared using the nonparametric Kruskal–Wallis test to compare three unmatched groups followed by Dunn’s multiple comparisons test to further analyze the differences between groups. If real-time PCR failed to detect rickettsial DNA, a value of 0 was given. Analysis was performed using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA).
RESULTS
In the in vitro cell culture assay, the bacterial concentration of each well at time 0 measured 8.3 × 103 bacteria/well. On day 3, mean bacterial loads were the following for varying concentrations of tigecycline: 1.0 × 107 bacteria/well (untreated control), 2.6 × 103 bacteria/well (4 µg/mL), 1.6 × 103 bacteria/well (2 µg/mL), 5.7 × 103 bacteria/well (1 µg/mL), 2.0 × 103 bacteria/well (0.5 µg/mL), 1.2 × 105 bacteria/well (0.25 µg/mL), 5.7 × 106 bacteria/well (0.13 µg/mL), and 6.2 × 105 bacteria/well (0.06 µg/mL). On day 5, bacterial loads were the following for varying concentrations of tigecycline: 6.2 × 108 bacteria/well (untreated control), 4.5 × 103 bacteria/well (4 µg/mL), 1.6 × 103 bacteria/well (2 µg/mL), 1.8 × 103 bacteria/well (1 µg/mL), 9.8 × 102 bacteria/well (0.5 µg/mL), 2.0 × 103 bacteria/well (0.25 µg/mL), 2.3 × 107 bacteria/well (0.13 µg/mL), and 1.9 × 107 bacteria/well (0.06 µg/mL). Thus, R. rickettsii was consistently inhibited by tigecycline at concentrations of ≥ 0.5 µg/mL. There was no cytopathic effect noted in Vero cells at any concentration of tigecycline.
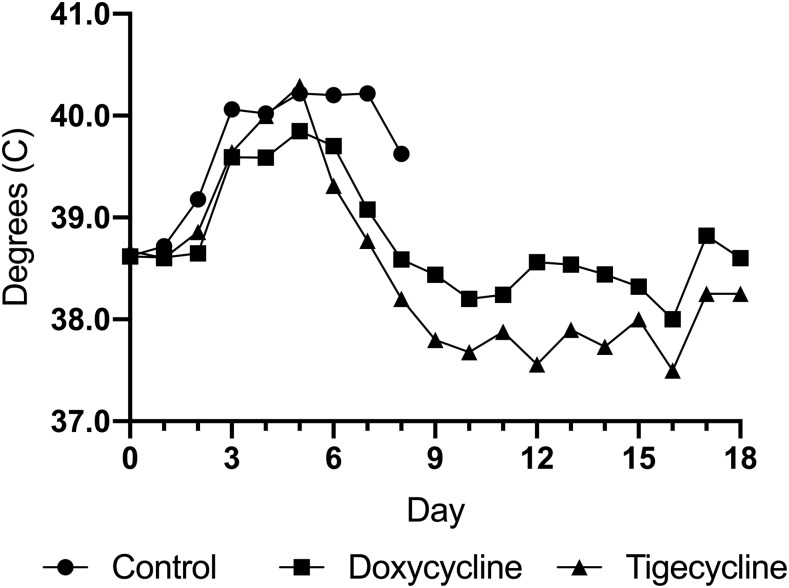
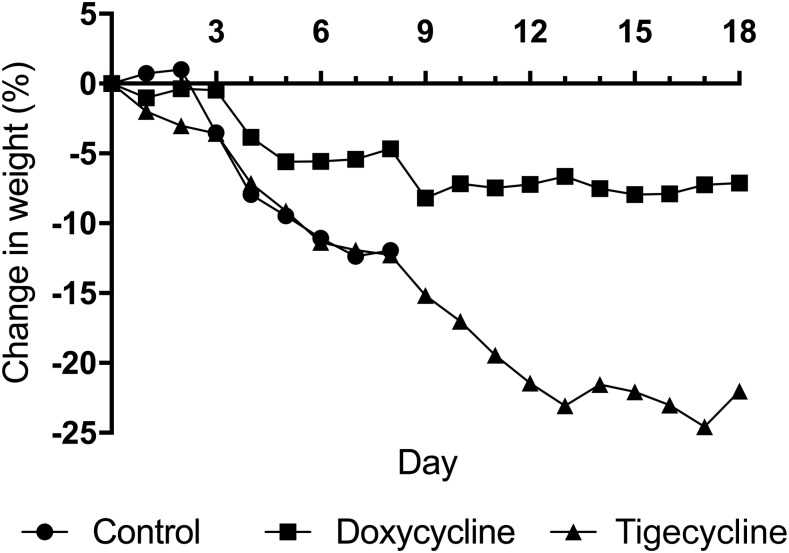
Animals infected with R. rickettsii developed fever (≥ 40.0°C) between days 3 and 4 after inoculation. Animals also lost weight and developed scrotal reaction as is typical for the guinea pig model of lethal RMSF. After initiation of treatment with either tigecycline or doxycycline, animals defervesced (Figure 1). By day 7 after inoculation (treatment day 3), no animal in the treatment group was febrile. Swelling and erythema of scrota subsided and returned to normal in animals of both treatment groups. Whereas animals in the doxycycline group experienced stabilization in their weight, those in both the tigecycline group and untreated controls lost weight throughout their course (Figure 2). Untreated controls remained febrile and progressively developed worsening signs of illness (lethargy, hunched back, and ruffled fur). Animals were necropsied at day 8, as in previous experiments, guinea pigs were typically moribund between days 8 and 10 using the same dose and stock of R. rickettsii. Indeed, two of the five untreated controls were moribund on day 8.
Figure 1.
Mean temperature in tigecycline-treated, doxycycline-treated, and untreated (control) Rickettsia rickettsii–infected guinea pigs.
Figure 2.
Mean change in weight in tigecycline-treated, doxycycline-treated, and untreated (control) Rickettsia rickettsii–infected guinea pigs.
The bacterial loads from blood collected at the time of necropsy demonstrated few organisms in the tigecycline groups compared with the untreated controls: Median of 0 bacteria/µL for tigecycline recipients and 119 bacteria/µL for untreated controls (P = 0.005) were observed (Table 1). The bacterial loads in tissues were less in the tigecycline group compared with untreated controls: liver (0 versus 2.9 × 104 copies/mg) (P = 0.038), lung (0 versus 8.3 × 103 copies/mg) (P = 0.019), skin (2.6 × 102 versus 2.2 × 105 copies/mg) (P = 0.048), spleen (0 versus 1.3 × 104 copies/mg) (P = 0.014), and testes (0 versus 1.0 × 105 copies/mg) (P = 0.038). There were no significant differences in the bacterial loads between doxycycline-treated versus tigecycline-treated guinea pigs (Table 1).
Table 1.
Bacterial loads from blood and tissues collected on day 8 after Rickettsia rickettsi inoculation (day 4 of treatment).
| Bacterial load*/(interquartile range) | Summary of adjusted P values for Dunn’s multiple comparisons test | |||||
|---|---|---|---|---|---|---|
| Tigecycline | Doxycycline | Control | Tigecycline vs. control | Doxycycline vs. control | Tigecycline vs. doxycycline | |
| Blood | 0 | 0 | 119 (41–274) | 0.005 | 0.005 | NS |
| Liver | 0 (0–225) | 0 | 2.9 × 104 (6.6 × 103–3.6 × 105) | 0.038 | 0.003 | NS |
| Lung | 0 | 0 | 8.3 × 103 (590–1.1 × 106) | 0.019 | 0.019 | NS |
| Skin | 260 (39–1.2 × 104) | 55 (0–5.7 × 103) | 2.2 × 105 (4.9 × 104–3.6 × 105) | 0.048 | 0.011 | NS |
| Spleen | 0 (0–29) | 0 (0–2) | 1.3 × 104 (4.7 × 103–1.3 × 106) | 0.014 | 0.011 | NS |
| Testes | 0 (0–31) | 0 | 1.0 × 105 (5.2 × 104–2.5 × 105) | 0.038 | 0.033 | NS |
NS = not significant.
* Expressed as bacteria/µL of blood or bacteria/mg of tissue.
In a subset of five guinea pigs in each treatment arm allowed finishing 7 days of antibiotic, with intention of monitoring in the days following antibiotic cessation, those in the tigecycline group continued to lose weight (Figure 2). This weight loss was despite the absence of the typical signs of R. rickettsii infection in this animal model (i.e., fever, ruffled fur, scrotal erythema and edema, and necrotic ear tips or digits) and consistent with another study of tigecycline in guinea pigs, which hypothesized a toxic effect of the antibiotic on this animal species.15 To differentiate the possibility of toxicity rather than infection, bacterial loads were tested in this subset of guinea pigs. Necropsies were performed on two tigecycline-treated guinea pigs which appeared lethargic and moribund on days 13 and 16 following infection and on two guinea pigs at the end of observation (day 18 after infection). Tissues collected at the time of these necropsies revealed no detected bacteria in the blood, liver, lung, spleen, and testes. Tissues collected from two doxycycline-treated guinea pigs at the end of experimental observations (day 18) revealed no detected bacteria in the blood, liver, lung, spleen, and testes.
DISCUSSION
These results demonstrate that tigecycline is active against R. rickettsii in vitro using a cell culture assay system. In the guinea pig model for fatal RMSF, tigecycline treatment was effective in allowing the immune system to clear the bacterium from tissues as similar to doxycycline. Furthermore, between days 8 and 10, when R. rickettsii–infected guinea pigs were expected to succumb to infection, treatment with tigecycline resolved fever and ameliorated signs of severe infection.
As an obligately intracellular organism, cultivation of Rickettsia spp. requires cell culture techniques.16 Methods to determine the antimicrobial susceptibility for these agents are not standardized nor validated as in the case for more typical bacteria.17 Historically, rickettsial susceptibility has been demonstrated with techniques using Rickettsia-infected animals, embryonated eggs, cell culture–based plaque assay, and cell culture–based quantitative real-time PCR.18–21 Various SFG and typhus group species are inhibited by tetracyclines (MIC 0.06–0.25 µg/mL), chloramphenicol (MIC 0.25–2.0 µg/mL), and fluoroquinolones (MIC 0.25–1.0 µg/mL).19,22,23 As a bacteriostatic agent with a structure similar to minocycline, we expected to find physiologically relevant concentrations of tigecycline, which would inhibit the growth of R. rickettsii. Indeed, we determined that the MIC of tigecycline against R. rickettsii to be 0.5 µg/mL. In phase 1 and phase 3 studies of tigecycline, the maximum serum drug concentrations have been recorded to be 0.40 to 0.98 µg/mL.24,25 Analysis of data from various clinical cohorts studying the use of tigecycline reports that MIC breakpoints ranging from 0.12 to 1 µg/mL are associated with cure rates of 83–100% for infections with S. aureus, Streptococci, Gram-negative bacteria, and anaerobes.25 Thus, with the MIC of 0.5 µg/mL reported here, it seems biologically plausible that tigecycline would be effective for those infected with R. rickettsii.
In the guinea pig animal model of lethal RMSF, we chose a tigecycline dosing regimen based on a previous study which examined several different doses and frequencies.15 We chose a twice daily regimen to be congruent with the twice daily dosing of doxycycline in guinea pigs. It was noted that guinea pigs treated with tigecycline continued to lose weight, despite the normalization of temperature and other signs of illness consistent with this animal model for fatal RMSF. This effect was noted in the study of tigecycline treatment of L. pneumophila, where death occurred in uninfected guinea pigs administered more than 5 days of tigecycline. In our study, R. rickettsii DNA was absent from tissues collected from animals that previously completed 7 days of tigecycline treatment. This indicates that the moribund status of these guinea pigs was probably related to a drug-related toxic phenomenon, rather than an infectious process.
A shortcoming of this study is its inability to translate the in vitro susceptibility and activity in an animal model to humans. Unfortunately, because of the relative infrequency of RMSF and the effectiveness of doxycycline, whose efficacy in rickettsial diseases is well established, head-to-head clinical trials to evaluate newer antibiotics are not foreseeable. It must be noted that the apparent susceptibility of rickettsiae to antibiotics does not necessarily correlate with clinical response. For example, the MIC of rifampin against Rickettsia species ranges from 0.06 to 1.0 µg/mL,19 but in a study of Mediterranean spotted fever, 5 days of rifampin was associated with treatment failures compared with those receiving a 1-day course of doxycycline.26 To our knowledge, there is no documented experience using tigecycline during RMSF. The drug has been successfully used in treatment of a woman from southern Italy with murine typhus (caused by Rickettsia typhi),27 but it must be noted that infection with R. typhi is less severe than that of R. rickettsii (preantibiotic era case fatality rates of 1% and 23%, respectively).3,28
The availability of tigecycline in Colombia, yet not of parenteral doxycycline (a less-expensive agent), may at casual thought seem peculiar, but the drug’s touted broad spectrum activity, its ability to overcome tetracycline-associated mechanisms of resistance, and its resultant activity against relatively resistant bacteria (e.g., MRSA and VRE) are compelling reasons to keep the drug in the antibiotic armamentarium. In the case of doxycycline, an inexpensive and bioavailable oral drug with few indications for intravenous use, demand for the parenteral form is likely low. Although unavailability of parenteral doxycycline was once a problem in Brazil29 and presently a problem in Mexico,30 we are unsure of how many countries in Latin America lack intravenous doxycycline.
Tetracyclines, especially doxycycline, are the mainstay of treatment for RMSF.6 Their effectiveness has been established by decades of cumulative clinical experience, rather than controlled trials. The only alternative, chloramphenicol, is an inferior agent. It is associated with a higher case fatality rate when compared with tetracyclines (7.6% versus 1.5%, [OR, 5.5; 95% CI, 3.9–7.7).31 Unrelated to dose, chloramphenicol can also cause rare but fatal aplastic anemia.32 Although the oral form is available throughout much of the developing world, it is no longer available in the United States. Other drugs, such as fluoroquinolones and newer generation macrolides (e.g., clarithromycin and azithromycin), have been demonstrated to be effective in mild cases of Mediterranean spotted fever (caused by Rickettsia conorii),33–35 but this infection is less severe than that caused by R. rickettsii. Thus, they cannot be recommended for RMSF.
In conclusion, these results demonstrate that tigecycline is active against R. rickettsii in vitro and in vivo using the guinea pig model for fatal RMSF. When faced with the difficult situation when the absorption of enteral doxycycline is compromised and intravenous alternatives are unavailable, tigecycline may be considered. In areas where RMSF is endemic, the availability of parenteral doxycycline would be ideal.
REFERENCES
- 1.Yu X-J, Walker DH, 2005. Family I. Rickettsiaceae. Brenner DJ, Kreig NR, Stanley JT, eds. Bergey’s Manual of Systematic Bacteriology. New York, NY: Springer, 96–116. [Google Scholar]
- 2.Parola P, et al. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker DH, Paddock CD, Dumler JS, 2008. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am 92: 1345–1361. [DOI] [PubMed] [Google Scholar]
- 4.Amancio FF, Amorim VD, Chamone TL, Brito MG, Calic SB, Leite AC, Fraga GL, Ferraz ML, 2011. Epidemiological characteristics of Brazilian spotted fever in Minas Gerais state, Brazil, 2000–2008. Cad Saude Publica 27: 1969–1976. [DOI] [PubMed] [Google Scholar]
- 5.Straily A, Drexler N, Cruz-Loustaunau D, Paddock CD, Alvarez-Hernandez G, 2016. Notes from the field: community-based prevention of Rocky Mountain spotted fever–Sonora, Mexico, 2016. MMWR Morb Mortal Wkly Rep 65: 1302–1303. [DOI] [PubMed] [Google Scholar]
- 6.Biggs HM, et al. 2016. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis–United States. MMWR Recomm Rep 65: 1–44. [DOI] [PubMed] [Google Scholar]
- 7.Mahon WA, Wittenberg JV, Tuffnel PG, 1970. Studies on the absorption and distribution of doxycycline in normal patients and in patients with severely impaired renal function. Can Med Assoc J 103: 1031–1034. [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts DJ, Hall RI, 2013. Drug absorption, distribution, metabolism and excretion considerations in critically ill adults. Expert Opin Drug Metab Toxicol 9: 1067–1084. [DOI] [PubMed] [Google Scholar]
- 9.Bauer G, Berens C, Projan SJ, Hillen W, 2004. Comparison of tetracycline and tigecycline binding to ribosomes mapped by dimethylsulphate and drug-directed Fe2+ cleavage of 16S rRNA. J Antimicrob Chemother 53: 592–599. [DOI] [PubMed] [Google Scholar]
- 10.Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E, 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis 41 (Suppl 5): S354–S367. [DOI] [PubMed] [Google Scholar]
- 11.Ellis-Grosse EJ, Babinchak T, Dartois N, Rose G, Loh E, 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin–aztreonam. Clin Infect Dis 41 (Suppl 5): S341–S353. [DOI] [PubMed] [Google Scholar]
- 12.Tanaseanu C, et al. 2008. Integrated results of 2 phase 3 studies comparing tigecycline and levofloxacin in community-acquired pneumonia. Diagn Microbiol Infect Dis 61: 329–338. [DOI] [PubMed] [Google Scholar]
- 13.Zhanel GG, Homenuik K, Nichol K, Noreddin A, Vercaigne L, Embil J, Gin A, Karlowsky JA, Hoban DJ, 2004. The glycylcyclines: a comparative review with the tetracyclines. Drugs 64: 63–88. [DOI] [PubMed] [Google Scholar]
- 14.Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH, 2004. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 42: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelstein PH, Weiss WJ, Edelstein MA, 2003. Activities of tigecycline (GAR-936) against Legionella pneumophila in vitro and in guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother 47: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanton LS, Walker DH, Bouyer DH, 2019. Rickettsia and Orientia. Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW, eds. Manual of Clinical Microbiology. Washington, DC: ASM Press, 1149–1162. [Google Scholar]
- 17.Dumler JS, 2012. Clincial disease: current treatment and new challenges. Palmer GH, Azad AF, eds. Intracellular Pathogens II: Rickettsiales. Washington, DC: ASM Press, 1–39. [Google Scholar]
- 18.McDade JE, 1969. Determination of antibiotic susceptibility of Rickettsia by the plaque assay technique. Appl Microbiol 18: 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolain JM, Maurin M, Vestris G, Raoult D, 1998. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother 42: 1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolain JM, Stuhl L, Maurin M, Raoult D, 2002. Evaluation of antibiotic susceptibilities of three rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob Agents Chemother 46: 2747–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smadel JE, Jackson EB, Cruise AB, 1949. Chloromycetin in experimental rickettsial infections. J Immunol 62: 49–65. [PubMed] [Google Scholar]
- 22.Maurin M, Raoult D, 1997. Bacteriostatic and bactericidal activity of levofloxacin against Rickettsia rickettsii, Rickettsia conorii, ‘Israeli spotted fever group rickettsia’ and Coxiella burnetii. J Antimicrob Chemother 39: 725–730. [DOI] [PubMed] [Google Scholar]
- 23.Raoult D, Drancourt M, 1991. Antimicrobial therapy of rickettsial diseases. Antimicrob Agents Chemother 35: 2457–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotfried MH, Horn K, Garrity-Ryan L, Villano S, Tzanis E, Chitra S, Manley A, Tanaka SK, Rodvold KA, 2017. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother 61: e01135–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacGowan AP, 2008. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother 62 (Suppl 1): i11–i16. [DOI] [PubMed] [Google Scholar]
- 26.Bella F, Espejo E, Uriz S, Serrano JA, Alegre MD, Tort J, 1991. Randomized trial of 5-day rifampin versus 1-day doxycycline therapy for Mediterranean spotted fever. J Infect Dis 164: 433–434. [DOI] [PubMed] [Google Scholar]
- 27.Luciani F, Cione E, Corsonello A, Guido F, De Santis S, Cannataro R, Perri M, Caroleo MC, Cannataro AM, 2014. Spotted fever from Rickettsia typhi in an older woman: a case report from a geographic area where it would not be expected. Int J Infect Dis 27: 10–12. [DOI] [PubMed] [Google Scholar]
- 28.Stuart BM, Pullen RL, 1945. Endemic (murine) typhus fever: clinical observations of 180 cases. Ann Intern Med 23: 17. [Google Scholar]
- 29.del Sa DelFiol F, Junqueira FM, da Rocha MC, de Toledo MI, Filho SB, 2010. Rocky Mountain spotted fever in Brazil [article in Portuguese]. Rev Panam Salud Publica 27: 461–466. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Hernandez G, Roldan JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD, 2017. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis 17: e189–e196. [DOI] [PubMed] [Google Scholar]
- 31.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE, 2001. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis 184: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 32.Wallerstein RO, Condit PK, Kasper CK, Brown JW, Morrison FR, 1969. Statewide study of chloramphenicol therapy and fatal aplastic anemia. JAMA 208: 2045–2050. [PubMed] [Google Scholar]
- 33.Meloni G, Meloni T, 1996. Azithromycin vs. doxycycline for Mediterranean spotted fever. Pediatr Infect Dis J 15: 1042–1044. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz Beltran R, Herrero Herrero JI, 1992. Evaluation of ciprofloxacin and doxycycline in the treatment of Mediterranean spotted fever. Eur J Clin Microbiol Infect Dis 11: 427–431. [DOI] [PubMed] [Google Scholar]
- 35.Raoult D, Gallais H, De Micco P, Casanova P, 1986. Ciprofloxacin therapy for Mediterranean spotted fever. Antimicrob Agents Chemother 30: 606–607. [DOI] [PMC free article] [PubMed] [Google Scholar]