ABSTRACT
BACKGROUND:
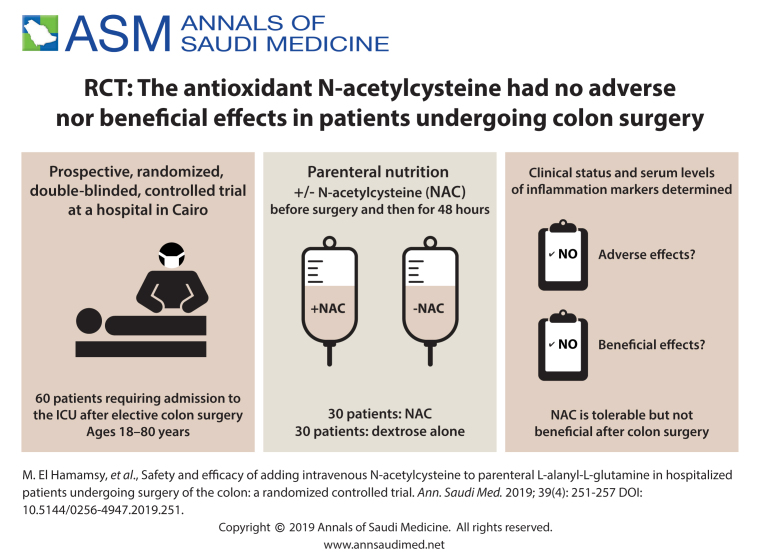
Colon surgery can cause systemic inflammatory response syndrome (SIRS). There is a recent trend towards the use of antioxidant agents in the prevention or alleviation of the severity of postoperative SIRS, but its use is controversial as studies have shown conflicting results.
OBJECTIVES:
Investigate the efficacy and tolerability of perioperative intravenous administration of N-acetylcysteine (NAC) as an antioxidant and anti-inflammatory agent in patients undergoing colon surgery.
DESIGN:
Randomized, double-blinded, and controlled clinical trial.
SETTING:
Surgical critical care unit in Egypt.
PATIENTS AND METHODS:
Sixty patients who required admission to the ICU following colon surgery were enrolled in the study between July 2015 and October 2016. Eligibility included the need for parenteral nutrition for at least 5 days due to failure of or contraindication to enteral nutrition. Patients were randomly allocated using a computer-generated list to a loading dose of NAC followed by continuous infusion started one hour prior to induction, and continued over 48 hours, or to the control group, who received the same volume of dextrose 5%. Allocation was concealed using opaque, sealed envelopes under pharmacy control. The researcher, the anesthesiologist, the surgeon, and patients were blinded to the treatment allocation.
MAIN OUTCOME MEASURES:
Clinical and laboratory evaluation for manifestations of SIRS, serum levels of tumor necrosis factor alpha and malondialdehyde, and occurrence of side effects in the study group.
SAMPLE SIZE:
60 patients with mean (SD) ages of 56 (15.1) years in the study group (n=30) and 57.7 (12.3) years in the control group (n=30).
RESULTS:
There was a significant difference in the mean serum level of ALT (22.6 (9.9) U/L in the study group vs. 31.1 (17.8) U/L in the control group, P=.028) after treatment with NAC, but differences between the groups in the serum level of tumor necrosis factor alpha and malondialdehyde after treatment were not significant. Serum levels of malondialdehyde increased in both groups after treatment P<.001. There was no statistically significant difference from baseline or between the groups after treatment in other clinical data and laboratory parameters following NAC administration, and only 6.6% of the patients in the study group experienced mild side effects.
CONCLUSIONS:
Preoperative administration of NAC is safe, but its efficacy as an antioxidant and anti-inflammatory agent was not statistically significant and requires further investigation in a larger sample.
LIMITATIONS:
Single-center study, small sample size, and short duration of NAC administration.
CLINICAL TRIALS REGISTRY:
CONFLICT OF INTEREST:
None.
INTRODUCTION
Colon surgery plays a pivotal role in the treatment of both benign or malignant colonic diseases,1 but is the major activator of systemic inflammatory response syndrome (SIRS).2 N-acetylcysteine (NAC) has an anti-inflammatory effect through inhibition of the release of pro-inflammatory cytokines.3 NAC is a strong scavenger of oxygen free radicals, and creates new stores of glutathione, which mitigates the release of malondialdehyde (MDA) as a byproduct.4 Adding NAC to L-alanyl L-glutamine reinforces the effect of the latter in patients suffering from oxidative stress and inflammatory responses, especially intensive care patients.5,6 However, few studies have assessed the addition of NAC to L-alanyl L-glutamine.7,8 Moreover, the use of NAC in the intensive care unit is still controversial as many studies have shown conflicting results.8 Therefore, we aimed to evaluate the efficacy of NAC addition to L-alanyl L-glutamine in patients undergoing colon surgery by assessing both the changes in serum levels of MDA as a marker of peroxidation and tumor necrosis factor alpha (TNF-α) as a marker of inflammation two days after surgery. We also evaluated safety.
PATIENTS AND METHODS
This prospective, randomized, double-blinded, controlled clinical trial involved 60 patients, aged between 18 and 80 years, of American Society of Anesthesiologist (ASA) physical status classification IIIII, who underwent elective colon surgeries and were admitted to the surgical intensive care unit at Ain Shams Specialized Hospital, Cairo, Egypt, from July 2015 to October 2016. The trial was registered at the US National Institutes of Health (https://clinicaltrials.gov/ct2/show/NCT03589495). The study protocol was reviewed and approved by the ethical committee of the Faculty of Pharmacy, Ain Shams University, and written informed consent was obtained from all participants. The study participants required ICU admission after surgery and required total parenteral nutrition (isonitrogenous and isocaloric) for at least 5 days due to failure or contraindication of enteral nutrition. Patients were excluded if they had persistent hemo-dynamic instability (systolic blood pressure ?80 mm Hg), renal impairment, hepatic insufficiency, severe or uncontrolled sepsis, persistent metabolic acidosis, head trauma, heart failure, and any sensitivity to the components of L-alanyl L-glutamine (Dipeptiven, Fresenius Kabi, Germany) or NAC. Eligible patients were randomly allocated before surgery into the control and study groups using a computer-generated list. Sealed opaque envelopes were supplied, and opened by a pharmacist to allocate each patient into a group. The anesthesia unit was supplied with the infusions prepared by the pharmacy unit. The researcher, the anesthesiologist, the surgeon, and patients were blinded to the treatment allocation.
The study group received an IV bolus of NAC (Hidonac, Zambon, S.P.A, Vicenza, Italy) (100 mg/kg dissolved in dextrose 5%) infused over 15 minutes as a loading dose, followed by continuous infusion of 50 mg/kg/day dissolved in dextrose 5% starting 1 hour before induction of anesthesia,9 and continuing for 48 hours after the operation in the ICU.10 The control received an equal volume of dextrose 5% given at the same rate and duration as in the study group as a placebo.10 Demographic data was collected before surgery. Venous blood samples were obtained before surgery (baseline laboratory evaluation), and on day two after surgery as in previous studies that made the postoperative evaluations between 1 hour to 72 hours after surgery.11,12
Safety assessments
Clinical data was recorded for any perioperative side or adverse effects like nausea, vomiting, tachycardia, hypotension and cough, and for vital parameters like mean arterial pressure, heart rate, body temperature, and respiratory rate. Laboratory parameters were evaluated through blood sampling, and by analysis of total leukocyte count, platelet count, serum alanine amino-transferase (ALT) as a necroinflammatory marker of hepatocyte injury, serum creatinine and blood urea nitrogen for kidney function assessment, serum electrolytes (sodium and potassium).
Efficacy assessments
Clinical evaluation of manifestations of SIRS included laboratory markers of inflammation and oxidative stress. Serum TNF-α, an inflammatory response parameter, was determined using sandwich enzyme linked immunosorbent assay detection using a human TNF-α commercial kit (Sinogeneclon company, Ltd, Hangzhou, China). Serum MDA, an oxidative stress marker, was determined by a colorimetric method using a commercial kit (Bio Diagnostic, Giza, Egypt). The postoperative length of both ICU and hospital stay were recorded.
Statistical analysis
Data management and analysis were performed using SPSS statistics software version 22 (Armonk, NY: IBM Corp, for Windows). Numerical data were summarized using the mean and standard deviation (SD) while categorical data were summarized as numbers and percentages. The latter data were assessed using the chi-square χ2 test to compare between the two groups. The t test or paired t test was used to compare means of continuous data as appropriate. P values <.05 were considered significant.
The sample size was calculated according to the oxidative stress marker MDA.13 Depending on a previous study performed on patients undergoing major abdominal surgery, it was found that MDA was 0.4 (0.03) and 0.37 (0.03) (μmol/L) in the control and NAC groups, respectively.13 Assuming the statistical power is 90% and α was set to .05, and by using PASS 11th release NCSS, LLC. Kaysville, Utah, USA,14 we recruited 30 cases into each group.
RESULTS
Sixty patients were randomized after three were excluded for refusal to sign the informed consent (Figure 1). The control and study groups were similar with respect to baseline demographics, comorbidities, and ASA score (Table 1). Both the study and control groups underwent similar colonic surgical procedures (Table 2). None of the clinical and laboratory parameters differed significantly in the control and study groups preoperatively (Table 3), and with the exception of ALT, were not significantly different postoperatively (Table 4). The mean postoperative serum level of ALT was lower in the study group than in the control group (P=.028) (Figure 2).
Figure 1.
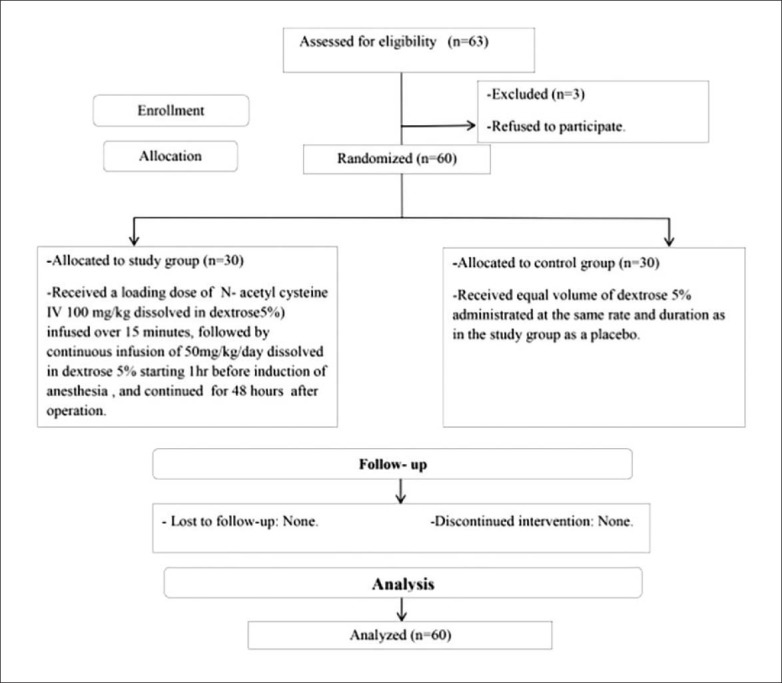
Patient flow chart.
Table 1.
Baseline demographics, comorbidities, and ASA score of the two groups.
| Parameter | Study group (n=30) | Control group (n=30) |
|---|---|---|
| Gender (n, %) | ||
| Male | 16 (53.3) | 13 (43.3) |
| Female | 14 (46.6) | 17 (56.6) |
| Age (y) | 56.2 (15.1) | 57.7 (12.3) |
| Weight (kg) | 58.4 (5.3) | 59.1 (5.3) |
| Height (cm) | 1.7 (0.08) | 1.7 (0.07) |
| BMI (kg/m2) | 21.2 (1.3) | 20.9 (0.9) |
| Comorbidities Hypertension | 13 (43.0) | 14 (46.6) |
| Diabetes mellitus | 10 (33.3) | 11 (36.6) |
| Chronic obstructive pulmonary disease | 5 (16.6) | 6 (20) |
| Ischemic heart disease | 6 (20.0) | 5 (16.6) |
| ASA II | 10 (33.3) | 7 (23.3) |
| ASA III | 20 (66.6) | 23 (76.6) |
Values are n (%) or mean (standard deviation), ASA: American society of anesthesiologist physical status classification system.
Table 2.
Types of surgical procedures.
| Surgical procedure | Study group (n=30) | Control group (n=30) | P |
|---|---|---|---|
| Total colectomy | 12 (40) | 11 (36.6) | .79 |
| Left hemicolectomy | 6 (20) | 5 (16.6) | .74 |
| Right hemicolectomy | 6 (20) | 4 (13.3) | .48 |
| Transverse colectomy | 2 (6.6) | 3 (10) | .64 |
| Sigmoid colectomy | 4 (13.3) | 7 (23.3) | .32 |
Values are number (percentage).
Table 3.
Clinical and laboratory parameters at baseline.
| Parameter | Study group (n=30) | Control group (n=30) | P |
|---|---|---|---|
| Mean arterial pressure (mm Hg) | 85.1 (13.2) | 89.8 (9.3) | .11 |
| Heart rate (bpm) | 88.3 (12.4) | 86.9 (11.02) | .63 |
| Temperature (°C) | 37.3 (0.4) | 37.2 (0.5) | .70 |
| Respiratory rate (breaths/min) | 14.1 (1.8) | 14.1 (1.8) | .94 |
| Total leukocyte count (109/L) | 11.9 (6.5) | 10.3 (3.5) | .23 |
| Platelet (109/L) | 260.8 (117.6) | 263.2 (126.2) | .94 |
| Alanine aminotransferase (U/L) | 19.9 (8.3) | 24.3 (10.7) | .085 |
| Serum creatinine (μmol/L) | 78.7 (32.2) | 73.7 (23.8) | .49 |
| Blood urea nitrogen (mmol/L) | 4.3 (1.6) | 4.0 (1.9) | .59 |
| Serum sodium (mmol/L) | 137.2 (3.6) | 135.8 (5.2) | .23 |
| Serum potassium (mmol/L) | 4.0 (0.5) | 4.0 (0.5) | .84 |
| TNF-α (ng/L) | 171.3 (68.7) | 167.4 (61.9) | .81 |
| MDA (μmol/L) | 0.8 (0.3) | 0.6 (0.3) | .14 |
Values are means (standard deviation), TNF-α = tumor necrosis factor alpha, MDA = malondialdehyde.
Table 4.
Clinical and laboratory parameters after treatment.
| Parameter | Study group (n=30) | Control group (n=30) | P |
|---|---|---|---|
| Mean arterial pressure (mm Hg) | 88.0 (7.7) | 91.4 (8.9) | .12 |
| Heart rate (bpm) | 85.6 (11.7) | 88.0 (6.4) | .34 |
| Temperature (°C) | 37.3 (0.4) | 37.2 (0.3) | .32 |
| Respiratory rate (breaths/min) | 14.2 (1.8) | 14.8 (1.5) | .15 |
| Total leukocyte count (109/L) | 13.6 (6.1) | 14.1 (5.6) | .72 |
| Platelet (109/L) | 233.7 (76.1) | 239.6 (113.8) | .81 |
| Alanine aminotransferase (U/L) | 22.6 (9.9) | 31.1 (17.8) | .028 |
| Serum creatinine (μmol/L) | 71.9 (27.1) | 70.4 (18.6) | .80 |
| Blood urea nitrogen (mmol/L) | 4.6 (1.6) | 4.4 (3.0) | .65 |
| Serum sodium (mmol/L) | 137.6 (3.2) | 135.7 (5.1) | .09 |
| Serum potassium (mmol/L) | 3.7 (0.5) | 4.0 (0.4) | z.09 |
| TNF-α (ng/L) | 130.1 (95.4) | 152.5 (98.8) | .37 |
| MDA (μmol/L) | 2.7 (1.8) | 2.8 (1.5) | .95 |
| Postoperative length of ICU stay (days) | 5.4 (1.2) | 5.7 (1.6) | .33 |
| Postoperative length of hospital stay (days) | 9.1 (3.2) | 11.4 (5.8) | .07 |
Values are means (standard deviation). TNF-α tumor necrosis factor alpha, MDA malondialdehyde.
Figure 2.
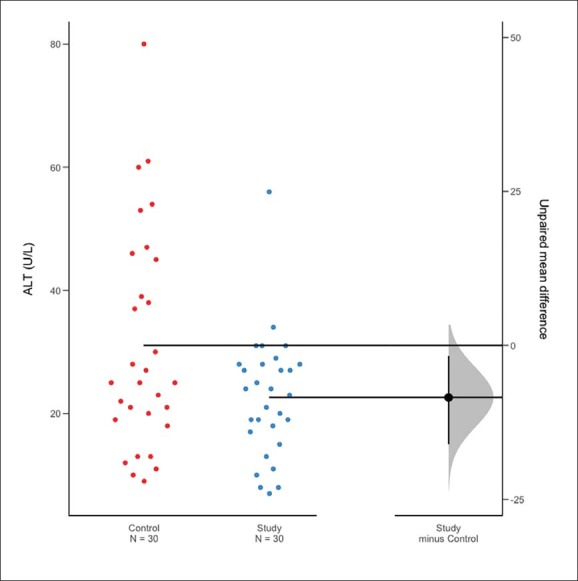
Estimation plot of differences in means for in alanine aminotransferase (ALT) in control and study groups after treatment with intravenous N-acetylcysteine and parenteral L-alanyl-L-glutamine.22
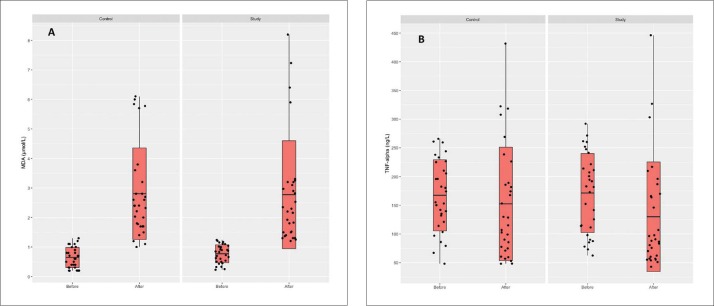
There were no differences between the two groups in the clinical manifestations of SIRS. MDA values increased after treatment in both groups compared to before treatment, but the difference between the two groups after treatment was not significant (P=.952) (Table 4, Figure 3A). TNF-α levels also declined, but the difference between groups postoperatively was not statistically significant (P=.376) (Table 4, Figure 3B).
Figure 3.
Serum levels of malondialdehyde (A) and tumor necrosis factor alpha (B) before and after treatment with N-acetylcysteine in the control and study groups (comparison of before and after within the two groups statistically significant for malondialdehyde [P<.001 for both groups; comparisons between the control and study groups statistically nonsignificant].
There were no significant differences in preoperative and postoperative clinical and laboratory parameters within the study group, consistent with its safety, and tolerability (Table 5). Postoperatively, only 6.6% of the patients in the study group experienced a few mild side effects; one patient suffered from tachycardia, and the other patient suffered from hypotension during the study. These side effects were mild and did not interfere with the study. Although there was a shorter postoperative length of ICU and hospital stay in the study group the differences were not statistically significant (Table 4).
Table 5.
Clinical and laboratory parameters before and after receiving NAC.
| Parameter | Before treatment (n=30) | After treatment (n=30) | P |
|---|---|---|---|
| Mean arterial pressure (mm Hg) | 85 (13.2) | 88 (7.7) | .32 |
| Heart rate (bpm) | 88.3 (12.4) | 85.6 (11.7) | .43 |
| Temperature (°C) | 37.3 (0.4) | 37.2 (0.3) | .47 |
| Respiratory rate (breaths/min) | 14.1 (1.7) | 14.2 (1.8) | .79 |
| Total leukocyte count (109/L) | 11.8 (6.4) | 13.6 (6.1) | .23 |
| Platelet (109/L) | 260.8 (117.6) | 233.7 (76.1) | .18 |
| Alanine aminotransferase (U/L) | 19.9 (8.3) | 22.6 (9.9) | .193 |
| Serum creatinine (μmol/L) | 78.6 (32.2) | 71.9 (27.1) | .10 |
| Blood urea nitrogen (mmol/L) | 4.2 (1.6) | 4.6 (1.7) | .14 |
| Serum sodium (mmol/L) | 137.2 (3.6) | 137.8 (3.6) | .66 |
| Serum potassium (mmol/L) | 3.9 (0.4) | 3.7 (0.5) | .07 |
| TNF-α (ng/L)) | 130.1 (95.4) | 152.5 (98.8) | .37 |
| MDA (μmol/L) | 2.7 (1.8) | 2.8 (1.5) | .95 |
Values are means (standard deviation).
DISCUSSION
The current study evaluated the safety and the potential effect of perioperative, short-term administration of NAC intravenously in patients undergoing colon surgeries. Our results showed no statistically significant evidence for NAC against surgical oxidative stress and inflammatory response; however, it showed an adequate safety profile. Early studies showed a robust safety profile and tolerability with infrequent and mild side effects such as flushing, hypotension, and angioedema.15,16 This was evident in our study, in which about 6.6% of the total number of patients who received NAC suffered from mild, well tolerated, and non-serious side effects, as only two patients (out of 30) suffered from hypotension and tachycardia, which were mild and did not affect our investigation.
We studied the effect of NAC on clinical manifestations, hemodynamic changes associated with postoperative SIRS (e.g. mean arterial pressure, heart rate, body temperature), and laboratory parameters. The current study concluded that there was no difference between the two groups, which is consistent with other studies that evaluated the effect of perioperative administration of NAC following surgery. Other studies also found no statistically significant difference between the NAC and placebo groups in mean arterial pressure, heart rate, platelet count, and serum creati-nine.8,17
In the current study, a lower postoperative increase in the serum level of ALT occurred in the study group, with a significant difference between the two groups, which may indicate that NAC protects the hepatocyte from injury by oxygen free radicals and inflammatory mediators through its anti-inflammatory and antioxidant effect. This is consistent with an animal study that showed the ability of NAC to preserve the integrity of cell membranes and protect the cell from oxidative stress.18 Beyaz et al reported significant postoperative decreases in ALT levels at the 1st and 24th hour versus preoperative values with short-term infusion of NAC infusion in laparoscopic surgery.11 Another study found a significant postoperative decrease in the ALT level on day 1 and 3.12 In another study NAC had no significant effect on the liver profile.8
Both in vitro and in vivo studies have reported a strong anti-inflammatory effect of NAC with scavenging of free radicals leading to a decrease in the oxidative stress on cells and attenuation of release of proinflammatory cytokines.19 We used TNF-α as a laboratory marker for evaluation of the anti-inflammatory effect of NAC. TNF-α levels declined more postoperatively in the study group than in the control group, but the difference was not statistically significant. This is consistent with a previous study that showed that postoperative levels of inflammatory markers at 4, 8, 12, and 24 hours were lower in the NAC group, but these differences did not reach statistical significance.17 Another study also found a reduction of the plasma inflammatory cytokines TNF-α and interleukin-1b.20 Postoperative TNF-α declined in both the study and the control group compared to the preoperative level. This finding is similar to results by Engel and colleagues who found that the postoperative TNF-α production on day 2 was lower than the preoperative level.21
Excessive release of reactive oxygen species and lipid peroxide formation participates in the tissue insult, cell anoxia and organ hypoperfusion that may occur during surgery.6 The disequilibrium between lipid peroxidation and glutathione leads to oxidative stress. MDA was evaluated as a lipid peroxidation marker.11 Previous studies revealed the ability of NAC to reduce the MDA level compared to control.13,20 Other researchers have reported an increased MDA level in study and control groups postoperatively; administration of NAC did not result in a decrease in serum MDA levels,11 which is similar to our results.
The effect of NAC on the length of hospital stay has varied with several studies showing no statistically significant differences in the hospital length of stay.17,20 However, others reported a shorter hospital stay after surgery in NAC-supplemented groups.12 The results of our study showed shorter hospital stays in the study group than the control, but the difference was not statistically significant. The current study has several limitations, including the small sample size, short duration of NAC administration, and short period of patient follow up. Therefore, we recommend further multicenter studies on a large number of patients with different NAC doses over short- and long-term duration of administration.
In conclusion, it is safe to give intravenous NAC in addition to L-alanyl L-glutamine over a short period in patients undergoing colon surgeries. However, we found no significant effect on the elevated MDA levels postoperatively. In addition, there was no significant reduction of the TNF-α level after surgery, reflecting the lack of clinical significance of NAC as an antioxidant and anti-inflammatory agent.
Funding Statement
None.
REFERENCES
- 1.Scardapane A, Brindicci D, Fracella M, Angelelli G. Post colon surgery complications: Imaging findings. Eur J Radiol. 2005; 53: 397–409. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein S, Osei Bonsu S, Aslam R, Yee F. Multidetector CT of the postoperative colon: review of normal appearances and common complications. Radio Graphics. 2013; 33: 515–32. [DOI] [PubMed] [Google Scholar]
- 3.Deniz M, Borman H, Seyhan T, Haberal M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns. 2012; 39(2): 320–25. [DOI] [PubMed] [Google Scholar]
- 4.Mokhtari V, Afsharian P, Shahhoseini M, Kalanta S, Moini A. A Review on Various Uses of N-acetylcysteine. Cell J. 2017; 19(1) 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, et al. Reducing deaths due to oxidative stress (the REDOXS study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc. 2006; 65: 250–263. [DOI] [PubMed] [Google Scholar]
- 6.Van Stijn MFM, Ligthart Melis GC, Boelens PG, Scheffer PG, Teerlink T, Twisk JWR, et al. Antioxidant enriched enteral nutrition and oxidative stress after major gastrointestinal tract surgery. World J Gastroenterol. 2008; 14(45): 6960–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler F, Seddiki L, Marion letellier R. Effects of L glutamine supplementation alone or with antioxidants on hydrogen peroxide induced injury in human intestinal epithelial cells. E SPEN, the European e Journal of Clinical Nutrition and Metabolism. 2011; 6: 211–16. [Google Scholar]
- 8.Molnar Z., Szakmany T, Marton S. Lack of Effect of Prophylactic N acetylcysteine on postoperative organ dysfunction following major abdominal tumor surgery: A randomized, placebo controlled, double blinded clinical trial. Anaesth Intensive Care. 2003; 31(3): 267–71. [DOI] [PubMed] [Google Scholar]
- 9.Santiago FM, Bueno P, Olmedo C, Muffak Granero K, Comino A, Serradilla M, et al. Effect of N-acetylcysteine administration on intraoperative plasma levels of interleukin -4 and Interleukin-10 in liver transplant recipients. Transplantation Proc. 2008; 40(9): 2978–80. 10.1016/j.transproceed.2008.08.103 [DOI] [PubMed] [Google Scholar]
- 10.Ozaydin M, Icli A, Yucel H, Akcay S, Peker O, Erdogan D, et al. Metoprolol vs. carvedilol or carvedilol plus N-acetylcysteine on postoperative atrial fibrillation: a randomized, double-blind, placebo-controlled study. Eur Heart J. 2013, 34: 597–604. [DOI] [PubMed] [Google Scholar]
- 11.Beyaz S, Yelken B, Kanbak G. The effects of N-acetylcysteine on hepatic function during isoflurane anesthesia for laparoscopic surgery patients. Indian J Anesth. 2011; 55(6): 567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim Eand Sharawy A. Effectiveness of intravenous infusion of N-acetylcysteine in cirrhotic patients undergoing major abdominal surgeries. Saudi J Anaesth. 2015; 9(3): 272–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuyumcu A, Akyol A, Buyuktuncer Z, Ozmen M, Besler H. Improved oxidative status in major abdominal surgery patients after N-acetylcysteine supplementation. Nutr J. 2015; 14(4): 1–9. 10.1186/1475-2891-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machin D, Campbell M, Fayers P, Pinol A. Sample size tables for clinical studies. 2nd Edition Malden MA: Blackwell Science; 1997. [Google Scholar]
- 15.Liu XH, Xu CY, Fan GH. Efficacy of N-acetylcysteine in preventing atrial fibrilla-tion after cardiac surgery: a meta-analysis of published randomized controlled trials. BMC Cardiovasc Disord. 2014; 14(52): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. Drug evaluation N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008; 8(12): 1955–62. [DOI] [PubMed] [Google Scholar]
- 17.El hamamsy I, Stevens L, Carrier M, Pellerin M, Demers P, Cartier R, et al. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: A randomized, double-blind, placebo controlled clinical trial. J Thorac Cardiovasc Surg. 2007; 133: 7–12. [DOI] [PubMed] [Google Scholar]
- 18.Pastor A, Collado P, Almar M, Gonzalez JG. Antioxidant enzyme status in biliary obstructed rats: effects of N acetylcysteine. J Hepatol. 1997; 27: 363–70. [DOI] [PubMed] [Google Scholar]
- 19.Sadowska AM, Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose effects: A review. Pulm Pharmacol Ther. 2007; 20(1): 9–22. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud KM and Ammar AS. Effect of N-acetylcysteine on cardiac injury and oxidative stress after abdominal aortic aneurysm repair: A randomized controlled trial. Acta Anaesthesiol Scand. 2011; 55: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 21.Engel J, Menges T, Martens F, Kwapisz M, Hempelmann G, Pitz S, et al. Role of glutamine administration on T-cell derived inflammatory response after cardiopulmonary bypass. Clin Nutr. 2009; 28:15–20. [DOI] [PubMed] [Google Scholar]
- 22.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: Everyday data analysis with estimation plots. bioRxiv [Internet]. 2018. [cited 2019 Feb 11];377978 Available from: https://www.biorxiv.org/content/10.1101/377978v1 [DOI] [PubMed]