ABSTRACT
Background
Infrared (IR) analysis is an emerging technology that may be a useful tool for milk banks to manage the nutrient variability in donor human milk.
Objective
To evaluate the accuracy, reliability, and comparability of commercial infrared analyzers for measuring human milk macronutrients in a milk bank setting.
Methods
Three nonprofit milk banks received blinded test kits of human milk that had been assessed using reference methods. Four infrared instruments were used to measure macronutrients as follows: 1 filtered mid-IR, 2 Fourier-transformed full-spectra mid-IR, and 1 near-IR. Twenty-five unique samples were read concurrently for the accuracy arm. An identical sample was read daily for 1 mo for the reliability arm.
Results
Values for R2 describing relationships with reference methods for total fat, crude protein, and lactose, were as follows: filtered mid-IR, 0.98, 0.94, and 0.48; Fourier-transformed full-spectra mid-IR, 0.97, 0.93, and 0.36 for instrument 1 and 0.98, 0.98, and 0.31 for instrument 2; and near-IR 0.93, 0.93, and 0.12. There was no significant difference between instruments for crude protein and total fat measurements. There were significant differences in carbohydrate measurements between instruments. For 1 mo of daily measurements in the reliability arm, CVs for filtered mid-IR were ≤4.6%, for Fourier-transformed full spectra mid-IR were ≤1.7%, and for near-IR were ≤5.1%.
Conclusions
Infrared analysis is an accurate and reliable method for measuring crude protein and total fat in a milk bank setting. Carbohydrate measurements are less accurate and are significantly different between instruments, which will likely lead to differences in derived calorie values.
Keywords: human milk, infrared, donor milk, milk bank, macronutrients
Introduction
There is significant variability in the macronutrient content of human milk between women at the same stage of lactation, with fat often reported as the most variable macronutrient (1–3). While macronutrient variability is of limited concern with ad libitum feeding of term infants, it may have a significant impact on preterm infants who are fed enterally at prescribed rates and are at risk for poor growth (4). The American Academy of Pediatrics and the Canadian Pediatric Society recommend that preterm infants who do not have access to their mother's own milk receive pasteurized donor human milk (DHM) from a milk bank due to its protective effect against necrotizing enterocolitis (NEC) (5–7). Milk banks screen donors serologically and for lifestyle risk factors, pool milk between multiple donors, pasteurize milk, and conduct postpasteurization microbiological tests before dispensing DHM to neonatal intensive care units (NICUs) (8). Three
small studies suggest that the fat content of DHM can also be highly variable (3, 9, 10), which may contribute to the poor growth observed in preterm infants fed DHM (7, 11, 12).
Knowing the macronutrient content of a donor's milk could improve the ability of milk banks to strategically combine donors to reach nutrient goals for DHM going to vulnerable infants in the NICU. Infrared (IR) analyzers are tools that have been used in the dairy industry to measure the macronutrient content of bovine milk (13). Some milk banks within the Human Milk Banking Association of North America (HMBANA) have adapted IR analyzers for use with human milk. Recently, the US FDA approved the first IR human milk macronutrient test system as a medical device (14). HMBANA milk banks operate as food manufacturers, a role that differs operationally and regulatorily from clinical entities that operate medical devices.
There is a growing body of research that suggests that, with appropriate sample handling, IR analyzers are accurate and reliable for measuring fat and protein in human milk (15–23); however, the majority of these studies were conducted in laboratory or clinical settings. Therefore, the purpose of this study was to 1) evaluate the accuracy of commercially available IR analyzers for measuring macronutrients in DHM in a milk bank setting; 2) compare results between a variety of IR technologies used at multiple milk banks; and 3) evaluate the reliability of commercially available IR analyzers for measuring macronutrients over 30 d in a milk bank setting.
Methods
This method validation study was classified as non–human subject research and provided an exempt status by the Institutional Review Board at the University of North Carolina Greensboro (UNCG). It was also approved by the HMBANA Research Committee.
Test kit preparation
Human milk test kits were prepared in the laboratory at the University of North Carolina Greensboro (UNCG). De-identified raw human milk used to prepare the kits was received from 3 HMBANA milk banks (NorthernStar Mothers Milk Bank, Calgary, Alberta; Northwest Mothers Milk Bank, Portland, OR; and Mothers' Milk Bank North Texas, Fort Worth, TX) whose donors consent to having their milk used for research purposes. The milk was from 12 unique, approved donors and had been classified as research milk due to various deferral requirements (e.g., donor use of medications). Test kit samples were prepared by thawing milk in a warming oven and shaking water baths, and pooling milk from 1 to 2 donors to reach volumes ranging from 200 to 1800 mL, with higher volumes needed for samples used in the reliability arm. Samples that contained milk from 2 donors used different combinations of donor milks, with the goal of creating a wide range of nutrients within the test kits. Each sample was continuously stirred with a magnet during the process of preparing test kit aliquots to ensure consistency. The test kits consisted of 10 unique calibration samples, with macronutrient values provided to the milk bank for instrument calibration; 25 unique, blinded samples for the accuracy arm of the study; and 30 identical, blinded samples for the reliability arm of the study. All test kits contained the same samples for calibration and the accuracy arm. Because IR instruments use up the sample in the process of analysis and samples cannot be remeasured, large sample volumes were required to create 30 d of identical samples for the reliability arm; therefore, samples for the reliability arm of the study were identical within a milk bank, but different between milk banks. Each sample in the test kit contained sufficient volume to be measured in duplicate. Completed test kits were frozen and shipped overnight on dry ice to participating milk banks and to reference labs. Due to volume limitations, only a subset of the 25 unique test kit samples in the accuracy arm were analyzed by reference methods: crude protein and nonprotein nitrogen (n = 20), total fat (n = 20), and lactose (n = 25).
Milk bank setting 1
The NorthernStar Mothers Milk Bank (Calgary, Alberta, Canada) has been operating since 2012 and processes approximately 200,000 oz (5,914 L) of DHM annually. Two human milk test kits were sent to the NorthernStar milk bank for testing on a near-IR instrument (Near) (Unity SpectraStar XL, Unity Scientific) and on a filtered mid-IR instrument (FiltMid) (Miris HMA Human Milk Analyzer, Miris). Sample handling processes are summarized in Table 1.
TABLE 1.
Summary of calibration and sample handling by instrument1
| FiltMid | FTMid1 | FTMid2 | Near | |
|---|---|---|---|---|
| Operator | Trained milk bank technician | Trained milk bank technician | Trained milk bank technician | Trained milk bank technician |
| Calibration set | Manufacturer calibration samples | Human milk samples (10) analyzed by USDA | Study calibration samples (10) | Study calibration samples (10) |
| Volume required for duplicate readings | 6 mL | 50 mL | 60 mL | 6 mL |
| Thawing | Overnight in refrigerator | Overnight in refrigerator | Overnight in refrigerator | Overnight in refrigerator |
| Warming | To 40°C in a bead warmer (heater model JBN5 US, Miris) | Temperatures at analysis 4°–8°C. Pilot study on effect of temperature showed no difference in results at starting temperatures of 4°C, 24°C, and 40°C (unpublished data). | To 20°C in water bath (Precision 183, Fisher Scientific) | To 40°C in bead warmer (heater model JBN5 US, Miris) |
| Mixing | Per manufacturer instructions | Sonicated at 75% amplitude 2 min with 6-mm probe submerged ∼75% into sample (VCX 130, Sonics and Materials) | Sonicated at 75% amplitude 3 min with 6-mm probe submerged ∼75% into sample (VCX 130, Sonics and Materials) | Sonicated at 50% amplitude for 15 s (ultrasonic processor model CV18, Miris); sample gently inverted 10 times before loading |
FiltMid (Miris HMA), FTMid1 (MilkoScan), FTMid2, (LactoScope), and Near (SpectraStar). FiltMid, filtered midinfrared; FTMid, Fourier-transformed full-spectra midinfrared; Near, near infrared.
Milk bank setting 2
The Mothers' Milk Bank of North Texas has been operating since September 2004 and processes approximately 600,000 oz (17,742 L) of DHM annually. One human milk test kit was sent to the North Texas milk bank for testing on a Fourier-transformed full spectra mid-IR instrument (FTMid1) (MilkoScan FT120, FOSS). Due to loss of study calibration samples, calibration was done with in-house calibration samples created by the North Texas milk bank and analyzed by the USDA Federal Milk Market. Sample handling processes are summarized in Table 1.
Milk bank setting 3
Northwest Mothers Milk Bank has been operating since 2013 and processes approximately 375,000 oz (11,089 L) of DHM annually. One human milk test kit was sent to the Northwest milk bank for testing on a Fourier-transformed full spectra mid-IR instrument (FTMid2) (LactoScope FTA; Perten Instruments). Additionally, Perten Instruments set up a custom channel on the LactoScope to estimate lactose using the calibration curve data, as IR technology does not distinguish between lactose and indigestible carbohydrates. Sample handling processes are summarized in Table 1.
Measurement
Reference methods were used to measure the macronutrients in the test kits as follows. Total nitrogen and nonprotein nitrogen (NPN) were measured by Eurofins using the Kjeldahl method (AOAC 991.20 and AOAC 991.21, respectively) to assess the protein in human milk. Total nitrogen was converted to Crude Protein using a protein conversion factor for milk foods of 6.38 (24, 25). True protein was calculated for reference samples as (crude protein − measured NPN). Total fat was measured by the USDA Federal Milk Market using the Mojonnier ether extraction method. Lactose was measured in triplicate at UNCG using AOAC method 2006.06 adapted to run in a 96-well plate. This method uses the enzymes β-galactosidase, galactose mutarose, and β-galactosidase dehydrogenase (K-LACGAR, Megazyme International) to convert lactose to β-d-galactose, which oxides NAD+ to NADH. This enzymatic approach has been reported as accurate with HPLC methods (26). Average CVs for lactose measurements in triplicate were 3.2%. It is important to note that IR technology, which was originally developed for the dairy industry, measures crude protein, which does not account for NPN (e.g., urea and nucleotides). In bovine milk, the amount of NPN is limited (<5%), while in human milk, NPN averages 20–30% and can be >50% (17, 27–29). Similarly, IR technology measures total carbohydrates and does not differentiate between lactose and human milk oligosaccharides (HMOs). Bovine milk contains limited indigestible carbohydrates while in human milk, HMOs can account for 15–25% of carbohydrates (30). In the context of analyzing human milk in a food manufacturing setting, we chose to measure lactose for our reference values instead of total carbohydrates because metabolizable nutrients are recommended for deriving calorie values in foods (24).
Data analysis
Accuracy for IR instruments was defined based on information published in the Miris HMA Human Milk Analyzer User Manual since this device has been approved for clinical use by the FDA: total fat ±12% of reference methods; crude protein, true protein, and carbohydrates ± 15% of reference methods (31). Coefficients of determination (R2) were calculated to assess relationships with reference methods and analyzer results were plotted as percentage differences from reference methods. Descriptive statistics on macronutrient measurements were computed by analyzer type and differences between analyzers were evaluated using an ANOVA with a Tukey's honest significant difference test for multiple comparisons. Intra-assay CVs were computed to assess the reliability of instruments over 1 mo of replicate measures. Regression analysis was used to probe for significant changes over time. All analysis was conducted with SAS Enterprise Edition 9.4 (SAS Corporation).
Results
Assessment of accuracy to reference methods
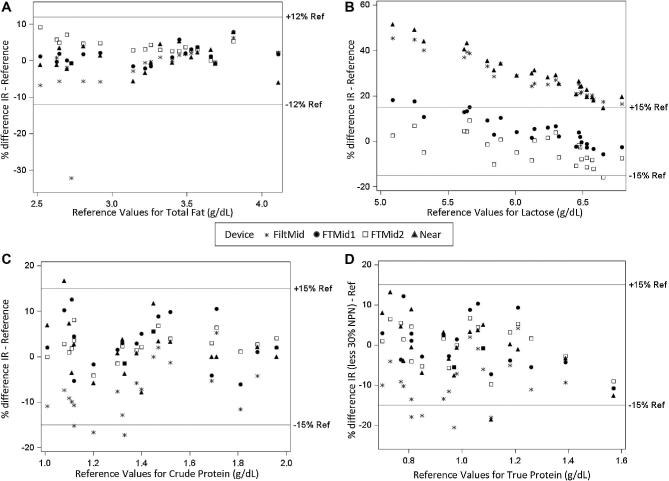
According to the reference methods used, the study samples contained the following ranges of macronutrients: total fat 2.52–4.11 g/dL (n = 20), crude protein 1.01–1.96 g/dL (n = 20), true protein 0.70–1.57 g/dL (n = 20), and lactose 5.09–6.79 g/dL(n = 25). Scatter plots of percentage differences between IR measurements and reference methods are presented in Figure 1. There was a high degree of accuracy (±15%) for crude protein measurements across all analyzers, with a small number of samples falling slightly outside the range with the filtered mid-IR (3/20, 15%) and near-IR instruments (1/20, 5%). None of the IR instruments measure true protein. Instead, true protein values were derived via a calculation by reducing crude protein by a flat percentage. Accurate true protein values could be obtained by applying a 30% deduction to crude protein on the Fourier-transformed full-spectra mid-IR instruments and the near-IR instrument, while a 20% deduction was appropriate on the filtered mid-IR instrument. Similarly, there was a high degree of accuracy (±12%) for total fat measurements across all analyzers. Only the Fourier-transformed full-spectra mid-IR instruments reported carbohydrate values that fell within ±15% of reference values for lactose, and there was a trend to overreporting at lower lactose concentrations. The filtered mid-IR and near-IR instruments reported carbohydrate values that were 20–50% higher than reference lactose values, with greater differences at lower lactose concentrations.
FIGURE 1.
Accuracy of infrared instruments compared with the reference methods FiltMid (Miris HMA), FTMid1 (MilkoScan), FTMid2, (LactoScope), and Near (SpectraStar). FiltMid, filtered midinfrared; FTMid, Fourier-transformed full-spectra midinfrared; Near, near infrared.
Relationship with reference methods, reported as R2 values from regression analysis for total fat, crude protein, and lactose, were as follows: FiltMid, 0.91, 0.94, and 0.48; FTMid1, 0.97, 0.93, and 0.36, FTMid2, 0.98, 0.98, and 0.31, and Near, 0.93, 0.93, and 0.12. One sample measured multiple times on FiltMid was >30% below reference values for fat, suggesting the fat loss may have occurred in kit preparation. When this sample was excluded, the R2 value for fat was 0.98 on FiltMid.
Comparison of results between analyzers
We did not observe a significant difference between the crude protein or total fat value obtained with different infrared instruments (P > 0.05). There was a significant difference in the carbohydrate measurements obtained between infrared instruments (P < 0.0001) (Table 2).
TABLE 2.
Comparison of macronutrient results by infrared analyzer1
| FiltMid (n = 25) | FTMid1 (n = 24) | FTMid2 (n = 25) | Near (n = 24) | P value | |
|---|---|---|---|---|---|
| Crude protein, g/dL | 1.29 ± 0.32 (0.90–2.00) | 1.43 ± 0.28 (1.03–2.00) | 1.42 ± 0.30 (1.01–2.04) | 1.40 ± 0.26 (1.07–1.96) | 0.3254 |
| Fat, g/dL | 3.34 ± 0.65 (1.85–4.65) | 3.44 ± 0.58 (2.55–4.72) | 3.46 ± 0.49 (2.71–4.60) | 3.44 ± 0.55 (2.49–4.80) | 0.8771 |
| Carbohydrate, g/dL | 7.78a ± 0.17 (7.40–8.10) | 6.38b ± 0.22 (5.89–6.72) | 5.88c ± 0.37 (5.06–6.55) | 7.90a ± 0.16 (7.62–8.17) | <0.0001 |
Values represent mean ± SD (range) unless otherwise indicated. Data were evaluated with ANOVA and Tukey's honest significant difference for multiple comparisons. Values in the same row with the same superscript are not significantly different. FiltMid (Miris HMA), FTMid1 (MilkoScan), FTMid2, (LactoScope), and Near (SpectraStar). FiltMid, filtered midinfrared; FTMid, Fourier-transformed full-spectra midinfrared; Near, near infrared.
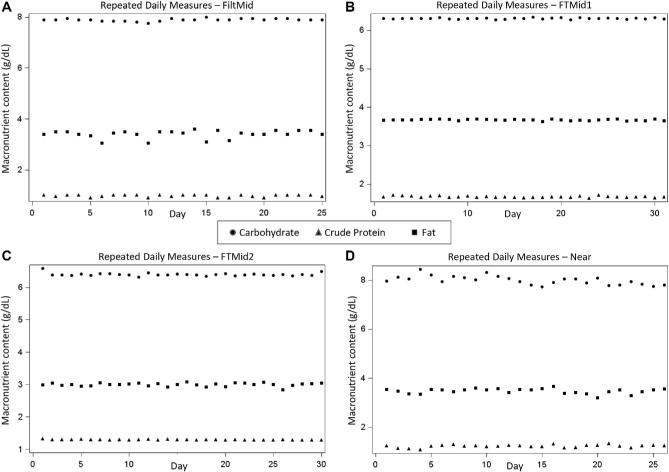
Assessment of reliability over 1 mo
Macronutrients measured daily over 1 mo are plotted by IR instrument in Figure 2. The only significant (P < 0.05) time-related trends that we observed were a decline in crude protein in the Fourier-transformed full-spectra mid-IR instrument (LactoScope) (slope = −0.0008 g/d; −0.06% daily change from reference value) and a decline in lactose values in the Near instrument (slope = −0.0147 g/day; −0.22% daily change from reference value). These temporal changes were very small, resulting in approximately 2–6% total change over the course of 30 d. The intra-assay CVs for 1 mo of daily measurements for crude protein, fat, and carbohydrates, respectively, were as follows: FiltMid, 4.2%, 4.6%, and 0.7%; FTMid1, 1.2%, 0.4%, and 0.3%; FTMid2, 0.9%, 1.7%, and 0.8%; Near, 5.1%, 3.0%, and 2.2%.
FIGURE 2.
Reliability of macronutrient measurements over 1 mo of daily use, by infrared instrument. Each milk bank received a different set of test samples, so macronutrient values between analyzers were not expected to be the same. FiltMid (Miris HMA), FTMid1 (MilkoScan), FTMid2, (LactoScope), and Near (SpectraStar). FiltMid, filtered midinfrared; FTMid, Fourier-transformed full-spectra midinfrared; Near, near infrared; ProCrude, crude protein.
Discussion
We observed high levels of accuracy in measuring total fat and crude protein in raw human milk across 3 different milk banks using a variety of commercially available infrared technologies, including devices developed specifically for human milk (Miris HMA), and devices developed for the dairy industry (MilkoScan, LactoScope, SpectraStar) and adapted by milk banks to measure human milk. Our findings are in agreement with others that have reported accuracy in measuring total fat and crude protein using near-IR (16, 18, 21), filtered mid-IR (15, 18–20, 23), and full-spectra mid-IR instruments (17, 32), when devices have been calibrated to human milk. Limited studies have been conducted in a milk bank setting (19, 23, 32, 33), and few studies have compared multiple types of infrared technology (18).
IR analyzers can only estimate true protein values using crude protein measurements. The average nonprotein nitrogen composition of our reference samples was 28% (range 20–34%). We found that a 20% reduction in crude protein was appropriate for the filtered mid-IR instrument and that a 30% reduction was needed for the other instruments, suggesting that conversion factors to obtain accurate true protein values may differ by instrument. In contrast to our findings, a few studies have described poor accuracy when using IR technology to measure crude protein and fat in human milk (34–36). Existing research using infrared analyzers for measuring macronutrients in human milk differs with respect to sample thawing, warming, and homogenization methods, as well as instrument calibration, which may explain differing conclusions (37).
We observed significant differences in the accuracy and comparability of carbohydrate measurements across devices. The Fourier-transformed full-spectra mid-IR devices mostly fell into ±15% of the reference values for lactose, while the near-IR and filtered mid-IR devices reported values that were 20–50% higher than reference lactose values. This is likely because, while IR technology measures total carbohydrates and cannot differentiate between lactose and HMOs, the full-spectra instrument calibration equations developed by the milk banks attempts to derive lactose values from total carbohydrate measurements based on known lactose values of the calibration samples. None of the instruments in our study showed strong agreement with reference methods for lactose (R2 < 0.5). Others have reported low accuracy in measuring lactose in human milk with infrared analysis (15, 17, 18, 32, 34).
During approximately 1 month of daily use, we found all IR devices provided consistent results, with small, but statistically significant, temporal changes in some components (in the range of 0.06% to 0.22%); however, these values are not likely to be clinically significant. The small temporal changes we did observe highlight the importance of monitoring instruments daily and recalibrating as needed to identify and respond to potential instrument drifts.
In the current regulatory environment, milk banks operate as food manufacturers, and thus they are subject to the Code of Federal Regulation, Title 21, Food and Drugs (38). Among other things, this regulation requires milk banks to follow Good Manufacturing Practices (part 110) and to maintain clean and accurate equipment (part 110.40). Our study provides evidence that a variety of infrared analyzers can be used in a milk bank setting to accurately measure crude protein and total fat. Use of these instruments may enable milk banks to strategically pool milk from different donors to reach protein and fat targets for DHM. Significant differences in the measurements and accuracy of lactose between devices may lead to differences in calorie calculations, which is an important area for future research given the nutritional vulnerability of infants receiving DHM.
Limitations
We used a small number of reference samples that did not include more extreme concentrations of fat and protein. This is an important area for future research, including testing cream products that have a higher percentage of total solids, which may impact IR accuracy. We defined accuracy based on thresholds for an instrument that has been approved as a clinical device by the FDA. Different levels of accuracy may be more appropriate for manufacturers of medical foods. There was one outlier for total fat (>30% below reference value) in the filtered mid-IR instrument; this sample was measured multiple times with similar results, suggesting that the error may have occurred during preparation of the test kits, highlighting how important drawing a representative sample is for generating meaningful results. We did not measure total carbohydrates, as we felt lactose was a better indicator of the energy available to an infant; therefore, instruments that measure total carbohydrates may have shown better performance results if they and been assessed against total carbohydrate reference values.
Conclusions
A variety of infrared instruments, including near-IR, filtered mid-IR, and full-spectra mid-IR, can accurately measure crude protein and total fat in a milk bank setting when instruments have been calibrated to human milk and when appropriate sample handling protocols are used. Infrared instruments may be useful tools to help milk banks determine how to combine milk from multiple donors to achieve target fat and protein amounts. Calculations to arrive at true protein measurements may differ between instruments. There are significant differences in carbohydrate measurements between instruments that may lead to differences in calorie values. Research is needed regarding the accuracy of IR devices to derive calorie values for human milk.
Acknowledgements
We thank Dr. Christoph Fusch for consultation on study design; Kelsey Smith, MA, for conducting analyses on milk handling methods that informed interpretation of study findings; Miris Corporation for making an evaluation instrument available to NorthernStar Mothers Milk Bank; and PerkinElmer for providing instrument customization to Northwest Mothers Milk Bank.
The authors’ responsibilities were as follows—MTP, JF, SS, LM, AV: designed and conducted the research; MTP, EAB: contributed to the analysis and interpretation of data; MTP: wrote the paper and had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This quality improvement study was funded by Mothers' Milk Bank of North Texas, NorthernStar Mothers Milk Bank, and Northwest Mothers Milk Bank through research funds donated to the Perrin Lab at the University of North Carolina Greensboro.
Author disclosures: MTP and AV serve on the Board of Directors for the Human Milk Banking Association of North America in an unpaid capacity. EAB serves on the Research Advisory Board of the Mother's Milk Bank Northeast. JF, SS, and LM report no conflicts of interest.
Abbreviations used: DHM, donor human milk; FiltMid, filter midinfrared; FTMid, Fourier transformed full-spectra midinfrared; HMBANA, Human Milk Banking Association of North America; HMO, human milk oligosaccharide; IR, infrared; Near, near infrared; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; NPN, nonprotein nitrogen; UNGC, University of North Carolina Greensboro.
References
- 1. Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr 1991;54(1):69–80. [DOI] [PubMed] [Google Scholar]
- 2. Nommsen LA, Lovelady CA, Heinig MJ, Lönnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING Study. Am J Clin Nutr 1991;53(2):457–65. [DOI] [PubMed] [Google Scholar]
- 3. Perrin MT, Fogleman AD, Newburg DS, Allen JC. A longitudinal study of human milk composition in the second year postpartum: implications for human milk banking. Matern Child Nutr 2017;13(1). doi: 10.1111/mcn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, Buzas JS, Bertino E, Gagliardi L, Bellu R. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 2015;136(1):e84–92. [DOI] [PubMed] [Google Scholar]
- 5. Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics 2017;139(1):e20163440. [DOI] [PubMed] [Google Scholar]
- 6. Kim JH, Unger S; Canadian Paediatric Society. Position statement—human milk banking. Paediatr Child Health 2010;15(9):595–8. [PMC free article] [PubMed] [Google Scholar]
- 7. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2014;(4):CD002971. doi: 10.1002/14651858.CD002971.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Human Milk Banking Association of North America. Guidelines for the establishment and operations of a donor human milk bank. Fort Worth, TX: 2018. [Google Scholar]
- 9. Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lact 2018;34(1):120–9. [DOI] [PubMed] [Google Scholar]
- 10. Moukarzel S, Soberanes L, Dyer RA, Albersheim S, Elango R, Innis SM. Relationships among different water-soluble choline compounds differ between human preterm and donor milk. Nutrients 2017;9(4):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brownell EA, Matson AP, Smith KC, Moore JE, Esposito PA, Lussier MM, Lerer TJ, Hagadorn JI. Dose-response relationship between donor human milk, mother's own milk, preterm formula, and neonatal growth outcomes. J Pediatr Gastroenterol Nutr 2018;67(1):90–6. [DOI] [PubMed] [Google Scholar]
- 12. Colaizy TT, Carlson S, Saftlas AF, Morriss FH. Growth in VLBW infants fed predominantly fortified maternal and donor human milk diets: a retrospective cohort study. BMC Pediatrics 2012;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biggs D. Infrared milk analyzer. J Dairy Sci 1972;55(5):650–1. [DOI] [PubMed] [Google Scholar]
- 14. FDA permits marketing of a diagnostic test to aid in measuring nutrients in breast milk [Internet]. FDA 2018[cited 2019 Jul 10]. Available from: http://www.fda.gov/news-events/press-announcements/fda-permits-marketing-diagnostic-test-aid-measuring-nutrients-breast-milk. [Google Scholar]
- 15. Menjo A, Mizuno K, Murase M, Nishida Y, Taki M, Itabashi K, Shimono T, Namba K. Bedside analysis of human milk for adjustable nutrition strategy. Acta Paediatrica 2009;98(2):380–4. [DOI] [PubMed] [Google Scholar]
- 16. Sauer CW, Kim JH. Human milk macronutrient analysis using point-of-care near-infrared spectrophotometry. J Perinatol 2011;31(5):339–43. [DOI] [PubMed] [Google Scholar]
- 17. Smilowitz JT, Gho DS, Mirmiran M, German JB, Underwood MA. Rapid measurement of human milk macronutrients in the neonatal intensive care unit: accuracy and precision of fourier transform mid-infrared spectroscopy. J Hum Lact 2014;30(2):180–9. [DOI] [PubMed] [Google Scholar]
- 18. Fusch G, Rochow N, Choi A, Fusch S, Poeschl S, Ubah AO, Lee SY, Raja P, Fush C. Rapid measurement of macronutrients in breast milk: How reliable are infrared milk analyzers? Clin Nutr 2015;34(3):465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Billard H, Simon L, Desnots E, Sochard A, Boscher C, Riaublanc A, Alexandre-Gouabau MC, Boquien CY. Calibration adjustment of the mid-infrared analyzer for an accurate determination of the macronutrient composition of human milk. J Hum Lact 2016;32(3):19–27. [DOI] [PubMed] [Google Scholar]
- 20. Zhu M, Yang Z, Ren Y, Duan Y, Gao H, Liu B, Ye W, Wang J, Yin S. Comparison of macronutrient contents in human milk measured using mid-infrared human milk analyser in a field study vs. chemical reference methods. Matern Child Nutr 2017;13(1). doi: 10.1111/mcn.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotrri G, Fusch G, Kwan C, Choi D, Choi A, Al Kafi N, Rochow N, Fusch C. Validation of correction algorithms for near-IR analysis of human milk in an independent sample set-effect of pasteurization. Nutrients 2016;8(3):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groh-Wargo S, Valentic J, Khaira S, Super DM, Collin M. Human milk analysis using mid-infrared spectroscopy. Nutr Clin Pract 2016;31(2):266–72. [DOI] [PubMed] [Google Scholar]
- 23. Buffin R, Decullier E, De Halleux V, Loys CM, Hays S, Studzinsky F, Jurdes E, Rigo J, Picaud JC. Assessment of human milk composition using mid-infrared analyzers requires calibration adjustment. J Perinatol 2017;37(5):552–7. [DOI] [PubMed] [Google Scholar]
- 24. Food and Agriculture Organization of the United Nations. Food energy—methods of analysis and conversion factors [Internet]. 2002. Available from: http://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf. [Google Scholar]
- 25. Wu X, Jackson RT, Khan SA, Ahuja J, Pehrsson PR. Human milk nutrient composition in the United States: current knowledge, challenges, and research needs. Curr Dev Nutr 2018;2(7):nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen RG, Neville M. Human lactation: milk components and methodologies. New York, NY: Plenum Press; 1985. [Google Scholar]
- 27. Lönnerdal B, Forsum E, Hambraeus L. A longitudinal study of the protein, nitrogen, and lactose contents of human milk from Swedish well-nourished mothers. Am J Clin Nutr 1976;29(10):1127–33. [DOI] [PubMed] [Google Scholar]
- 28. Feng P, Gao M, Burgher A, Zhou TH, Pramuk K. A nine-country study of the protein content and amino acid composition of mature human milk. Food Nutr Res 2016;60:31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen RG. Handbook of milk composition [Internet]. London, UK: Academic Press; 1995. [Google Scholar]
- 30. Coppa GV, Gabrielli O, Pierani P, Catassi C. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 1993;91(3):637. [PubMed] [Google Scholar]
- 31. Miris HMA Human Milk Analyzer User Manual [Internet]. 2017. Available from: https://www.mirissolutions.com/support/user-manuals. [Google Scholar]
- 32. Michaelsen KF, Pedersen SB, Skafte L, Jaeger P, Peitersen B. Infrared analysis for determining macronutrients in human milk. J Pediatr Gastroenterol Nutr 1988;7(2):229–35. [DOI] [PubMed] [Google Scholar]
- 33. Casadio YS, Williams TM, Lai CT, Olsson SE, Hepworth AR, Hartmann PE. Evaluation of a mid-infrared analyzer for the determination of the macronutrient composition of human milk. J Hum Lact 2010;26(4):376–83. [DOI] [PubMed] [Google Scholar]
- 34. Silvestre D, Fraga M, Gormaz M, Torres E, Vento M. Comparison of mid-infrared transmission spectroscopy with biochemical methods for the determination of macronutrients in human milk. Matern Child Nutr 2014;10(3):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parat S, Groh-Wargo S, Merlino S, Wijers C, Super DM. Validation of mid-infrared spectroscopy for macronutrient analysis of human milk. J Perinatol 2017;37(7):822–6. [DOI] [PubMed] [Google Scholar]
- 36. Corvaglia L, Battistini B, Paoletti V, Aceti A, Capretti MG, Faldella G. Near-infrared reflectance analysis to evaluate the nitrogen and fat content of human milk in neonatal intensive care units. Arch Dis Child Fetal Neonatal Ed 2008;93(5):372–5. [DOI] [PubMed] [Google Scholar]
- 37. Fusch G, Kwan C, Kotrri G, Fusch C. “Bed side” human milk analysis in the neonatal intensive care unit: a systematic review. Clin Perinatol 2017;44(1):209–67. [DOI] [PubMed] [Google Scholar]
- 38. US FDA, CFR–Code of Federal Regulations–Title 21 [Internet]. Washington, DC: US Department of Health and Human Services [cited 2019 Jul 10]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart = 110.