Abstract
Anthropogenic noise is a recognized global pollutant, affecting a wide range of nonhuman animals. However, most research considers only whether noise pollution has an impact, ignoring that individuals within a species or population exhibit substantial variation in responses to stress. Here, we first outline how intrinsic characteristics (e.g., body size, condition, sex, and personality) and extrinsic factors (e.g., environmental context, repeated exposure, prior experience, and multiple stressors) can affect responses to environmental stressors. We then present the results of a systematic search of the anthropogenic-noise literature, identifying articles that investigated intraspecific variation in the responses of nonhuman animals to noise. This reveals that fewer than 10% of articles (51 of 589) examining impacts of noise test experimentally for intraspecific variation in responses; of those that do, more than 75% report significant effects. We assess these existing studies to determine the current scope of research and findings to-date, and to provide suggestions for good practice in the design, implementation, and reporting of robust experiments in this field. We close by explaining how understanding intraspecific variation in responses to anthropogenic noise is crucial for improving how we manage captive animals, monitor wild populations, model species responses, and mitigate effects of noise pollution on wildlife. Our aim is to stimulate greater knowledge and more effective management of the harmful consequences of this global pollutant.
Keywords: biological responses, environmental stressors, experiments, management, mitigation, noise pollution
To understand fully how noise pollution affects animals, we must consider differences between individuals. Less than 10% of published experimental studies on noise effects investigate variation due either to individual characteristics (such as size and sex) or external factors (such as current context or previous exposure). We explain how greater consideration of individual variation is vital for understanding man-made noise as a global pollutant and for developing mitigation strategies to protect animals.
INTRODUCTION
Human population growth, rapid urbanization and infrastructure development, greater resource exploration and extraction, and the expansion of transportation networks have all contributed to the increased production of anthropogenic noise, altering terrestrial and aquatic soundscapes worldwide (Kight and Swaddle 2011; Shannon et al. 2015; Buxton et al. 2017). Many human activities generate noise within the hearing ranges of other animals, at sound levels above those found naturally and with different acoustic characteristics from abiotic and biotic sounds (Hildebrand 2009). Those man-made additions to the acoustic environment that contain little or no useful information and which have negative consequences on wildlife represent a well-recognized form of pollution. A wide variety of anthropogenic noise sources have been shown to affect invertebrate, fish, amphibian, bird, and mammal behavior (e.g., disrupting vocal communication, foraging, antipredator responses, and parental care), physiology (e.g., causing stress, hearing damage, and immune-system impairment), and development (e.g., reducing growth and causing morphological malformations), with resulting fitness consequences (for recent reviews, see Morley et al. 2014; Shannon et al. 2015; Kunc et al. 2016). However, most research has only considered whether noise pollution has an effect and the nature of its impact. Typically, empirical studies are inherently based on the assumption that conspecifics are ecologically equivalent, reporting responses as a mean cohort effect. Such a simplification ignores intraspecific (within-species) variation (Radford et al. 2016a).
Considerable variation exists between individuals of the same species for both intrinsic and extrinsic reasons, causing differences in the way that conspecifics look, behave, and respond to natural selection pressures, such as predation risk, food availability, and novel environments (Bolnick et al. 2003). It is therefore inevitable that when presented with anthropogenic stressors, individuals from the same species will respond in different ways (Bolnick et al. 2011). These varied responses may define the difference between success and failure; the likelihood of mortality or the ability to emigrate, to adapt through genetic changes, or to respond via phenotypic plasticity (Engås et al. 1996; Höglund et al. 2008; Cripps et al. 2014). Intraspecific variation in responses can also have far-reaching impacts on the population dynamics, community structure and ecosystem function of entire groups of animals (Post et al. 2008; Rudman et al. 2015; Charette and Derry 2016; Des Roches et al. 2017). Indeed, in some cases intraspecific variation can have a greater influence than interspecific differences on overall community responses to environmental change (Crutsinger et al. 2006; Siefert and Ritchie 2016; Raffard et al. 2019). Furthermore, varied responses set the stage for future evolution, as the cohort of individuals capable of reproducing following an anthropogenic stress event defines the evolutionary potential of the postdisturbance population (Medina et al. 2007; Bijlsma and Loeschcke 2012). To consider only “mean” responses to anthropogenic stressors is therefore to underappreciate the likely consequences of the disturbance; a lack of population-level impacts may be masking more subtle but important within-population changes. Conversely, consideration of intraspecific variation facilitates a more comprehensive understanding of the impacts of anthropogenic stressors on animals, the likely consequences for wider ecosystems, and the best management strategies to address these changes.
In this review, we begin by outlining the existence and importance of intraspecific variation in response to environmental stressors, and why its consideration with respect to anthropogenic noise is needed. We explain how variation arising from intrinsic characteristics (e.g., body size, body condition, sex, and personality) and extrinsic factors (e.g., environmental context, repeated exposure, prior experience, and multiple stressors) can affect responses in biological systems, and the consequences of such variation. We then report on a systematic review of the literature relating to the impacts of anthropogenic noise on nonhuman animals. We provide a comprehensive list of experimental studies that have investigated intraspecific variation in responses to noise, and offer qualitative and quantitative summaries of the scope and findings of that research. Moreover, we draw on an assessment of those existing studies when making suggestions for best practice in designing and implementing robust experimental research that would benefit the field moving forwards. Finally, we explain how a greater focus on intraspecific variation in response to anthropogenic noise is crucial for improving the management of animals in captivity, monitoring the impacts on wild populations, modeling species responses, and mitigating the effects on wildlife. Our aim is to stimulate a greater understanding of the importance of intraspecific variation when determining both the impacts of anthropogenic noise and how best to mitigate this global pollutant.
THE EXISTENCE AND IMPORTANCE OF INTRASPECIFIC VARIATION
Intraspecific variation is caused by a range of intrinsic characteristics and extrinsic factors. Fundamentally, genotypic and epigenetic differences between individuals underpin intrinsic phenotypic characteristics (e.g., body size, body condition, sex, and personality) that vary within a population (Skinner 2015). Individuals with different characteristics may respond differently to environmental and anthropogenic stressors in terms of both their behavior and physiology (as discussed in detail below). These responses may be the consequence of, or be mediated by, differences in life-history trade-offs and strategies (Stearns 1997; Zera and Harshman 2001). Considerable intraspecific differences also arise due to extrinsic factors, including variation in the environmental context in which a stressor is experienced, repeated exposure to or prior experience of a stressor, and the presence and magnitude of multiple stressors. Many of these extrinsic factors (such as repeated exposure or prior experience) are likely underpinned by the flexibility in behavioral and/or physiological responses to sustained exposure to stressors; phenotypic plasticity is often the first line of defense when organisms are confronted with environmental change (Chevin et al. 2010; Wong and Candolin 2015). In this section, we describe several general mechanisms by which intraspecific variation can exert an influence on responses to environmental stressors and highlight the importance of considering this variation for an understanding of responses to anthropogenic disturbances such as noise. We do not provide an exhaustive list of potential characteristics and factors, but use illustrative examples where there is strong existing evidence for an influence.
Intrinsic characteristics
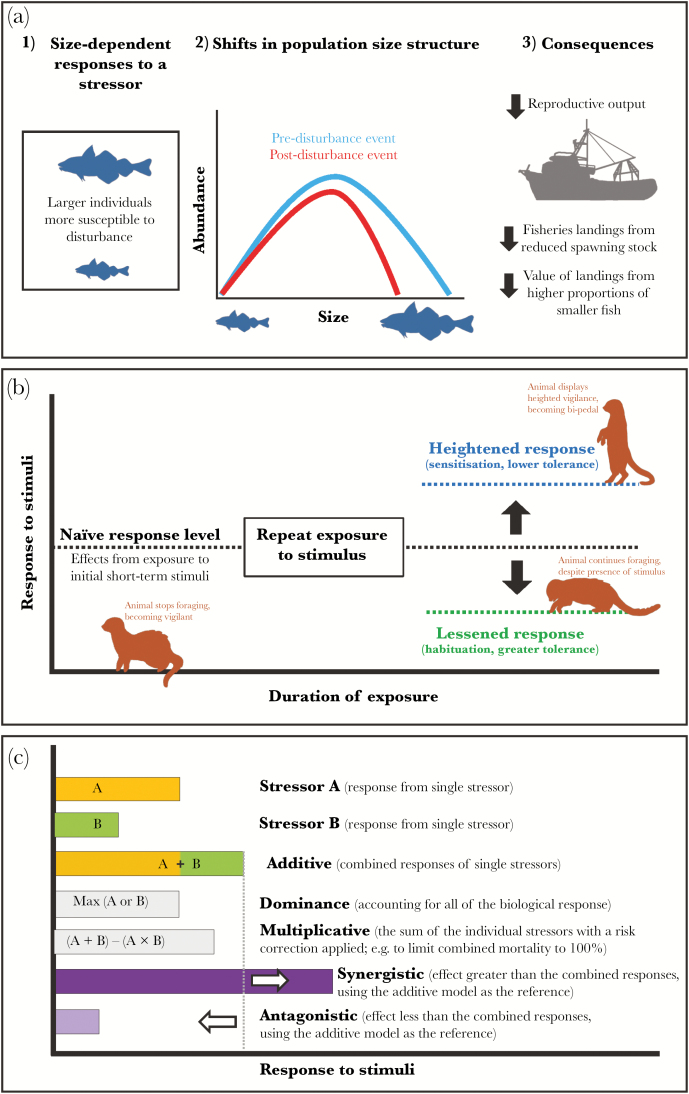
Variation in body size, which often scales with age (Sebens 1987), affects responses to environmental stressors due to fundamental differences in physiological mechanisms, morphology, and behavior (Spear et al. 1996; Ortiz-Santaliestra et al. 2006; Pörtner and Knust 2007). Size-dependent selection can drive changes in the demographic structure of populations (Figure 1a); for example, selective harvesting of larger individuals by commercial fishing fleets can cause shifts toward higher proportions of younger age classes among the spawning stock (Ottersen et al. 2006). In many taxa, body size correlates with fecundity (Shine 1988) and so stressors that truncate population size-structure may impact population reproductive potential and recruitment success, while also reducing the capacity to cope with further stress in variable environments (Kiffney and Clements 1996; Walther et al. 2002; Planque et al. 2010).
Figure 1.
Importance of considering intraspecific variation in responses to environmental change. (a) Body size can affect responses to environmental stress with potential consequences for population-size structure and reproductive output, and thus implications for human economics and food security. (b) Prior experience of a stimulus may lead to a change in the level of response exhibited. (c) Multiple stressors can result in a variety of different response levels depending how the individual stressors interact (Côté et al. 2016). Images in figure drawn by WiseArt.net.
Conspecific individuals differ in body condition as a result of the heterogeneous nature of food availability and from inherent variation in routine metabolic rates (Metcalfe and Monaghan 2001; Killen et al. 2011). Variation in body condition results in considerable differences in behavioral and physiological functioning (Duckworth et al. 2001), and can therefore affect responses to environmental stress. Animals in poor condition with low energy reserves may display more risk-prone behaviors (Caraco et al. 1990), be unable to maintain optimal physiological functioning when challenged by a stressor, or fail to recover from additional environmental stresses (Sokolova et al. 2012; Sokolova 2013). Considering variation in body condition is important for the development of management and mitigation strategies to alleviate anthropogenic stress. For instance, during periods of reduced foraging opportunities or increased physiological stress, animals may be more at risk from anthropogenic stressors, and thus mitigation strategies become increasingly important at these times.
Sex-dependent effects occur in animals due to differences in morphology, biochemical processes, and hormonal profiles (McClellan-Green et al. 2007; Palanza 2017). For example, if there are sex differences in baseline levels of stress-induced hormones, which influence individual responses to disturbances (Pottinger et al. 1996; Dalla et al. 2011), then males and females may respond differently to the same environmental stressor. In some species, sex-mediated responses to stress may be an adaptive mechanism associated with different energetic requirements (Afonso et al. 2003). Different effects of environmental stress on each sex may have profound population-level consequences, including with respect to sex ratios which may become altered by, for instance, sex-specific mortality (Grüebler et al. 2008) or impacts on temperature-dependent sex determination (Parrott and Blunt 2005; Jensen et al. 2018). Potential impacts will be species-specific and related to mating strategy, but could include a decline in reproductive output affecting overall population viability (White et al. 2017; Jensen et al. 2018).
Intraspecific variation in animal personality—defined by a suite of behavioral traits consistent across time and environmental context—has been shown in multiple taxa (Sih et al. 2004; Réale et al. 2007). Personality covaries with physiological and neuroendocrinological mechanisms, determining an individual’s coping style; that is, how they deal behaviorally and physiologically with environmental stress (Carere et al. 2010). Personality type can affect how individuals perform in changing environments: in some systems, bold, fast-exploring, proactive individuals may do well in less-risky, stable environments, whereas slow-exploring, reactive behavioral types may perform better in high-risk environments and in situations of environmental change as they may have greater behavioral flexibility (Guillette et al. 2011; Sih et al. 2012). Maintenance of variation is important for adaptability to future environmental fluctuations (Dall et al. 2004; Sih et al. 2012). Furthermore, intraspecific variation in personality can have important implications for ecological processes, with variation in predator personalities shown to influence the composition of prey communities (Royauté and Pruitt 2015).
Extrinsic factors
The current environmental context, including food availability and predation risk, can affect behavior exhibited by individual organisms (Lima and Dill 1990; Sih et al. 2004). Behavioral variability due to different environmental contexts reflects a trade-off between the risk from a stressor and the benefit gained from continuing a current activity (Lima and Dill 1990). For instance, many animals reduce their foraging during periods of increased predation risk (Clarke 1983; Lima and Dill 1990), and anthropogenic disturbances can influence predation risk (Chalfoun et al. 2002). Treating context as homogenous across studies compromises the quality of documented information about predicted responses to environmental stress.
Behavioral and physiological responses can change with repeated exposure to stressors, across a range of taxa (Figure 1b) (Burger and Gochfeld 1999; Bejder et al. 2009). These modifications can also be transferred to offspring through epigenetic mechanisms (Dias and Ressler 2014). Moreover, anthropogenic stressors vary across time and space (Hildebrand 2009; Mekonnen and Hoekstra 2015), meaning that individuals within a population or in different populations are likely to experience different conditions from one another. Variation in this prior experience can influence current responses, resulting in stronger effects due to sensitization, or weaker effects due to increased tolerance or habituation (Bejder et al. 2009). Using data based on short-term responses from assays that do not link directly to fitness may therefore under- or over-estimate realized impacts on populations (Bejder et al. 2009).
Organisms are rarely exposed to stressors in isolation, due to the multitude of anthropogenic threats faced by animals worldwide; these threats include light and chemical pollution, changing climates, hypoxia, acidification of marine and freshwater systems, and habitat destruction and fragmentation (Millennium Ecosystem Assessment 2005; McBryan et al. 2013). The effects of multiple stressors can be additive or multiplicative, or one stressor can dominate another; additionally, interactions may be synergistic or antagonistic (Figure 1c) (Côté et al. 2016; Gunderson et al. 2016). Understanding responses to single stressors does not always allow realistic predictions of responses to multiple stressors (Darling and Côté 2008); populations may show no adverse effects to particular pollutants in isolation, but the addition of another stressor may cause a markedly different response (Relyea and Mills 2001) or even stress individuals beyond their physiological limit (Fasola et al. 2015).
STATE OF KNOWLEDGE WITH RESPECT TO ANTHROPOGENIC NOISE
We performed a systematic search of the peer-reviewed literature that has investigated the impacts of anthropogenic noise on nonhuman animals (see Supplementary Material for methods), with three main aims. First, we identified the number and scope of studies examining intraspecific variation in response to anthropogenic noise. Second, we used the resulting comprehensive list of experimental studies to compare findings relating to different intrinsic characteristics and extrinsic factors. Finally, we drew on an assessment of those existing studies to make suggestions for best practice in the design and implementation of experimental research that would benefit the field moving forwards.
Research focus to-date
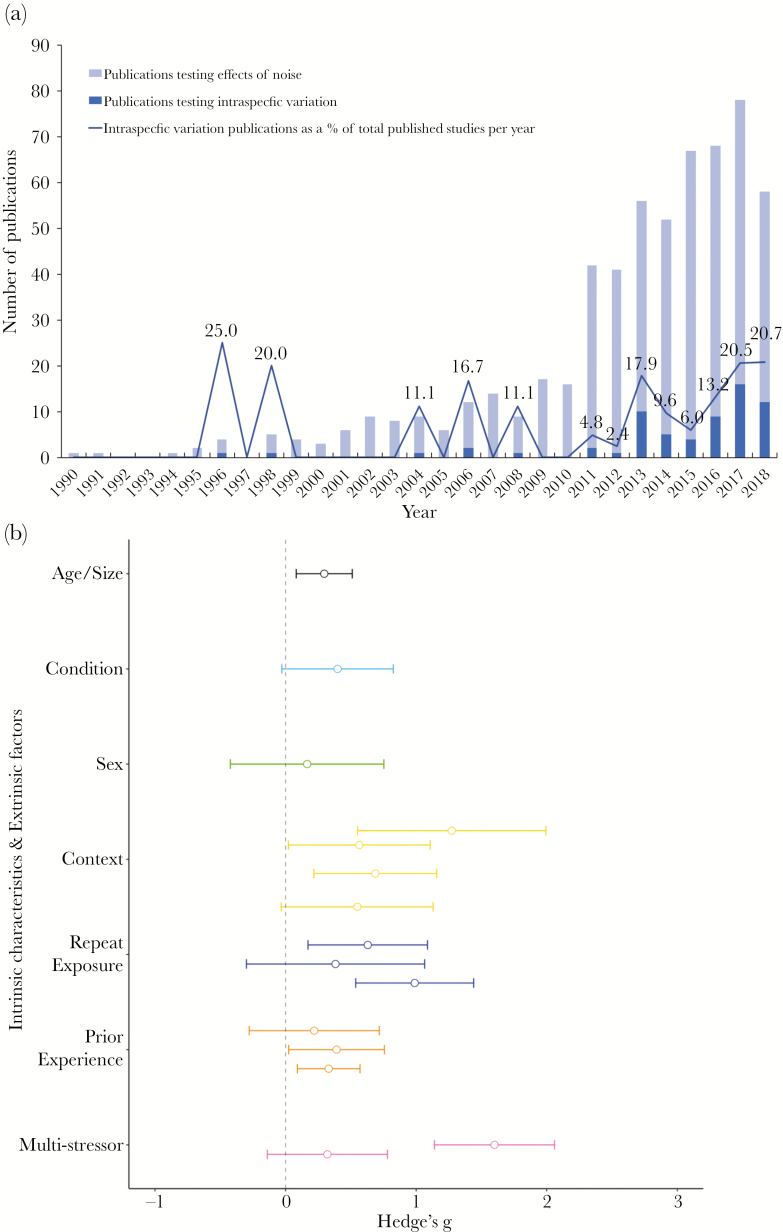
The body of literature investigating the effects of anthropogenic noise on nonhuman animals has increased rapidly in the last decade (Figure 2a; Shannon et al. 2015). The proportion of peer-reviewed studies considering intraspecific variation has also been growing, especially since 2013, although the absolute number still remains low (Figure 2a). It is possible (as in all research fields) that a publication bias exists toward articles that find an effect; there may be some that set out to test for intraspecific variation but, on finding no evidence, subsequently pooled results to report a general effect of noise. From our literature search, we identified 65 articles that have tested intraspecific variation in response to anthropogenic noise. These comprise 51 experimental studies (detailed in Supplementary Table S1) and 14 observational studies (Supplementary Table S2), representing 8.7% and 2.4%, respectively of all articles published on anthropogenic noise that met our criteria. At present, the majority of noise studies testing intraspecific variation have considered extrinsic factors (n = 41, 71%), of which repeated exposure (n = 16) has been examined the most, followed by environmental context (n = 13), prior experience (n = 8), and multiple stressors (n = 4). Intrinsic characteristics are less well-represented in our identified articles (n = 17; 29%); where an article documents consideration of two or more sources of intraspecific variation (n = 7), they are included multiple times in this assessment. Among intrinsic characteristics, sex (n = 8) has been investigated the most often, followed by body size/age (n = 6), body condition (n = 2), and personality (n = 1).
Figure 2.
(a) Number of peer-reviewed publications per year that have investigated the effects of anthropogenic noise, and intraspecific response variation, in nonhuman animals. (b) Standardized effect sizes (Hedge’s g) for experimental studies in Supplementary Table S1 (calculated where possible and using illustrative examples where studies present more than one response metric) for different sources of intraspecific variation. Points and associated error bars represent composite standardized effect sizes (CES) and associated 95% confidence intervals from individual studies. CES were calculated in the following way: the weight of each group per study (1/variance of the effect size) was determined, and subsequently multiplied by the individual effect size (ES × weight); the composite effect size was then determined by dividing the sum of the effect size × weight by the sum of the weights. 95% confidence intervals were calculated with the following equation . All CES are presented as positive integers to enable illustrative comparisons across studies.
The experimental studies conducted to-date span a broad taxonomic range and have considered a variety of response measures. Fish are the most well-documented taxa (n = 22, 42% of experimental studies investigating intraspecific variation), followed by birds (n = 14, 27%), mammals (n = 8, 15%; aquatic: n = 5, terrestrial: n = 3), arthropods (n = 5, 10%; aquatic: n = 3, terrestrial: n = 2), and amphibians (n = 3, 6%); one study considered both amphibians and arthropods. Shannon et al. (2015) identified birds and marine mammals as by far the most-studied taxa in terms of noise impacts in general; the relative preponderance of fish studies on intraspecific variation likely reflects a recent upsurge in their consideration in anthropogenic-noise research (Kunc et al. 2016). In general, there is a strong taxonomic bias toward vertebrates, despite invertebrates making up 97% of known animals, having great ecosystem and commercial importance, and offering the opportunity for valuable experimental tractability (Morley et al. 2014). With regards to specific response measures, the majority of experimental noise studies considering intraspecific variation have focused solely on behavioral responses (n = 37, 73%), compared with eight (16%) for physiological measures and six (12%) where both behavior and physiology data have been collected. Only two studies (4%) have directly measured fitness impacts; while fitness estimates are often logistically more challenging to determine, they are what is ultimately required to assess population consequences.
Current knowledge base
There are some qualitative differences in findings between different sources of intraspecific variation in the experimental work conducted to-date; any conclusions drawn at this stage need to be cautious, due to the small number of relevant studies in each case. Of the 58 measured aspects of intraspecific variation detailed in Supplementary Table S1, 44 (76%) are reported as having a significant effect on the response to anthropogenic noise. Overall, intrinsic characteristics (13 out of 17 cases; 76%) and extrinsic factors (31 out of 41; 76%) were equally likely to have a significant influence. However, at the level of specific characteristics and factors, there were considerable differences. All studies considering body size (n = 6) and personality (n = 1) reported significant effects on responses to noise, with environmental context and repeated exposure having a significant influence in 92% and 81% of studies, respectively. There is greater variation between studies considering each of sex (63%), prior experience (63%), and body condition (50%) as aspects of intraspecific variation. Only 25% of studies investigating multiple stressors reported an alteration in the effect of anthropogenic noise in the presence of an additional stressor.
We also considered, where possible, the standardized and composite effect sizes of intraspecific variation found in experimental anthropogenic-noise studies (Supplementary Table S1). Only rarely were the effect sizes for individual categories of interest or the intraspecific variation itself reported. We therefore attempted to calculate effect sizes ourselves, but this proved difficult due to a lack of relevant information. In the future, it would be useful if studies either included the means, standard errors/deviations, and sample sizes or the raw data to allow for accurate calculation of effect sizes from more complex experimental designs (repeated measures), or if they reported explicitly the statistical tests comparing the categories in question; only 29% (15/51) of studies in Supplementary Table S1 provided even some of this information. Consideration of the composite effect sizes we were able to calculate (n = 15) for different intrinsic characteristics and extrinsic factors indicated that no particular source of intraspecific variation causes an obviously greater magnitude in response differences to noise than any other (Figure 2b). A large composite effect size represents either a single characteristic (e.g., male or female) that is substantially more sensitive to noise than its equivalent opposite, or two or more characteristics that are affected by noise compared with the baseline/control conditions. In the four sources of intraspecific variation for which composite effect sizes could be calculated for more than one study, only one (multiple stressors) shows no overlap in confidence intervals, suggesting a substantial difference in response between studies. The different effect sizes for the two studies assessing multiple stressor impacts of noise may be related to the use of different response metrics (behavioral and physiological), which has been shown to affect the overall magnitude in response across the anthropogenic noise literature (Cox et al. 2018). From the remaining three sources, all three (prior experience, repeated exposure, and context) comprised studies where effect sizes overlapped with each other. Clearly, formal meta-analytic comparisons will only become possible with a greater number of suitable studies in the future.
Future experimental studies
To improve our understanding of intraspecific variation in responses to noise, more and robust experimental tests are required. We suggest that a series of key decisions can aid the design and implementation of such tests; these are not mutually exclusive. Some of these decisions are broadly applicable to most, if not all, research fields and are frequently discussed. For instance, consideration of the relative advantages and disadvantages of captive versus field-based work, the need for suitable controls and sample sizes, and avoidance of pseudoreplication. We outline the importance of these fundamental concepts in Supplementary Table S3, highlighting existing examples of good practice from experimental articles on intraspecific variation in noise responses. Decisions that have more specific relevance to the study of anthropogenic noise are described in detail below.
Rigorous anthropogenic-noise research needs to involve suitable acoustic measurements, including consideration of what is known about the hearing thresholds of the study species. Full characterization of the sound field is required (rather than just the reporting of single decibel values) and should be presented in the appropriate domain for the species in question (Francis and Barber 2013; McKenna et al. 2016; Nedelec et al. 2016). For aquatic studies on fish and invertebrates, this includes reporting acoustic metrics in both particle-motion and sound-pressure domains (Nedelec et al. 2016); for terrestrial studies, the correct frequency weighting for the taxa needs to be applied (McKenna et al. 2016). Of studies in Supplementary Table S1, only 62% clearly do this (11 out of 25 fish and aquatic invertebrate studies; 17 out of 20 terrestrial studies). It has been suggested that sound measurements be recorded with both a Z-frequency weighting (flat response) and a weighting more appropriate for the study species (Francis and Barber 2013); only two examples of this approach exist in Supplementary Table S1 (LaZerte et al. 2016, 2017). Determining the best sound-characterization approach should ideally be informed by knowledge of the hearing range of the species being studied; in some species at least, there can be ontogenetic changes in hearing thresholds (Kenyon 1996; Wright et al. 2011). However, care needs to be taken when using published hearing thresholds; for example, the validity of many published fish-hearing measurements has recently been called into question (Hawkins et al. 2015).
In addition to clear and detailed reporting of acoustic metrics, it is important to consider the advantages and disadvantages of using loudspeaker playback (the methodology employed in 43 of the studies in Supplementary Table S1) versus real noise sources (nine studies in Supplementary Table S1) for experiments on the impacts of anthropogenic noise. Loudspeaker playback enables isolation of noise as the stressor, free from visual disturbances and other potential confounds (e.g., wake effects from passing boats or ships). However, use of loudspeakers results in sound fields that can differ considerably from those in real-world situations (Okumura et al. 2002; Quadros et al. 2014; Slabbekoorn 2016). Additionally, loudspeakers tend to provide only a point sound-source which is unrealistic for many types of anthropogenic noise, including vehicle traffic; this can be overcome with the use of multiple loudspeakers to create, for example, a phantom road (McClure et al. 2013). To provide acoustic validity, real-noise sources are required but this presents a number of challenges. First, it is often logistically difficult to obtain access to real noise sources. Second, robust experimental design requires multiple exemplars of the noise source to minimize pseudoreplication (Kroodsma et al. 2001). The latter is particularly problematic when testing for impacts of noise in aquatic environments (e.g., commercial shipping or pile-driving), however motorboats offer the opportunity for well-controlled experiments (Simpson et al. 2016). While captive experiments are constrained to using loudspeaker playback, large-scale mesocosm and field-based studies allow the potential use of both acoustic methods, and therefore facilitate a beneficial comparison. Using both methods allows a demonstration of the effect of an actual human activity while also assessing the importance of the noise component alone, but there is only one study in Supplementary Table S1 that combines the use of both loudspeaker playback and real noise sources (Harding et al. 2018).
Most published work investigating intraspecific variation in anthropogenic-noise effects has focused on either behavioral and/or physiological responses (see “Research focus to-date”). So, the range of response measures could be profitably broadened, especially given the rapid advancement in genetic-sequencing technologies and reductions in processing costs (Connon et al. 2018). It is increasingly possible to use methods such as restriction site-associated DNA sequencing to explore genotypic variation (Paris et al. 2015), and transcriptomics to reveal the genetic effects of stressors (Pespeni et al. 2013), as well as determine potential plasticity and evolutionary adaptability (Munday et al. 2013); the use of these methods is in its infancy in anthropogenic-noise studies (see Chen et al. 2018 for an example). Ultimately, though, a full understanding of population and ecosystem consequences will require consideration of impacts on individual fitness. Ideally studies would measure these directly rather than extrapolating from, for instance, short-term behavioral and physiological responses. Clearly measuring fitness is logistically challenging, but Casper et al. (2013) and Potvin and Macdougall-Shackleton (2015) have demonstrated that it can be feasible with respect to intraspecific variation in response to anthropogenic noise.
APPLICATIONS FOR CAPTIVE AND WILD ANIMAL POPULATIONS
We believe that improving current understanding of intraspecific variation in responses to anthropogenic noise will increase our capability to manage captive animals effectively, monitor impacts on wild populations, model species responses, and mitigate the effects of noise pollution. Below, we provide specific suggestions as to how and why increasing our understanding can be valuable in each of these areas.
Management
Incorporating intraspecific variation in noise responses into the management of captive animals could provide benefits to welfare, food productivity, and experimental control in research studies. Captive systems are often inherently noisy (e.g., transportation and construction noise in zoos raise ambient sound levels, as do pumps and aerators in aquaculture facilities; Bart et al. 2001; Shepherdson et al. 2004), but can potentially be quieted by noise-reduction techniques (Davidson et al. 2007). Elevated noise in captivity can cause stress and negatively affect growth, condition, and survival (Banner and Hyatt 1973; Shepherdson et al. 2004; Anderson et al. 2011). However, the effects of noise exposure may differ between cohorts or life-stages; for instance, animals may be especially vulnerable early in life (Davidson et al. 2007, 2009; de Soto et al. 2013; Nedelec et al. 2014). It is widely accepted that the feeding regimes needed to meet the energy and nutrient requirements of growing animals will change with age, and so diets are prepared accordingly for captive animals (Gardner et al. 1988; Wecke and Liebert 2013). If there are age-specific responses to noise, then tailoring noise-reduction management techniques to particular life-stages may similarly be beneficial to animal welfare, growth rates, and productivity; in aquaculture, this could arise through, for instance, a better feed-conversion efficiency (Davidson et al. 2007). Moreover, acoustic noise in captive research systems should be minimized to remove unwanted statistical variation, potential confounding factors and possible biases in response data (Sabet et al. 2016). Importantly, if intraspecific variation in response to noise exists, individuals from more or less susceptible cohorts should be split evenly between different experimental treatments to avoid biases.
Monitoring
Considering intraspecific variation is crucial when monitoring the responses of wild animal populations to anthropogenic noise. If intrinsic characteristics cause undetected or unconsidered response variation, then population assessments and predictions about resilience to environmental stress may be misleading (Mimura et al. 2017). For instance, European eels (Anguilla anguilla) in poorer body condition were shown to be more affected than those in better condition by ship noise (Purser et al. 2016). Depending on when impact assessments are made, noise responses may appear more or less severe as a result of temporal fluctuations in body condition within populations (Brosset et al. 2015). Studies focusing on population averages may also provide inaccurate impact assessments when responses to noise change with repeated exposure or prior experience (Radford et al. 2016b; Harding et al. 2018). Monitoring populations near to and far from human activities would allow cohorts with varying degrees of noise exposure to be assessed (Harding et al. 2018), but in doing so it is important to control for habitat type and other anthropogenic disturbances, to avoid potential confounds and isolate noise as the stressor (Francis et al. 2012). Additionally, incorporating long-term acoustic-monitoring data, such as those from the Ocean Noise Reference Station Network (Haver et al. 2018), into environmental impact assessments (EIAs) could allow for consideration of prior experience and how exposure to different noise types can lead to specific or generalized response changes (Radford et al. 2016b). Including context-based and cumulative-impact evaluations into EIAs and monitoring could further reduce uncertainty when predicting behavioral and fitness effects (Ellison et al. 2012; Nowacek et al. 2015; Harris et al. 2018).
Interactions between species are highly flexible and can be influenced by intraspecific variation (Pruitt et al. 2012; Lichtenstein et al. 2016). For example, variation in the levels of an aggressive phenotype in a population of Anelosimus studiosus spiders was shown to alter relationships with heterospecifics and affect reproductive performance (Pruitt et al. 2012). Incorporating intraspecific variation of this nature may prove important for accurately determining the impact of noise on interactions between species—both antagonistic/conflict relationships (Simpson et al. 2016; Nedelec et al. 2017b) and those of a more cooperative or mutualistic nature (Nedelec et al. 2017a)—and at the community level (Moran et al. 2016). In a hypothetical example, whilst a given predator–prey relationship may appear to be affected by noise in favor of one party (Simpson et al. 2016), repeated exposure may adjust the balance of the relationship if, say, the prey becomes more tolerant and the predator continues to display its original response. Identifying the potential impacts of anthropogenic noise on the functional diversity of community assemblages (type, range, and abundance of organismal traits in a community; Díaz et al. 2007) will develop greater insight into how fundamental ecological processes and ecosystem stability may be affected. Predictions about impacts on functional diversity would be improved by considering intraspecific variation (Cianciaruso et al. 2009) because there can be differences in functional roles depending on such intrinsic characteristics as size and age (Bonaldo and Bellwood 2008). Intraspecific variation can have greater effects on indirect ecological interactions than removal or replacement of a species (Des Roches et al. 2017), and influence the strength of trophic cascades in a community (Post et al. 2008).
Modeling
Modeling species responses to anthropogenic noise would likely be improved by inclusion of intraspecific variation. Predictive modeling can be used for EIAs and for projecting future species distributions and understanding functional and community-level changes (Chevin et al. 2010; Rossington et al. 2013). Empirical investigations of physiological and behavioral responses are valuable for parameterizing both population-persistence models and individual- and trait-based models, and for interpreting presence/absence and abundance data in combination with environmental variables in species-distribution models (SDMs) (Chevin et al. 2010; Koenigstein et al. 2016). Failure to capture intraspecific variation in baseline parameters may artificially reduce model uncertainty, but will also reduce the value of predictions when deciding mitigation measures and developing management strategies. Mechanistic population models can include multiple size/age classes and variation in phenotypic traits, while individual-based models can be expanded to include genotype (Moran et al. 2016). For example, including tree growth data as a measure of intraspecific variation improved the quality of pine tree distribution models relative to models that treated all trees as equal, and produced different future predictions as a result (Oney et al. 2013). Indeed, incorporating intraspecific variation into SDMs in this manner has been shown to alter predicted responses of species to environmental change in several cases (Valladares et al. 2014), and is therefore likely also to be important when modeling responses to anthropogenic noise.
Mitigation
Strategies for mitigating the impacts of anthropogenic noise should include the identification of traits that make particular individuals within a population especially vulnerable. For example, male natterjack toads (Epidalea calamita) exhibit high site-fidelity during breeding seasons, as they remain located at a particular pond from which they call to attract free-ranging females (Sinsch 1992). In a hypothetical local noise-pollution scenario, mobile females might move out of a noisy area to nearby quieter habitat, but site-attached males may be unable to do so, with potentially important consequences for population demography and implications for the management of noise (Husté et al. 2006). Using only knowledge of short-term responses may also have detrimental consequences if, for instance, individuals habituate to stimuli over time and can compensate for initial impacts; mitigation measures required initially to avoid acute effects on survival (Simpson et al. 2016) might subsequently become unnecessary, and their continued implementation could be conservative. By contrast, there may be declines in growth and fitness consequences for individuals inhabiting areas exposed to chronic noise, even if no acute changes in behavior or physiology were initially displayed (Slabbekoorn et al. 2010).
In general, there is a need to determine both the spatial and temporal scale of noise impact, including the establishment of noise-exposure threshold levels (those at which different biological responses are predicted; Farcas et al. 2016). Incorporation of intraspecific variation into these threshold levels will improve their accuracy; the result might then be multiple spatial zones where different subsets of a population are likely to be affected. Additionally, impacts of noise during the breeding season might be especially detrimental, due to the increased fitness costs associated with reproductive failure (Nedelec et al. 2017b), and so noise mitigation during this period might have a greater population-level impact than at other times of year. As an existing conservation example, there is season-specific legislation on noise pollution with respect to marine mammal movement and behavioral patterns (Merchant et al. 2018; Pine et al. 2019). For instance, the Be Whale Wise regulations are a set of guidelines, developed collaboratively by government agencies, nonprofit organizations and local stakeholders, for boat users in the Salish Sea (USA and Canada). These guidelines instruct users to take extra care to reduce vessel noise in the area between May and September, which is the breeding and pupping seasons for marine mammals (Be Whale Wise 2019). Overall, the aim should be to use information about intraspecific variation in noise responses to enhance the effectiveness of mitigation strategies.
CONCLUSIONS
Over the last 10–15 years, a rapidly burgeoning literature has provided substantial evidence that nonhuman animals from a wide range of taxa and ecosystems are detrimentally affected by anthropogenic noise (Morley et al. 2014; Shannon et al. 2015; Kunc et al. 2016). While there is undoubted value in continuing to extend the geographic and taxonomic range of sampling (Shannon et al. 2015), there is also a need to expand the scope of studies. We argue that including greater consideration of intraspecific variation in responses would represent a profitable and important expansion in this regard. Doing so will generate a more complete and realistic understanding across all levels of biological organization, helping to prevent misinterpretations that can lead to over- or under-estimation of the impacts of noise exposure. While it is well-recognized that care should be taken when extrapolating results between species, as responses may differ substantially (Shannon et al. 2015; Kunc et al. 2016), similar caution should be applied within species. Moreover, inclusion of intraspecific response variation in studies will enable better translation into suitable and effective management recommendations and actions. With continued urbanization, energy-generation and transport-network expansion in a range of different ecosystems, it is likely that anthropogenic noise will become ever more prevalent across the globe. To understand its true impact and to mitigate its effects, we must pay careful attention to the variation that exists within populations and species.
Supplementary Material
FUNDING
This work was supported by a Natural Environment Research Council (NERC)–Marine Scotland CASE GW4+ DTP Studentship (NE/L002434/1) to H.R.H, a NERC–Australian Institute of Marine Science CASE GW4+ DTP Studentship (NE/L002434/1) to T.A.C.G and a NERC Research Grant (NE/P001572/1) to S.D.S and A.N.R.
We thank Sofia Jain at WiseArt.net for the images in Figure 1. We are grateful to Tom Bunce, Kieran McCloskey, Sophie Nedelec, Emma Weschke, and Katy Wong for valuable discussions, and to 2 anonymous referees for useful comments on the article.
Author contributions: H.R.H., T.A.C.G., E.E., S.D.S., and A.N.R. developed the structure and content of the review. H.R.H. wrote the initial manuscript, revised by A.N.R., with all authors contributing to produce the final version.
Data accessibility: There are no data associated with this article.
REFERENCES
- Afonso LOB, Basu N, Nakano K, Devlin RH, Iwama GK. 2003. Sex-related differences in the organismal and cellular stress response in juvenile salmon exposed to treated bleached kraft mill effluent. Fish Physiol Biochem. 29:173–179. [Google Scholar]
- Anderson PA, Berzins IK, Fogarty F, Hamlin HJ, Guillette LJ. 2011. Sound, stress, and seahorses: the consequences of a noisy environment to animal health. Aquaculture. 311:129–138. [Google Scholar]
- Banner A, Hyatt M. 1973. Effects of noise on eggs and larvae of two estuarine fishes. Trans Am Fish Soc. 102:134–136. [Google Scholar]
- Bart AN, Clark J, Young J, Zohar Y. 2001. Underwater ambient noise measurements in aquaculture systems: a survey. Aquac Eng. 25:99–110. [Google Scholar]
- Bejder L, Samuels A, Whitehead H, Finn H, Allen S. 2009. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar Ecol Prog Ser. 395:177–185. [Google Scholar]
- Be Whale Wise 2019. Marine wildlife guidelines [cited 2019 April 12]. Available from: http://www.bewhalewise.org/marine-wildlife-guidelines/.
- Bijlsma R, Loeschcke V. 2012. Genetic erosion impedes adaptive responses to stressful environments. Evol Appl. 5:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur D. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 26:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD. 2003. The ecology of individuals: incidence and implications of individual specialization. Am Nat. 161:1–28. [DOI] [PubMed] [Google Scholar]
- Bonaldo RM, Bellwood DR. 2008. Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar Ecol Prog Ser. 360:237–244. [Google Scholar]
- Brosset P, Ménard F, Fromentin J-M, Bonhommeau S, Ulses C, Bourdeix J-H, Bigot J-L, Van Beveren E, Roos D, Saraux C. 2015. Influence of environmental variability and age on the body condition of small pelagic fish in the Gulf of Lions. Mar Ecol Prog Ser. 529:219–231. [Google Scholar]
- Burger J, Gochfeld M. 1999. Role of human disturbance in response behavior of laysan albatrosses (Diomedea immutabilis). Bird Behav. 13:22–30. [Google Scholar]
- Buxton RT, McKenna MF, Mennitt D, Fristrup K, Crooks K, Angeloni L, Wittemyer G. 2017. Noise pollution is pervasive in U.S. protected areas. Science. 356:531–533. [DOI] [PubMed] [Google Scholar]
- Caraco T, Blanckenhorn WU, Gregory GM, Newman JA, Recer GM, Zwicker SM. 1990. Risk-sensitivity: ambient temperature affects foraging choice. Anim Behav. 39:338–345. [Google Scholar]
- Carere C, Caramaschi D, Fawcett TW. 2010. Covariation between personalities and individual differences in coping with stress : converging evidence and hypotheses. Curr Zool. 56:728–741. [Google Scholar]
- Casper BM, Halvorsen MB, Matthews F, Carlson TJ, Popper AN. 2013. Recovery of barotrauma injuries resulting from exposure to pile driving sound in two sizes of hybrid striped bass. PLoS One. 8:e73844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfoun AD, Thompson FR, Ratnaswamy MJ. 2002. Nest predators and fragmentation: a review and meta-analysis. Conserv Biol. 16:306–318. [Google Scholar]
- Charette C, Derry AM. 2016. Climate alters intraspecific variation in copepod effect traits through pond food webs. Ecology. 97:1239–1250. [DOI] [PubMed] [Google Scholar]
- Chen I-H, Chou L-S, Chou S-J, Wang J-H, Stott J, Blanchard M, Jen I-F, Yang W-C. 2018. Sound exposure-induced cytokine gene transcript profile changes in captive bottlenose dolphin (Tursiops truncatus) blood identified by a probe-based qRT-PCR. J Vet Med Sci. 80:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8:e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciaruso MV, Batalha MA, Gaston KJ, Petchey OL. 2009. Including intraspecific variability in functional diversity. Ecology. 90:81–89. [DOI] [PubMed] [Google Scholar]
- Clarke JA. 1983. Moonlight’s influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behav Ecol Sociobiol. 13:205–209. [Google Scholar]
- Connon RE, Jeffries KM, Komoroske LM, Todgham AE, Fangue NA. 2018. The utility of transcriptomics in fish conservation. J Exp Biol. 221:jeb148833. [DOI] [PubMed] [Google Scholar]
- Côté IM, Darling ES, Brown CJ. 2016. Interactions among ecosystem stressors and their importance in conservation. Proc R Soc B. 283:20152592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K, Brennan LP, Gerwing TG, Dudas SE, Juanes F. 2018. Sound the alarm: a meta-analysis on the effect of aquatic noise on fish behavior and physiology. Glob Chang Biol. 24:3105–3116. [DOI] [PubMed] [Google Scholar]
- Cripps G, Lindeque P, Flynn KJ. 2014. Have we been underestimating the effects of ocean acidification in zooplankton? Glob Chang Biol. 20:3377–3385. [DOI] [PubMed] [Google Scholar]
- Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science. 313:966–968. [DOI] [PubMed] [Google Scholar]
- Dall SRX, Houston AI, McNamara JM. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett. 7:734–739. [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. 2011. Sex differences in response to stress and expression of depressive-like behaviours in the rat. In Neill JC, Kulkarni J, editors. Biological basis of sex differences in psychopharmacology. Heidelberg, Berlin: Springer-Verlag; p. 97–118. [DOI] [PubMed] [Google Scholar]
- Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol Lett. 11:1278–1286. [DOI] [PubMed] [Google Scholar]
- Davidson J, Bebak J, Mazik P. 2009. The effects of aquaculture production noise on the growth, condition factor, feed conversion, and survival of rainbow trout, Oncorhynchus mykiss. Aquaculture. 288:337–343. [Google Scholar]
- Davidson J, Frankel AS, Ellison WT, Summerfelt S, Popper AN, Mazik P, Bebak J. 2007. Minimizing noise in fiberglass aquaculture tanks: noise reduction potential of various retrofits. Aquac Eng. 37:125–131. [Google Scholar]
- Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Schweitzer JA, Palkovacs EP. 2017. The ecological importance of intraspecific variation. Nat Ecol Evol. 2:57–64. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. 2014. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 17:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Lavorel S, Chapin FS III, Tecco PA, Gurvich DE, Grigulis K. 2007. Functional diversity—at the crossroads between ecosystem functioning and environmental filter. In: Canadell JG, Pataki D, Pitelka L, editors. Terrestrial ecosystems in a changing world. Heidelberg, Berlin: Springer-Verlag; p. 81–90. [Google Scholar]
- Duckworth RA, Mendonca MT, Hill GE. 2001. A condition dependent link between testosterone and disease resistance in the house finch. Proc Biol Sci. 268:2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison WT, Southall BL, Clark CW, Frankel AS. 2012. A new context-based approach to assess marine mammal behavioral responses to anthropogenic sounds. Conserv Biol. 26:21–28. [DOI] [PubMed] [Google Scholar]
- Engås A, Løkkeborg S, Ona E, Soldal AV. 1996. Effects of seismic shooting on local abundance and catch rates of cod (Gadus morhua) and haddock (Melanogrammus aeglefinus). Can J Fish Aquat Sci. 53:2238–2249. [Google Scholar]
- Farcas A, Thompson PM, Merchant ND. 2016. Underwater noise modelling for environmental impact assessment. Environ Impact Assess Rev. 57:114–122. [Google Scholar]
- Fasola E, Ribeiro R, Lopes I. 2015. Microevolution due to pollution in amphibians: a review on the genetic erosion hypothesis. Environ Pollut. 204:181–190. [DOI] [PubMed] [Google Scholar]
- Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front Ecol Environ. 11:305–313. [Google Scholar]
- Francis CD, Kleist NJ, Ortega CP, Cruz A. 2012. Noise pollution alters ecological services: enhanced pollination and disrupted seed dispersal. Proc Biol Sci. 279:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RW, Smith LW, Park RL. 1988. Feeding and management of dairy heifers for optimal lifetime productivity. J Dairy Sci. 71:996–999. [DOI] [PubMed] [Google Scholar]
- Grüebler MU, Schuler H, Müller M, Spaar R, Horch P, Naef-Daenzer B. 2008. Female biased mortality caused by anthropogenic nest loss contributes to population decline and adult sex ratio of a meadow bird. Biol Conserv. 141:3040–3049. [Google Scholar]
- Guillette LM, Reddon AR, Hoeschele M, Sturdy CB. 2011. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc Biol Sci. 278:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson AR, Armstrong EJ, Stillman JH. 2016. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Ann Rev Mar Sci. 8:357–378. [DOI] [PubMed] [Google Scholar]
- Harding HR, Gordon TAC, Hsuan RE, Mackaness ACE, Radford AN, Simpson SD. 2018. Fish in habitats with higher motorboat disturbance show reduced sensitivity to motorboat noise. Biol Lett. 14:20180441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Thomas L, Falcone EA, Hildebrand J, Houser D, Kvadsheim PH, Lam FPA, O Miller PJ, Moretti DJ, Read AJ, et al. 2018. Marine mammals and sonar: dose–response studies, the risk-disturbance hypothesis and the role of exposure context. J Appl Ecol. 55:396–404. [Google Scholar]
- Haver SM, Gedamke J, Hatch LT, Dziak RP, Van Parijs S, McKenna MF, Barlow J, Berchok C, DiDonato E, Hanson B, et al. 2018. Monitoring long-term soundscape trends in U.S. Waters: the NOAA/NPS Ocean Noise Reference Station Network. Mar Policy. 90:6–13. [Google Scholar]
- Hawkins AD, Pembroke AE, Popper AN. 2015. Information gaps in understanding the effects of noise on fishes and invertebrates. Rev Fish Biol Fisheries. 25:39–64. [Google Scholar]
- Hildebrand JA. 2009. Anthropogenic and natural sources of ambient noise in the ocean. Mar Ecol Prog Ser. 395:5–20. [Google Scholar]
- Höglund E, Gjøen H-M, Pottinger TG, Øverli Ø. 2008. Parental stress-coping styles affect the behaviour of rainbow trout Oncorhynchus mykiss at early developmental stages. J Fish Biol. 73:1764–1769. [Google Scholar]
- Husté A, Clobert J, Miaud C. 2006. The movements and breeding site fidelity of the natterjack toad (Bufo calamita) in an urban park near Paris (France) with management recommendations. Amphib Reptil. 27:561–568. [Google Scholar]
- Jensen MP, Allen CD, Eguchi T, Bell IP, LaCasella EL, Hilton WA, Hof CAM, Dutton PH. 2018. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr Biol. 28:154–159. [DOI] [PubMed] [Google Scholar]
- Kenyon TN. 1996. Ontogenetic changes in the auditory sensitivity of damselfishes (Pomacentridae). J Comp Physiol A. 179:553–561. [Google Scholar]
- Kiffney PM, Clements WH. 1996. Size-dependent response of macroinvertebrates to metals in experimental streams. Environ Toxicol Chem. 15:1352–1356. [Google Scholar]
- Kight CR, Swaddle JP. 2011. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol Lett. 14:1052–1061. [DOI] [PubMed] [Google Scholar]
- Killen SS, Marras S, Mckenzie DJ. 2011. Fuel, fasting, fear: routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. J Anim Ecol. 80:1024–1033. [DOI] [PubMed] [Google Scholar]
- Koenigstein S, Mark FC, Gößling-Reisemann S, Reuter H, Poertner H-O. 2016. Modelling climate change impacts on marine fish populations: process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish. 17:972–1004. [Google Scholar]
- Kroodsma DE, Byers BE, Goodale E, Johnson S, Liu WC. 2001. Pseudoreplication in playback experiments, revisited a decade later. Anim Behav. 61:1029–1033. [Google Scholar]
- Kunc HP, McLaughlin KE, Schmidt R. 2016. Aquatic noise pollution: implications for individuals, populations, and ecosystems. Proc Biol Sci. 283:20160839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaZerte SE, Otter KA, Slabbekoorn H. 2017. Mountain chickadees adjust songs, calls and chorus composition with increasing ambient and experimental anthropogenic noise. Urban Ecosyst. 20:989–1000. [Google Scholar]
- LaZerte SE, Slabbekoorn H, Otter KA. 2016. Learning to cope: vocal adjustment to urban noise is correlated with prior experience in black-capped chickadees. Proc Biol Sci. 283:20161058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein JLL, Pruitt JN, Modlmeier AP. 2016. Intraspecific variation in collective behaviors drives interspecific contests in acorn ants. Behav Ecol. 27:553–559. [Google Scholar]
- Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 68:619–640. [Google Scholar]
- McBryan TL, Anttila K, Healy TM, Schulte PM. 2013. Responses to temperature and hypoxia as interacting stressors in fish: implications for adaptation to environmental change. Integr Comp Biol. 53:648–659. [DOI] [PubMed] [Google Scholar]
- McClellan-Green P, Romano J, Oberdörster E. 2007. Does gender really matter in contaminant exposure? A case study using invertebrate models. Environ Res. 104:183–191. [DOI] [PubMed] [Google Scholar]
- McClure CJW, Ware HE, Carlisle J, Kaltenecker G, Barber JR. 2013. An experimental investigation into the effects of traffic noise on distributions of birds: avoiding the phantom road. Proc Biol Sci. 280:20132290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M, Shannon G, Fristrup K. 2016. Characterizing anthropogenic noise to improve understanding and management of impacts to wildlife. Endanger Species Res. 31:279–291. [Google Scholar]
- Medina MH, Correa JA, Barata C. 2007. Micro-evolution due to pollution: possible consequences for ecosystem responses to toxic stress. Chemosphere. 67:2105–2114. [DOI] [PubMed] [Google Scholar]
- Mekonnen MM, Hoekstra AY. 2015. Global gray water footprint and water pollution levels related to anthropogenic nitrogen loads to fresh water. Environ Sci Technol. 49:12860–12868. [DOI] [PubMed] [Google Scholar]
- Merchant ND, Faulkner RC, Martinez R. 2018. Marine noise budgets in practice. Conserv Lett. 11:e12420. [Google Scholar]
- Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 16:254–260. [DOI] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press. [Google Scholar]
- Mimura M, Yahara T, Faith DP, Vázquez-Domínguez E, Colautti RI, Araki H, Javadi F, Núñez-Farfán J, Mori AS, Zhou S, et al. 2017. Understanding and monitoring the consequences of human impacts on intraspecific variation. Evol Appl. 10:121–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EV, Hartig F, Bell DM. 2016. Intraspecific trait variation across scales: implications for understanding global change responses. Glob Chang Biol. 22:137–150. [DOI] [PubMed] [Google Scholar]
- Morley EL, Jones G, Radford AN. 2014. The importance of invertebrates when considering the impacts of anthropogenic noise. Proc Biol Sci. 281:20132683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecol Lett. 16:1488–1500. [DOI] [PubMed] [Google Scholar]
- Nedelec SL, Campbell J, Radford AN, Simpson SD, Merchant ND. 2016. Particle motion: the missing link in underwater acoustic ecology. Methods Ecol Evol. 7:836–842. [Google Scholar]
- Nedelec SL, Mills SC, Radford AN, Beldade R, Simpson SD, Nedelec B, Côté IM. 2017a. Motorboat noise disrupts co-operative interspecific interactions. Sci Rep. 7:6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec SL, Radford AN, Pearl L, Nedelec B, Mccormick MI, Meekan MG, Simpson SD. 2017b. Motorboat noise impacts parental behaviour and offspring survival in a reef fish. Proc Biol Sci. 284: 20170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec SL, Radford AN, Simpson SD, Nedelec B, Lecchini D, Mills SC. 2014. Anthropogenic noise playback impairs embryonic development and increases mortality in a marine invertebrate. Sci Rep. 4:5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek DP, Clark CW, Mann D, Miller PJ, Rosenbaum HC, Golden JS, Jasny M, Kraska J, Southall BL. 2015. Marine seismic surveys and ocean noise: time for coordinated and prudent planning. Front Ecol Environ. 13:378–386. [Google Scholar]
- Okumura T, Akamatsu T, Yan HY. 2002. Analyses of small tank acoustics: empirical and theoretical approaches. Bioacoustics. 12:330–332. [Google Scholar]
- Oney B, Reineking B, O’Neill G, Kreyling J. 2013. Intraspecific variation buffers projected climate change impacts on Pinus contorta. Ecol Evol. 3:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Santaliestra ME, Marco A, Fernández MJ, Lizana M. 2006. Influence of developmental stage on sensitivity to ammonium nitrate of aquatic stages of amphibians. Environ Toxicol Chem. 25:105–111. [DOI] [PubMed] [Google Scholar]
- Ottersen G, Hjermann DØ, Stenseth NC. 2006. Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish Oceanogr. 15:230–243. [Google Scholar]
- Palanza P, Parmigiani S. 2017. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci Biobehav Rev. 76:134–143. [DOI] [PubMed] [Google Scholar]
- Paris JR, King RA, Stevens JR. 2015. Human mining activity across the ages determines the genetic structure of modern brown trout (Salmo trutta L.) populations. Evol Appl. 8:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JL, Blunt BR. 2005. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ Toxicol. 20:131–141. [DOI] [PubMed] [Google Scholar]
- Pespeni MH, Sanford E, Gaylord B, Hill TM, Hosfelt JD, Jaris HK, LaVigne M, Lenz EA, Russell AD, Young MK, et al. 2013. Evolutionary change during experimental ocean acidification. Proc Natl Acad Sci. 110:6937–6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine MK, Schmitt P, Culloch RM, Lieber L, Kregting LT. 2019. Providing ecological context to anthropogenic subsea noise: assessing listening space reductions of marine mammals from tidal energy devices. Renew Sustain Energy Rev. 103:49–57. [Google Scholar]
- Planque B, Fromentin J-M, Cury P, Drinkwater KF, Jennings S, Perry RI, Kifani S. 2010. How does fishing alter marine populations and ecosystems sensitivity to climate? J Mar Syst. 79:403–417. [Google Scholar]
- Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 315:95–97. [DOI] [PubMed] [Google Scholar]
- Post DM, Palkovacs EP, Schielke EG, Dodson SI. 2008. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology. 89:2019–2032. [DOI] [PubMed] [Google Scholar]
- Pottinger TG, Carrick TR, Hughes SE, Balm PHM. 1996. Testosterone, 11-ketotestosterone, and estradiol-17β modify baseline and stress-induced interrenal and corticotropic activity in trout. Gen Comp Endocrinol. 104:284–295. [DOI] [PubMed] [Google Scholar]
- Potvin DA, Macdougall-Shackleton SA. 2015. Traffic noise affects embryo mortality and nestling growth rates in captive zebra finches. J Exp Zool. 323:722–730. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Cote J, Ferrari MCO. 2012. Behavioural trait variants in a habitat-forming species dictate the nature of its interactions with and among heterospecifics. Funct Ecol. 26:29–36. [Google Scholar]
- Purser J, Bruintjes R, Simpson SD, Radford AN. 2016. Condition-dependent physiological and behavioural responses to anthropogenic noise. Physiol Behav. 155:157–161. [DOI] [PubMed] [Google Scholar]
- Quadros S, Goulart VDL, Passos L, Vecci MAM, Young RJ. 2014. Zoo visitor effect on mammal behaviour: does noise matter? Appl Anim Behav Sci. 156:78–84. [Google Scholar]
- Radford AN, Lèbre L, Lecaillon G, Nedelec SL, Simpson SD. 2016b. Repeated exposure reduces the response to impulsive noise in European seabass. Glob Chang Biol. 22:3349–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford AN, Purser J, Bruintjes R, Voellmy IK, Everley KA, Wale MA, Holles S, Simpson SD. 2016a. Beyond a simple effect: variable and changing responses to anthropogenic noise. In: Popper AN, Hawkins A, editors. Effects of noise on aquatic life II. Advances in experimental medicine and biology. New York, NY: Springer; p. 901–907. [DOI] [PubMed] [Google Scholar]
- Raffard A, Santoul F, Cucherousset J, Blanchet S. 2019. The community and ecosystem consequences of intraspecific diversity: a meta-analysis. Biol Rev. 94:648–661. [DOI] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol Rev. 82:291–318. [DOI] [PubMed] [Google Scholar]
- Relyea RA, Mills N. 2001. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proc Natl Acad Sci USA. 98:2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossington K, Benson T, Lepper P, Jones D. 2013. Eco-hydro-acoustic modeling and its use as an EIA tool. Mar Pollut Bull. 75:235–243. [DOI] [PubMed] [Google Scholar]
- Royauté R, Pruitt JN. 2015. Varying predator personalities generates contrasting prey communities in an agroecosystem. Ecology. 96:2902–2911. [DOI] [PubMed] [Google Scholar]
- Rudman SM, Rodriguez-Cabal MA, Stier A, Sato T, Heavyside J, El-Sabaawi RW, Crutsinger GM. 2015. Adaptive genetic variation mediates bottom-up and top-down control in an aquatic ecosystem. Proc Biol Sci. 282:20151234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet SS, Wesdorp K, van Dooren D, Slabbekoorn H. 2016. Sound affects behavior of captive zebrafish: always consider the potential for acoustic effects on your laboratory tests. Proc Mtgs Acoust. 27:010010. [Google Scholar]
- Sebens KP. 1987. The ecology of indeterminate growth in animals. Annu Rev Ecol Syst. 18:371–407. [Google Scholar]
- Shannon G, Mckenna MF, Angeloni LM, Crooks KR, Fristrup KM, Brown E, Warner KA, Nelson MD, White C, Briggs J, et al. 2015. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol Rev. 91:982–1005. [DOI] [PubMed] [Google Scholar]
- Shepherdson DJ, Carlstead KC, Wielebnowski N. 2004. Cross-institutional assessment of stress responses in zoo animals using longitudinal monitoring of faecal corticoids and behaviour. Anim Welf. 13:S105–S113. [Google Scholar]
- Shine R. 1988. The evolution of large body size in females: a critique of Darwin’s “fecundity advantage” model. Am Nat. 131:124–131. [Google Scholar]
- Siefert A, Ritchie ME. 2016. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. Oecologia. 181:245–255. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q Rev Biol. 79:241–277. [DOI] [PubMed] [Google Scholar]
- Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol Lett. 15:278–289. [DOI] [PubMed] [Google Scholar]
- Simpson SD, Radford AN, Nedelec SL, Ferrari MCO, Chivers DP, McCormick MI, Meekan MG. 2016. Anthropogenic noise increases fish mortality by predation. Nat Commun. 7:10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsch U. 1992. Sex-biassed site fidelity and orientation behaviour in reproductive natterjack toads (Bufo calamita). Ethol Ecol Evol. 4:15–32. [Google Scholar]
- Skinner MK. 2015. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biol Evol. 7:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H. 2016. Aiming for progress in understanding underwater noise impact on fish: complementary need for indoor and outdoor studies. In: Popper AN, Hawkins AD, editors. Effects of noise on aquatic life II. Advances in experimental medicine and biology. New York, NY: Springer; p. 1057–1065. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol. 25:419–427. [DOI] [PubMed] [Google Scholar]
- Sokolova IM. 2013. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr Comp Biol. 53:597–608. [DOI] [PubMed] [Google Scholar]
- Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. 2012. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res. 79:1–15. [DOI] [PubMed] [Google Scholar]
- de Soto NA, Delorme N, Atkins J, Howard S, Williams J, Johnson M. 2013. Anthropogenic noise causes body malformations and delays development in marine larvae. Sci Rep. 3:2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LB, Ainley DG, Ribic CA. 1996. Incidence of plastic in seabirds from the tropical Pacific, 1984–91: relation with distribution of species, sex, age, season, year and body weight. Mar Environ Res. 40:123–146. [Google Scholar]
- Stearns SS. 1997. The evolution of life histories. Oxford: Oxford University Press. [Google Scholar]
- Valladares F, Matesanz S, Guilhaumon F, Araújo MB, Balaguer L, Benito-Garzón M, Cornwell W, Gianoli E, van Kleunen M, Naya DE, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett. 17:1351–1364. [DOI] [PubMed] [Google Scholar]
- Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature. 416:389–395. [DOI] [PubMed] [Google Scholar]
- Wecke C, Liebert F. 2013. Improving the reliability of optimal in-feed amino acid ratios based on individual amino acid efficiency data from N balance studies in growing chicken. Animals. 3:558–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JW, Cole BJ, Cherr GN, Connon RE, Brander SM. 2017. Scaling up endocrine disruption effects from individuals to populations: outcomes depend on how many males a population needs. Environ Sci Technol. 51:1802–1810. [DOI] [PubMed] [Google Scholar]
- Wong BBM, Candolin U. 2015. Behavioral responses to changing environments. Behav Ecol. 26:665–673. [Google Scholar]
- Wright KJ, Higgs DM, Leis JM. 2011. Ontogenetic and interspecific variation in hearing ability in marine fish larvae. Mar Ecol Prog Ser. 424:1–13. [Google Scholar]
- Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu Rev Ecol Syst. 32:95–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.