Summary
Background
Since 2014, England has seen increased scarlet fever activity unprecedented in modern times. In 2016, England's scarlet fever seasonal rise coincided with an unexpected elevation in invasive Streptococcus pyogenes infections. We describe the molecular epidemiological investigation of these events.
Methods
We analysed changes in S pyogenes emm genotypes, and notifications of scarlet fever and invasive disease in 2014–16 using regional (northwest London) and national (England and Wales) data. Genomes of 135 non-invasive and 552 invasive emm1 isolates from 2009–16 were analysed and compared with 2800 global emm1 sequences. Transcript and protein expression of streptococcal pyrogenic exotoxin A (SpeA; also known as scarlet fever or erythrogenic toxin A) in sequenced, non-invasive emm1 isolates was quantified by real-time PCR and western blot analyses.
Findings
Coincident with national increases in scarlet fever and invasive disease notifications, emm1 S pyogenes upper respiratory tract isolates increased significantly in northwest London in the March to May period, from five (5%) of 96 isolates in 2014, to 28 (19%) of 147 isolates in 2015 (p=0·0021 vs 2014 values), to 47 (33%) of 144 in 2016 (p=0·0080 vs 2015 values). Similarly, invasive emm1 isolates collected nationally in the same period increased from 183 (31%) of 587 in 2015 to 267 (42%) of 637 in 2016 (p<0·0001). Sequences of emm1 isolates from 2009–16 showed emergence of a new emm1 lineage (designated M1UK)—with overlap of pharyngitis, scarlet fever, and invasive M1UK strains—which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. Median SpeA protein concentration in supernatant was nine-times higher among M1UK isolates (190·2 ng/mL [IQR 168·9–200·4]; n=10) than M1global isolates (20·9 ng/mL [0·0–27·3]; n=10; p<0·0001). M1UK expanded nationally to represent 252 (84%) of all 299 emm1 genomes in 2016. Phylogenetic analysis of published datasets identified single M1UK isolates in Denmark and the USA.
Interpretation
A dominant new emm1 S pyogenes lineage characterised by increased SpeA production has emerged during increased S pyogenes activity in England. The expanded reservoir of M1UK and recognised invasive potential of emm1 S pyogenes provide plausible explanation for the increased incidence of invasive disease, and rationale for global surveillance.
Funding
UK Medical Research Council, UK National Institute for Health Research, Wellcome Trust, Rosetrees Trust, Stoneygate Trust.
Introduction
Scarlet fever is a classic exanthem of childhood caused by the bacterium Streptococcus pyogenes (group A streptococcus) that, until the beginning of the 20th century, was associated with frequent loss of life among children.1 By the start of the 20th century, long before widespread use of antibiotics, the incidence and severity of scarlet fever had begun to fall, a phenomenon that remains largely unexplained.2 One potential (untestable) hypothesis is that the streptococcal bacteria causing the disease might have undergone a pathogenetic change that led to a reduction in the invasive and septic sequelae of scarlet fever.
Since the 1940s, scarlet fever has followed a seasonal springtime pattern—peaking between March and May while remaining less frequent throughout the rest of the year—without the major cyclical epidemics observed in the early 20th century.3 Surges in invasive infections can periodically follow a similar seasonal pattern for reasons that are incompletely understood. In 2014, England had an unexpected surge in scarlet fever infections, with over 15 000 disease notifications—a marked increase in incidence compared with previous decades.3, 4 Despite having a major impact on public health resources,3 the increase in infections was not associated with any rise in the incidence of invasive disease. Even greater seasonal upsurges of scarlet fever were observed in 2015, when there were over 17 000 notifications, and in 2016, when there were over 19 000 notifications.3 In the spring of 2016, there was a 1·5-times increase in the number of laboratory-confirmed invasive S pyogenes infections compared with that in the previous 5 years, coinciding with the peak in scarlet fever notifications.3, 5 The absence of any association between scarlet fever notifications and increased invasive infection notifications in 20143 led us to speculate that the association of scarlet fever with invasive disease in 2016 might be strain dependent.
Research in context.
Evidence before this study
In March to May of 2016, an unexpected elevation in notifications of invasive Streptococcus pyogenes infections in England was seen, coinciding with a national increase in notifications of seasonal scarlet fever (a paediatric exanthem also caused by S pyogenes). Since 2014, scarlet fever notifications in England have reached unexpectedly high levels, peaking between March and May each year, although notifications of invasive S pyogenes infections in 2014 were within expected limits, in contrast to 2016. We aimed to test the hypothesis that the link between scarlet fever and invasive infection patterns might be strain-related and, in the process, identified the emergence of a new M1T1 lineage. We searched PubMed for clinical and laboratory studies published before March 1, 2019, using the search terms “scarlet fever” and “upsurge” or “mortality”, as well as “emm1” or “M1T1” and “streptococcus”. We also searched using the terms “SpeA” or “scarlet fever toxin” or “erythrogenic toxin” and “streptococcus” and “regulation”. We identified studies describing recent and historical trends in scarlet fever incidence, studies that described trends in strain types causing invasive infections, and studies that linked SpeA to dominant strains. We also found studies of toxin expression that reported links with phage induction, growth phase regulation, transcriptional regulators, proteolysis, and host proteins as potential regulators.
Added value of this study
Our study provides a molecular explanation for the association between increased incidence of scarlet fever and increased incidence of invasive S pyogenes infections, by identifying an emergent lineage of M1T1 S pyogenes (M1UK) that expanded rapidly to become the largest single contributor to both non-invasive and invasive infections in 2016. The findings raise the possibility that historical associations between epidemic waves of scarlet fever and invasive infections might also have been linked to strain pathogenicity, in addition to general population susceptibility. Genomic analysis confirmed that the strains that cause scarlet fever are no different to those that cause streptococcal pharyngitis and rarer invasive infections. Increases in one disease could lead to increases in all, particularly if the lineage involved is highly pathogenic. The emergent lineage was characterised by a number of genetic changes that were predictive of increased production of SpeA, and this increased production was confirmed by laboratory testing. Although this might be just one of many changes in the new lineage, increased production of SpeA is predicted to enhance bacterial fitness, as suggested by the increasing dominance of the new lineage in comparison to older M1T1 strains in England. The work highlights that group A streptococcal lineages can differ in pathogenicity.
Implications of all the available evidence
Scarlet fever notifications in England in the period 2014–18 are the highest seen since 1960, and incidence in young children exceeds that reported in other countries. It is uncertain whether the increase in scarlet fever is a result of practice change or other population or environmental factors; the new lineage described is not implicated in the initial upsurge. However, there is a need to curtail this increasing trend. Interventions targeted at the population at risk have the potential to reduce the reservoir of S pyogenes that can seed more harmful invasive infections. Research to identify the most appropriate intervention is underway and practice guidelines for streptococcal pharyngitis might need to take strain variation and wider population effects into account. Increased S pyogenes disease activity could provide a platform for strain evolution and expansion, highlighting an unforeseen consequence of modern epidemics. The genetic changes in the emergent M1T1 clone require detailed laboratory investigation to understand the wider phenotypic changes that have occurred and the molecular basis for these, including transmissibility and response to treatment. It is not known whether the new lineage will recede in due course, as other lineages have done, or if it will remain dominant in the population, and surveillance is needed. Detection of new lineage representatives outside the UK underlines a need for global surveillance and increased vigilance if strains with increased fitness and altered phenotype are detected.
Microbiological surveillance of S pyogenes upper respiratory tract infections in England is constrained, as most doctors do not routinely take samples for bacteriological diagnosis of sore throat. However, all S pyogenes isolates cultured from samples submitted from the population of northwest London are collected and archived by the Imperial College Infection Biobank, allowing longitudinal study of strains causing both non-invasive and invasive infections. Alongside the national reference laboratory, which systematically archives invasive S pyogenes isolates from across the country, the collection provides unique insight into the relationship between upper respiratory tract isolates and isolates from rare, but more severe and invasive, infections.
S pyogenes can be serotyped or genotyped on the basis of the M antigen, which is encoded by the emm gene. Changes in disease incidence are sometimes characterised by expansion of specific emm genotypes.4 Using a combination of epidemiological and bacteriological approaches, we set out to identify the emm genotypes responsible for S pyogenes infections during the 2014–16 scarlet fever seasons regionally, then extended the study nationally, to identify bacterial determinants that might explain the observed increase in invasive disease due to S pyogenes in 2016.
Methods
S pyogenes notifications
Cases of suspected scarlet fever are notified by clinicians to Public Health England on the basis of symptoms and signs consistent with scarlet fever, with or without laboratory confirmation of S pyogenes infection. Scarlet fever has been notifiable since 1899 in England. Since 2010, cases of invasive S pyogenes infections have also been notifiable to Public Health England, in accordance with statutory regulations.
S pyogenes isolates and emm genotyping
All non-invasive S pyogenes isolates submitted to the Diagnostic Laboratory at Imperial College Healthcare National Health Service Trust (London, UK) between Jan 1, 2009 and Dec 31, 2013, and between March 1 and May 31 each year during 2014–16 were cultured and stored frozen in glycerol (sampling rationale is detailed in the appendix p 29). This laboratory serves northwest London, a population of roughly 2 million people, representing approximately 3% of the population of England. Clinical data were linked to isolates and anonymised in accordance with research ethics approval (number 06/Q0406/20). Isolates were emm genotyped (appendix p 29). Laboratories from England and Wales are requested to submit sterile site and invasive S pyogenes isolates to the national reference laboratory, where emm genotyping is done on all submitted isolates. All isolates were cultured on Columbia blood agar (Oxoid, Basingstoke, UK) or in Todd-Hewitt broth (Oxoid) at 37°C with 5% CO2.
Genome sequencing
All non-invasive emm1 S pyogenes isolates collected from northwest London from 2009 to 2016 were subject to genome sequencing (appendix p 29), as were invasive emm1 isolates collected from England and Wales from March to May of 2013 and 2016. Comparative genomic analysis of invasive isolates was done with new (2013 and 2016) and existing (2014–15)6 genome sequence data (appendix p 12–27). DNA from S pyogenes isolates was prepared, sequenced, analysed, and compared with published data from the UK (2007–12), North America, Nordic regions, and southeast Asia6, 7, 8, 9, 10, 11 (appendix pp 28–29). All new genome sequence data generated in this study have been submitted to the European Nucleotide Archive) under the accession numbers listed in the appendix (pp 8, 12).
Quantification of speA production
Transcript expression of speA, encoding streptococcal pyrogenic exotoxin A (SpeA; also known as scarlet fever or erythrogenic toxin A), was quantified by real-time PCR using a standard curve method. SpeA protein expression in 5 × concentrated overnight supernatants was assessed with western blotting, and compared with a recombinant SpeA standard (appendix pp 29, 30). For SpeA protein testing, one or two isolates per year (2009–15) per lineage (M1global vs M1UK) were randomly selected by lot, after excluding any strains with mutations in the two-component regulator covRS (also known as csrRS), which is known to repress a number of virulence factors, including SpeA.12
Statistical analysis
Datasets were compared with a two-tailed Mann Whitney U test for continuous data or a χ2 test for categorical data, using GraphPad Prism 5.0 software. p values less than 0·05 were considered to indicate statistical significance. To assess trends in invasive disease incidence, Poisson regression in Stata (version 15) was done using mid-year population denominators for England from the Office for National Statistics.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
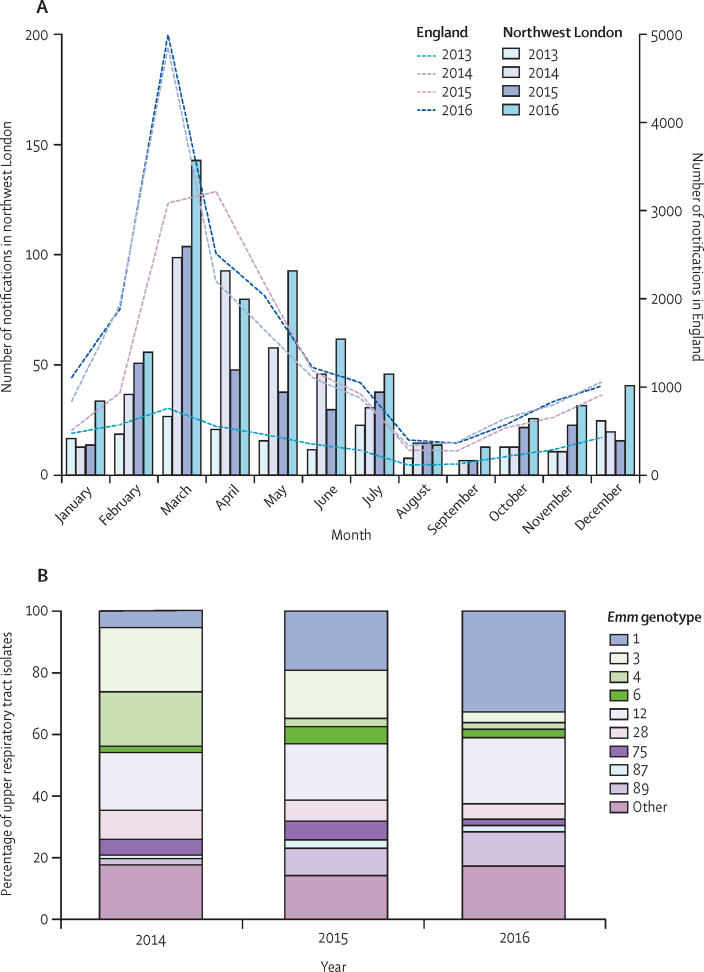
The northwest London population had a year-on-year rise in scarlet fever notifications between 2014 and 2016 that was representative of the country as a whole when compared with national notification data (figure 1A). We also compared notifications of invasive S pyogenes infections in northwest London with national data (appendix p 2), which showed a marked increase in S pyogenes invasive disease in 2016 in northwest London during the scarlet fever season that also mirrored the national pattern of increased notifications in 2016.
Figure 1.
2016 surge in scarlet fever associated with expansion of emm1 upper respiratory tract isolates of Streptococcus pyogenes
(A) Monthly notifications of scarlet fever in northwest London (bars) in 2013–16 showing the surge in notifications between March and May, peaking in 2016. National scarlet fever notifications (dashed lines) are shown for comparison. (B) emm genotyping of all upper respiratory tract isolates of S pyogenes from northwest London between March and May each year during 2014–16. emm1 strains emerged as the dominant upper respiratory tract genotype by 2016.
emm genotypes of all S pyogenes upper respiratory tract isolates obtained in northwest London during the scarlet fever seasons spanning 2015 and 2016 were ascertained and compared with existing data from 2014.4 emm1 upper respiratory tract strains increased in frequency year-on-year in the scarlet fever seasons, from five (5%) of 96 isolates in 2014, to 28 (19%) of 147 isolates in 2015 (p=0·0021 vs 2014 value), and 47 (33%) of 144 in 2016 (p=0·0080 vs 2015 value); in 2016, emm1 became the single most frequent S pyogenes genotype causing upper respiratory tract infections (figure 1B). The increase in emm1 strains in 2016 contrasted with the previously reported associations of emm3 and emm4 strains with the initial upsurge in scarlet fever in 2014.4 Brief clinical details were supplied for 243 of 387 upper respiratory tract isolates overall, among which 53 (22%) mentioned scarlet fever. Of these scarlet fever-associated isolates, those genotyped as emm1 increased from zero (0%) of 17 in 2014, to four (24%) of 17 in 2015, and seven (37%) of 19 in 2016 (p=0·0053 vs 2014 value).
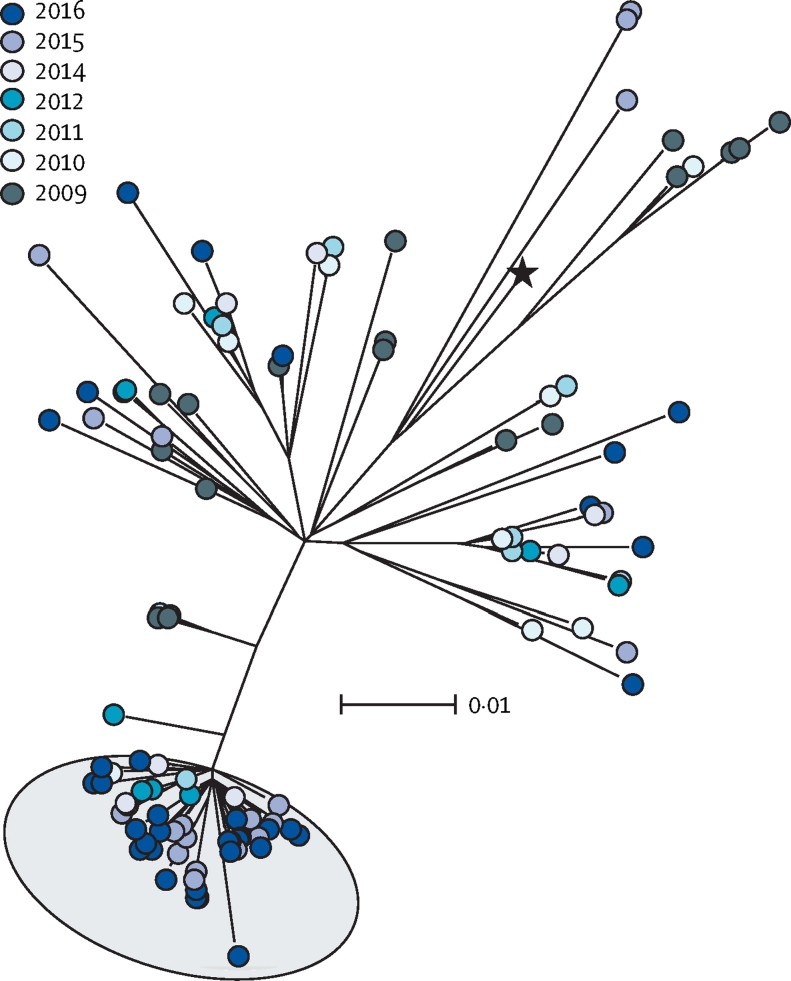
To identify any genetic basis for the expansion of emm1 S pyogenes among upper respiratory tract isolates collected in London, the genomes of all non-invasive emm1 isolates available from northwest London from 2009 to 2016 were sequenced (n=135). Single-nucleotide polymorphism (SNP)-based analysis of emm1 strains pointed to the emergence of a new emm1 lineage (designated M1UK), which could be differentiated from the contemporary emm1 population (M1global) by the presence of 27 core SNPs in regulatory and metabolic genes (figure 2, appendix p 7). The earliest member of the M1UK lineage was identified in 2010, and five intermediate isolates (with 13 or 23 of the unique SNPs) were detected between 2009 and 2012 (appendix p 3). Similarly to M1global, M1UK isolates were identified among all age groups, but included more cases of scarlet fever and evidence of recent transmissions than M1global (appendix p 3). From 2015 onwards, around two-thirds of non-invasive emm1 isolates from northwest London were within the new M1UK lineage (22 [71%] of 31 isolates in 2015, and 30 [65%] of 46 in 2016). Recombination and pan-genome analyses provided no evidence for gain or loss of transferable elements when comparing M1global and M1UK strains. Lineage-specific acquisition of antimicrobial-resistance genes was not detected; evidence of the mefA and msrD macrolide-resistance locus was found in just one M1UK isolate, while eight M1global isolates possessed antimicrobial-resistance genes (one isolate with the mefA and msrD locus, one with the tetracycline-resistance gene tetM, and six with the aminoglycoside-resistance gene aph3).
Figure 2.
Emergence of new emm1 lineage among non-invasive Streptococcus pyogenes isolates
Maximum likelihood phylogenetic tree constructed from core single-nucleotide polymorphisms (excluding prophage regions) of emm1 non-invasive isolates collected in northwest London in 2009–16 (n=135). Background shading in grey indicates the emergent lineage M1UK. 52 (85%) of 61 strains within the emergent lineage were isolated in either 2015 or 2016. The scale bar indicates the nucleotide substitutions per site. The black star indicates the reference strain MGAS5005. See appendix (p 3) for rooted tree with available metadata.
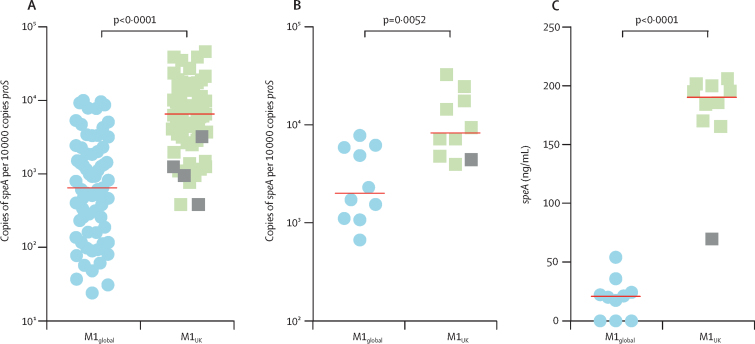
Scarlet fever is a toxin-mediated syndrome, historically associated with the expression of the phage-encoded erythrogenic toxin SpeA,13 the gene for which is possessed by all emm1 isolates studied in this investigation. Among the 27 M1UK lineage-defining SNPs, three non-synonymous mutations were identified in the gene rofA encoding the standalone transcriptional regulator RofA,14 which, alongside a homologue, nra, has been implicated as a repressor of SpeA production in some, but not all, streptococcal genotypes.15, 16 Real-time PCR analysis of all sequenced non-invasive emm1 isolates from northwest London (n=135) indicated that the emergent lineage (M1UK) was associated with significantly increased transcription of speA compared with other contemporary emm1 isolates (M1global), suggesting that differential SpeA production was a feature of the M1UK lineage (figure 3A).
Figure 3.
Expression of scarlet fever toxin SpeA in emergent M1UK isolates and other emm1 isolates
Phenotypic comparison of emergent M1UK isolates with M1global strains. (A) Quantification of absolute copy number of speA transcripts relative to house-keeping gene proS in all genome-sequenced non-invasive isolates from northwest London (n=135). Isolates were assigned to each lineage on the basis of SNPs (appendix pp 7–11). Quantification of speA transcript expression (B) and SpeA protein concentration in supernatant (C) from randomly selected M1UK and M1global strains lacking any mutations in covRS (n=10 per group). Median values are shown by horizontal lines in each graph. p values are from Mann-Whitney U tests. Grey squares denote strains that are intermediate members of the M1UK lineage. SpeA=streptococcal pyrogenic exotoxin type A.
To ascertain whether this difference in transcription translated into a difference in SpeA protein expression, we compared ten M1UK (including one intermediate strain harbouring only 13 of the 27 lineage-defining SNPs) and ten M1global isolates and corroborated our finding that M1UK isolates had enhanced speA transcription (figure 3B). Quantitative western blot analysis of SpeA protein in bacterial overnight culture supernatant showed nine-times greater median SpeA production in the emergent M1UK lineage (190·2 ng/mL [IQR 168·9–200·4]) than in the M1global strains (20·9 ng/mL [0·0–27·3]; p<0·0001; figure 3C). The M1UK strain representing an intermediate genotype (13 of 27 SNPs, including the rofA SNPs) did not show enhanced SpeA protein secretion (figure 3C).
The sequence for speA in non-invasive M1UK and M1global isolates was identical to that of the reference strain MGAS5005. Integration sites for the phage carrying speA in sequenced non-invasive M1UK and M1global isolates were consistent with those in the reference strain, and mapped sequences from M1UK and M1global isolates were very similar (if not identical) to the reference strain phage 5005.1; no evidence of translocated superantigens or duplication of speA was found.
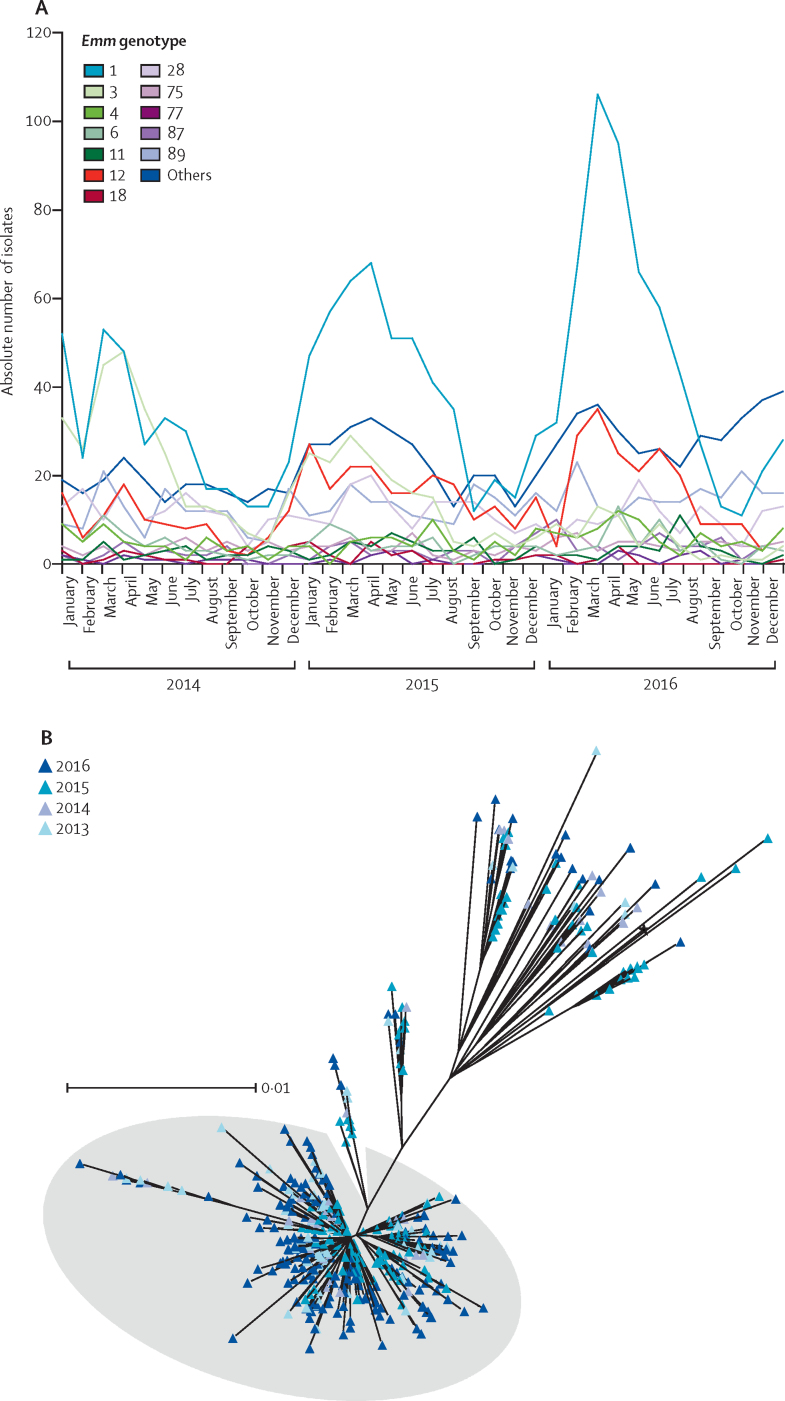
Although there was no rise in invasive disease notifications in 2014, the first year of elevated scarlet fever activity, a marked increase was seen nationally in the spring of 2016 compared with the same period (March to May) in the preceding 3 years (rate ratio 1·43 [95% CI 1·31–1·56], p<0·001; appendix p 2). Although invasive disease case numbers in northwest London were modest when considered on a monthly basis by comparison with national data (appendix p 2), the seasonal increase in notifications matched that observed nationally. emm genotyping of all invasive disease isolates referred to the national laboratory showed significant absolute and relative year-on-year increases in emm1, from 183 (31%) of 587 isolates between March and May 2015, to 267 (42%) of 637 in the same period in 2016 (p<0·0001 vs 2015 values), peaking March 2016 (figure 4A).
Figure 4.
Prevalence of emm1 strains among invasive Streptococcus pyogenes infections nationally during scarlet fever seasons and emergence of M1UK lineage over time
(A) emm genotypes of invasive S pyogenes isolates referred to national reference laboratory per month during 2014–16. Increases in total invasive disease cases were observed locally and nationally during March to May 2016 (appendix p 2). (B) Maximum likelihood phylogenetic tree constructed from core single-nucleotide polymorphisms (excluding prophage regions) of genome-sequenced invasive emm1 S pyogenes isolates in England and Wales (n=552) between March and May each year during 2013–16. Shading in grey indicates the emergent lineage M1UK. Clustering was not observed based on geographical origin, indicating emergence of the lineage on a national level. The black star indicates the reference strain MGAS5005. The scale bar represents the number of nucleotide substitutions per site.
To ascertain whether invasive S pyogenes infections might be affected by the emergent M1UK lineage, we compared the genome sequences of 552 invasive emm1 isolates (appendix p 12–27) in England and Wales from March to May each year during 2013–16. Focusing first on London, where sequence data from non-invasive isolates were available for comparison, SNP-based phylogenetic analysis showed intermingling of the 31 sterile-site invasive and 135 non-invasive isolates; 16 (84%) of 19 invasive strains obtained in 2015 and 2016 lay within the emergent M1UK lineage, compared with just five (42%) of 12 obtained between 2013 and 2014 (appendix p 4). Four (13%) of 31 invasive isolates were identical to, or no more than two SNPs different from, non-invasive isolates in the community, consistent with recent transmission.
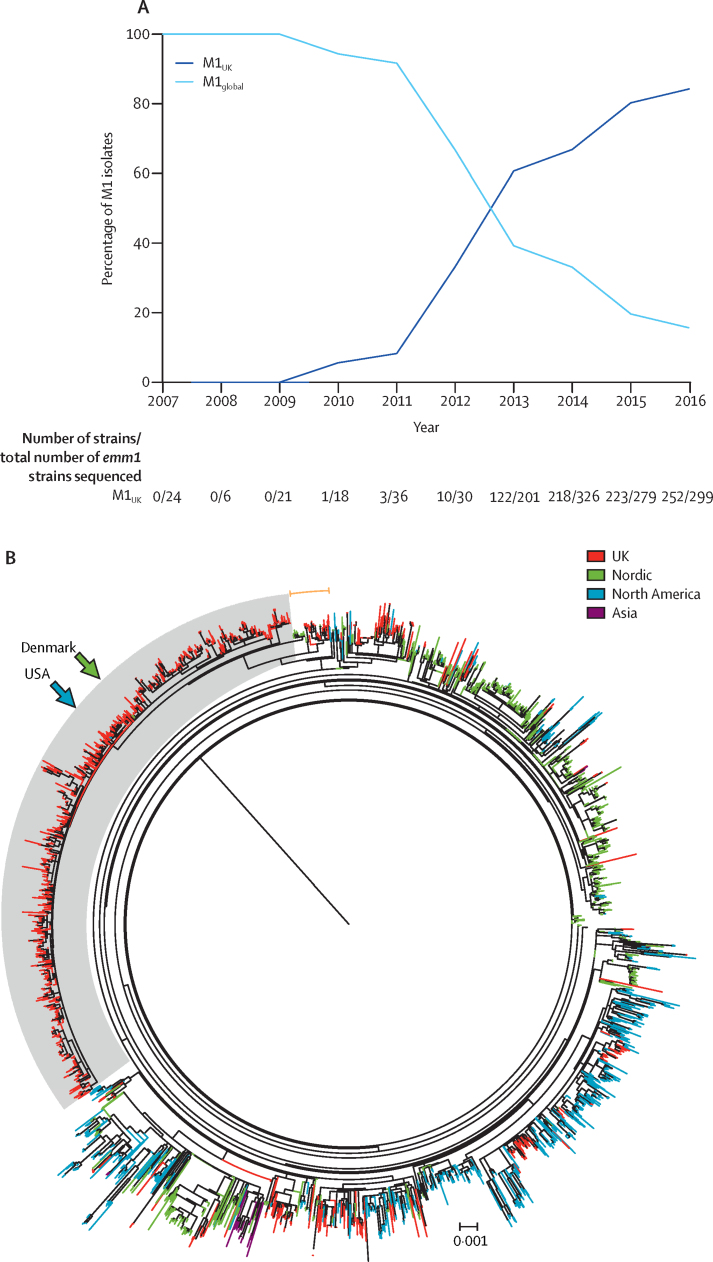
We then analysed the genome sequences of all 552 emm1 sterile site isolates collected between March and May each year from 2013 to 2016, obtained nationally from across different geographical locations in England and Wales. 425 (77%) of 552 invasive emm1 strains were within the new lineage (figure 4B), which was present in the UK invasive isolate population as early as 2013. Like M1global strains, M1UK strains were phylogenetically distinct from the historical UK emm1 scarlet fever speA-positive isolate NCTC8198 and emm1 speC-positive SF370 (appendix p 5). Longitudinal analysis of all available 1240 UK emm1 sequences (appendix p 28)6, 7, 8 obtained from invasive and non-invasive disease cases showed a yearly increase in M1UK such that, by 2016, M1UK strains represented more than 80% of all available emm1 isolates in the UK, outnumbering M1global strains (figure 5A).
Figure 5.
Longitudinal (A) and geographical (B) comparison of M1UK lineage with pandemic emm1 strains of Streptococcus pyogenes
(A) Proportions of M1UK and M1global isolates among total sequenced invasive and non-invasive emm1 S pyogenes isolates (n=1240) annually in the UK between 2007 and 2016. (B) M1UK lineage in a global context. Maximum likelihood phylogenetic tree constructed from core SNPs (excluding prophage regions) comparing all sequenced UK emm1 isolates with the global emm1 populations from North America, Nordic countries, and Asia (n=2800 isolates). Shading in grey indicates the emergent lineage M1UK; orange arc indicates intermediate isolates that lie outside M1UK but possess 13 or more of the 27 SNPs present in M1UK, including three SNPs in rofA. UK and international emm1 isolates arise throughout the tree, but isolates within the M1UK lineage are exclusively from the UK, except two single isolates from Denmark and the USA (arrows). The scale bar indicates the number of nucleotide substitutions per site. See appendix (p 6) for the unrooted tree. SNPs=single-nucleotide polymorphisms.
Phylogenetic comparison of UK emm1 sequences with available international sequences from North America, Nordic regions, UK, and southeast Asia (appendix p 28)9, 10, 11 confirmed that M1UK strains were distinct from the globally disseminated pandemic emm1 strains (figure 5B). A small number of intermediate isolates (possessing at least 13 of the 27 lineage-defining SNPs, including three SNPs in rofA) were identified in the UK and in Nordic countries, including Denmark (n=16), Finland (n=2), and Sweden (n=4; figure 5b, appendix p 6).10 M1UK isolates that possessed all 27 SNPs were, however, unique to the UK, except single sequences isolated in the USA in 20159 and in Denmark in 2012,10 underlining the potential of the new lineage to disseminate internationally (figure 5B, appendix p 6).
Discussion
The modern era of resurgent invasive S pyogenes infections was heralded by reports of virulent strains producing the scarlet fever toxin SpeA17 and subsequently dominated by the emm1 lineage.10 In this study, we showed that the originally polyclonal upsurge of scarlet fever in England has more recently been characterised by the emergence of a new emm1 S pyogenes lineage that produces significantly higher levels of SpeA than other contemporary emm1 strains. The new M1UK lineage showed an apparent fitness advantage within the population, manifest during the scarlet fever seasons of 2015 and 2016. Phylogenetic analysis showed the emergent lineage to be the dominant cause of invasive S pyogenes infections in England in 2016, and indicated that isolates from symptomatic throat infections and scarlet fever represent the major reservoir for invasive infections. The data support the hypothesis that transmission of virulent emm1 strains with enhanced ability to cause scarlet fever could underlie the contemporaneous rise in invasive S pyogenes disease.
An unprecedented year-on-year increase in scarlet fever notifications, the underlying basis for which remains unclear,3, 4 has been seen in England since 2014. Among children aged 1–4 years, the incidence of scarlet fever in 2018 reached 1488 per 100 000.18 The increase in scarlet fever activity has followed secular changes in factors such as household structure (including use of childcare) and health-care use,4 as well as health-care policies,19 although causal links have not been established. One unforeseen consequence of medical practice change was that the capacity to investigate such an upsurge was undermined by a reduction in diagnostic testing at the national level. In northwest London, however, where strains are collected for epidemiological analysis, a significant increase in genotype emm4 pharyngitis strains was observed during the 2014 upsurge in scarlet fever, while emm3 was the main genotype associated with physician-reported scarlet fever.4 emm1 was infrequent in 2014, contrasting with the increase we observed between 2015 and 2016, when genotype emm1 S pyogenes became the dominant cause of upper respiratory tract infections regionally, and invasive disease notifications nationally.
Our genome sequence analysis revealed the emergence of a new emm1 lineage, separated from all other emm1 strains by 27 unique core SNPs, including three within a gene encoding a potential SpeA regulator, RofA. SpeA is usually the only phage-encoded superantigen in contemporary emm1 S pyogenes, and has been implicated in the re-emergence of severe invasive S pyogenes infections in the 1980s.10, 17 Although the roles of any specific SNPs were not investigated in the current study, we hypothesise that increased SpeA production by M1UK strains might be an important contributor to the apparent fitness of the new lineage within the nasopharynx. As a phage-encoded superantigen, SpeA is hypothesised to trigger scarlet fever in susceptible children, and has been shown to permit nasopharyngeal infection in humanised models of murine streptococcal infection, plus potential induction of immunity when administered as a toxoid.20 SpeA can trigger B cell death and abrogate immunoglobulin secretion by the human tonsil, and might contribute to recurrent tonsillitis in children.21, 22 Thus, production of SpeA might augment the ability of S pyogenes to cause scarlet fever and paediatric pharyngitis. Whether population immunity to SpeA will lead to an eventual decline in the lineage will be of interest to monitor.
Griffiths type 1 S pyogenes strains23 (later designated serotype M1; genotype emm1) were the first to be classically associated with scarlet fever in the early 20th century. Although the oldest emm1 scarlet fever reference strains dating from the 1920s possess and produce phage-encoded SpeA24, emm1 strains circulating later in the mid-20th century lacked phage-encoded SpeA.10 The epidemic success of M1T1 clonal emm1 strains of S pyogenes that subsequently emerged in the 1980s is attributed to acquisition of a more active NADase–streptolysin O locus, as well as acquisition of phage-encoded speA2, an allele of speA, and the DNAse-encoding gene sda.10 It is, therefore, surprising that these epidemic emm1 strains, forming the M1global lineage, apparently produce very little SpeA in vitro. The three SNPs identified in the rofA gene might contribute to the greater abundance of SpeA produced by the emergent M1UK lineage strains, but appear insufficient alone to account for this change. Gene regulation could be affected by a number of the additional SNPs implicated. A shift to enhanced SpeA expression in the M1UK lineage might represent just one component of a new phase in the evolution of emm1, although we have not yet explored other aspects of bacterial fitness.
Since the late 2000s, increased scarlet fever notifications have been reported in China,25 South Korea,26, 27 and Hong Kong.28 The M1UK lineage is distinct from emm1 sequences identified in Asia, where different, non-emm1 genotypes have been reported as upsurge-associated, and incidence in very young children does not approach that reported in England.25 Although the acquisitions of novel prophages and antimicrobial resistance have been highlighted as selective pressures in China and Hong Kong,28 no evidence to suggest that these are factors in England, or that the emergence of a single lineage was a trigger for the original upsurge in England, has been reported.3
Sampling of the non-invasive reservoir of S pyogenes in our study was limited to a single region of England, and single seasons in 2014–16; thus, we cannot be certain about whether the non-invasive isolate emm genotype or M1UK proportions altered outside these periods, and the analysis might be skewed through inadvertent inclusion of isolates from outbreaks. The collection used in this study, however, is the only systematic longitudinal collection of non-invasive strains available, and spans 2009–16. None of the samples included was identified as outbreak-associated, although clinical details were imperfect.
Groups of isolates differing by two SNPs or less were identified among non-invasive M1UK isolates, suggesting recent transmission, whereas no such groups were observed among the M1global non-invasive isolates, albeit these were from 2009–2014 when emm1 isolates were much less frequently identified. Whether the findings reflect inadvertent sampling of small outbreaks or greater outbreak potential cannot be addressed using these data.
Regardless of the drawbacks of regional sampling, the detection of a new lineage and increase in invasive emm1 infections prompted sequencing of invasive emm1 isolates referred to the national reference laboratory, which confirmed emergence of the same lineage at a national level, and excluded any artefact introduced by regional sampling. Our phylogenetic analysis of invasive isolates focused on the same season, in order to understand a rise in invasive disease, but published genome-sequenced invasive strains from the UK outside these months also showed emergence of the same lineage. Although the new lineage outcompeted the contemporary emm1 strains that were sequenced during the period studied, we do not know whether the lineage's success will endure.
Genetic analyses were limited by the nature of high-throughput short-read sequencing data, which do not provide fully assembled genome sequences for each strain and the phages therein; other lineage-specific differences might yet be identified using different approaches. Our study also did not address the mechanistic basis for fitness in the new lineage or the molecular basis for increased SpeA expression; links to specific SNPs are by association only, and detailed experimental work is underway to address such questions.
Scarlet fever is a visible and readily recognisable manifestation of S pyogenes infection that affects children aged 4–6 years.3, 4 Importantly, however, the surges observed in scarlet fever are accompanied by seasonal increases in streptococcal sore throat and tonsillitis in the wider population, also predominantly among children.29 Although we cannot rule out the possibility that the precursors (ie, intermediates) or founders of the M1UK lineage were imported, we speculate that a generalised increase in S pyogenes activity in the wider population—which coincided with England's scarlet fever upsurges—might have provided the conditions required for adaptation and expansion of emm1 S pyogenes. Whether the M1UK lineage will be suited to other environments is unknown; management of streptococcal sore throat differs greatly between countries,30 as do other important factors such as climate. We previously reported the emergence of a new emm89 lineage that had lost the capsule locus but gained an active NADase–streptolysin O locus, in addition to four other major recombination events.31 This emm89 lineage has now been identified across several continents.32 The identification of two members of the new M1UK lineage among isolates outside the UK underlines the potential of such lineages to spread globally. Compared with other genotypes, emm1 S pyogenes has a recognised, heightened association with invasive infections.9 The expansion of such a lineage within the community reservoir of S pyogenes might be sufficient to explain England's recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate. Wider national and global surveillance will provide clearer understanding of the lineage's geographical reach and longer-term fitness, and permit enhanced public health readiness where necessary.
Acknowledgments
Acknowledgments
This work was supported by grants from the UK Medical Research Council (grant number MR/P022669/1); the UK National Institute for Health Research (NIHR) Health Protection Unit in Healthcare Associated Infections and Antimicrobial Resistance (grant number HPRU-2012-10047); an NIHR Biomedical Research Centre grant (SpyVAC); and the Conor Kerin Foundation. NNL is a Sir Henry Wellcome Postdoctoral Research Fellow funded by the Wellcome Trust (grant number 103197/Z/13/Z). EJ is a Rosetrees/Stoneygate 2017 Imperial College Research Fellow (number M683). SS acknowledges the NIHR Biomedical Research Centre grant awarded to Imperial College and the Biomedical Research Centre Infection Bioresource. The authors are grateful to colleagues in the Imperial College National Health Service Healthcare Trust Diagnostic Laboratory and Respiratory and Vaccine-Preventable Bacteria Reference Unit, Public Health England, and Juliana Coelho and Rebecca Guy (National Infection Service, Public Health England).
Contributors
SS, EJ, NNL, CET, VC, and TL contributed to the conception of this project. HKL, XZ, MM, MP, MA, LL, EJ, and JP were responsible for collection of laboratory and genomic data. JYC, TL, and VC were responsible for epidemiological data. EJ and NNL interpreted and analysed data. EJ and CET undertook bioinformatics analysis of whole genome sequence data. SS, NNL, and EJ prepared the manuscript; NNL and EJ contributed equally. All authors contributed to the interpretation of results and critical review of the manuscript.
Declaration of interests
JP is a consultant to Next Gen Diagnostics LLC. All other authors declare no competing interests.
Supplementary Material
References
- 1.Krause RM. Evolving microbes and re-emerging streptococcal disease. Clin Lab Med. 2002;22:835–848. doi: 10.1016/s0272-2712(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 2.Duncan SR, Scott S, Duncan CJ. Modelling the dynamics of scarlet fever epidemics in the 19th century. Eur J Epidemiol. 2000;16:619–626. doi: 10.1023/a:1007645110006. [DOI] [PubMed] [Google Scholar]
- 3.Lamagni T, Guy R, Chand M. Resurgence of scarlet fever in England, 2014–16: a population-based surveillance study. Lancet Infect Dis. 2018;18:180–187. doi: 10.1016/S1473-3099(17)30693-X. [DOI] [PubMed] [Google Scholar]
- 4.Turner CE, Pyzio M, Song B. Scarlet fever upsurge in England and molecular-genetic analysis in north-west London, 2014. Emerg Infect Dis. 2016;22:1075–1078. doi: 10.3201/eid2206.151726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health England Group A streptococcal infections: fourth update on seasonal activity, 2015/2016. May 6, 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/521550/hpr1616_SF-GAS2.pdf
- 6.Kapatai G, Coelho J, Platt S, Chalker VJ. Whole genome sequencing of group A streptococcus: development and evaluation of an automated pipeline for emm gene typing. PeerJ. 2017;5 doi: 10.7717/peerj.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner CE, Dryden M, Holden MT. Molecular analysis of an outbreak of lethal postpartum sepsis caused by Streptococcus pyogenes. J Clin Microbiol. 2013;51:2089–2095. doi: 10.1128/JCM.00679-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner CE, Bedford L, Brown NM. Community outbreaks of group A streptococcus revealed by genome sequencing. Sci Rep. 2017;7 doi: 10.1038/s41598-017-08914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chochua S, Metcalf BJ, Li Z. Population and whole genome sequence based characterization of invasive group A streptococci recovered in the United States during 2015. MBio. 2017;8:e01422–e01517. doi: 10.1128/mBio.01422-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasser W, Beres SB, Olsen RJ. Evolutionary pathway to increased virulence and epidemic group A streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA. 2014;111:e1768–e1776. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Zakour NL, Davies MR, You Y. Transfer of scarlet fever-associated elements into the group A streptococcus M1T1 clone. Sci Rep. 2015;5 doi: 10.1038/srep15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson K, Schlievert PM, Selander RK, Musser JM. Characterization and clonal distribution of four alleles of the speA gene encoding pyrogenic exotoxin A (scarlet fever toxin) in Streptococcus pyogenes. J Exp Med. 1991;174:1271–1274. doi: 10.1084/jem.174.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogg GC, Gibson CM, Caparon MG. The identification of rofA, a positive-acting regulatory component of prtF expression: use of an m gamma delta-based shuttle mutagenesis strategy in Streptococcus pyogenes. Mol Microbiol. 1994;11:671–684. doi: 10.1111/j.1365-2958.1994.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 15.Beckert S, Kreikemeyer B, Podbielski A. Group A streptococcal rofA gene is involved in the control of several virulence genes and eukaryotic cell attachment and internalization. Infect Immun. 2001;69:534–537. doi: 10.1128/IAI.69.1.534-537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinari G, Rohde M, Talay SR, Chhatwal GS, Beckert S, Podbielski A. The role played by the group A streptococcal negative regulator Nra on bacterial interactions with epithelial cells. Mol Microbiol. 2001;40:99–114. doi: 10.1046/j.1365-2958.2001.02373.x. [DOI] [PubMed] [Google Scholar]
- 17.Stevens DL, Tanner MH, Winship J. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 18.Public Health England Group A streptococcal infections: seasonal activity, 2017/18: third report. April 13, 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/699729/hpr1318_sf-gas.pdf
- 19.National Institute for Health and Care Excellence Respiratory tract infections—antibiotic prescribing. Prescribing antibiotics for self-limiting respiratory tract infections in adults and children in primary care. July, 2008. https://www.nice.org.uk/guidance/cg69/evidence/full-guideline-pdf-196853293 [PubMed]
- 20.Kasper KJ, Zeppa JJ, Wakabayashi AT. Bacterial superantigens promote acute nasopharyngeal infection by Streptococcus pyogenes in a human MHC class II-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies FJ, Olme C, Lynskey NN, Turner CE, Sriskandan S. Streptococcal superantigen-induced expansion of human tonsil T cells leads to altered T follicular helper cell phenotype, B cell death and reduced immunoglobulin release. Clin Exp Immunol. 2019;197:83–94. doi: 10.1111/cei.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dan JM, Havenar-Daughton C, Kendric K. Recurrent group A streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant TFH cells. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith F. The serological classification of Streptococcus pyogenes. J Hyg (Lond) 1934;34:542–584. doi: 10.1017/s0022172400043308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriskandan S, Moyes D, Buttery LK. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 1996;173:1399–1407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Chan TC, Yap LW. Resurgence of scarlet fever in China: a 13-year population-based surveillance study. Lancet Infect Dis. 2018;18:903–912. doi: 10.1016/S1473-3099(18)30231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DW, Kim SH, Park JW. Incidence and characteristics of scarlet fever, South Korea, 2008–2015. Emerg Infect Dis. 2017;23:658–661. doi: 10.3201/eid2304.160773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Cheong HK. Increasing number of scarlet fever cases, South Korea, 2011–2016. Emerg Infect Dis. 2018;24:172–173. doi: 10.3201/eid2401.171027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies MR, Holden MT, Coupland P. Emergence of scarlet fever Streptococcus pyogenes emm12 clones in Hong Kong is associated with toxin acquisition and multidrug resistance. Nat Genet. 2015;47:84–87. doi: 10.1038/ng.3147. [DOI] [PubMed] [Google Scholar]
- 29.Royal College of General Practitioners Research & Surveillance Centre Weekly returns service annual report 2016–2017. https://www.rcgp.org.uk/clinical-and-research/our-programmes/research-and-surveillance-centre.aspx
- 30.Chiappini E, Regoli M, Bonsignori F. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther. 2011;33:48–58. doi: 10.1016/j.clinthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Turner CE, Abbott J, Lamagni T. Emergence of a new highly successful acapsular group A streptococcus clade of genotype emm89 in the United Kingdom. MBio. 2015;6 doi: 10.1128/mBio.00622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Olsen RJ, Nasser W. A molecular trigger for intercontinental epidemics of group A streptococcus. J Clin Invest. 2015;125:3545–3559. doi: 10.1172/JCI82478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.