Abstract
Background
Conduct disorder (CD), which is characterized by severe aggressive and antisocial behavior, is linked to emotion processing and regulation deficits. However, the neural correlates of emotion regulation are yet to be investigated in adolescents with CD. Furthermore, it remains unclear whether CD is associated with deficits in emotional reactivity, emotion regulation, or both.
Methods
We used functional magnetic resonance imaging to study effortful emotion regulation by cognitive reappraisal in 59 female adolescents 15 to 18 years of age (30 with a CD diagnosis and 29 typically developing (TD) control adolescents).
Results
Behaviorally, in-scanner self-report ratings confirmed successful emotion regulation within each group individually but significant group differences in emotional reactivity and reappraisal success when comparing the groups (CD < TD). Functional magnetic resonance imaging results revealed significantly lower activation in left dorsolateral prefrontal cortex and angular gyrus in CD compared with TD adolescents during emotion regulation, but no group differences for emotional reactivity. Furthermore, connectivity between left dorsolateral prefrontal cortex and the bilateral putamen, right prefrontal cortex, and amygdala was reduced in CD compared with TD adolescents during reappraisal. Callous-unemotional traits were unrelated to neural activation, but these traits correlated negatively with behavioral reports of emotional reactivity.
Conclusions
Our results demonstrate reduced prefrontal brain activity and functional connectivity during effortful emotion regulation in female adolescents with CD. This sheds light on the neural basis of the behavioral deficits that have been reported previously. Future studies should investigate whether cognitive interventions are effective in enhancing emotion-regulation abilities and/or normalizing prefrontal and temporoparietal activity in female adolescents with CD.
Keywords: Conduct disorder, Dorsolateral prefrontal cortex, Emotion processing, Emotion regulation, Female adolescents, fMRI
Adolescence is a time of profound changes, marked by an increase in emotionality and a growing need to acquire socioaffective skills, such as the ability to effectively regulate one’s emotions 1, 2. Emotion regulation skills provide a means of controlling the intensity, duration, or extent of the experience evoked by an emotional stimulus or situation 3, 4. Proficient emotion regulation is typically attained in late adolescence or early adulthood (5) and has been linked to better social functioning and psychological and physical health 4, 6. Deficient emotion regulation, in contrast, has been suggested to be associated with childhood psychopathologies, including conduct disorder (CD) 7, 8, 9.
CD is a psychiatric disorder of childhood and adolescence that is characterized by repetitive and persistent aggressive and nonaggressive behaviors that violate others’ basic rights or major age-appropriate societal norms (10). Overall, the lifetime prevalence of CD is estimated at 9.5% (11) but differs between the sexes [i.e., 12.0% for male and 7.1% for female adolescents (11)]. The origin of behaviors characteristic of CD [e.g., irritability, anger outbursts, or intense emotional responses (12)] may be underpinned by variations in emotional reactivity 13, 14 and/or emotion-regulation difficulties 8, 15. Considerable heterogeneity observed within CD adolescents has led to attempts to subtype these individuals according to specific features. Most prominently, variations in callous-unemotional (CU) traits, psychopathy, or levels of anxiety have been suggested to impact the behavioral and neural characteristics associated with CD 15, 16, 17, 18, 19.
The neural substrates of emotion regulation have been studied in healthy 20, 21 and clinical 22, 23, 24 populations. Within the neuroimaging literature, effortful emotion-regulation strategies, such as cognitive reappraisal, have been the most commonly studied and effective strategies 3, 21, and these are core targets of various intervention approaches 25, 26, 27. While different forms of emotion regulation exist, this paper focuses on effortful emotion regulation via cognitive reappraisal. Effortful emotion regulation by cognitive reappraisal follows an initial reaction to an emotional stimulus that activates affect-related brain regions, such as amygdala, insula, and striatum. Based on data from healthy adolescents and adults 27, 28, 29, two models of emotion regulation have been proposed (30). The mediation hypothesis of emotion regulation suggests that emotion regulation is achieved by prefrontal-subcortical mediation effects 28, 30. According to this hypothesis, emotion regulation may include both 1) the activation of brain regions associated with cognitive control mechanisms, attention, and response inhibition (e.g., bilateral ventrolateral and dorsolateral prefrontal cortex [vlPFC/dlPFC]) or temporoparietal brain regions (e.g., angular/middle temporal gyrus) and 2) the modulation, or downregulation, of affective regions [e.g., amygdala, insula, or ventral striatum 27, 28, 29]. Alternatively, the direct pathway hypothesis describes emotion regulation primarily through activity in prefrontal and cortical brain regions, without further involvement of subcortical systems 28, 30.
While neuroimaging studies have examined the neural correlates of effortful emotion regulation in psychiatric populations or those who experienced childhood adversity 23, 24, 31, no data exist yet on CD. Meta-analytic studies of adolescents with CD or disruptive behavior have revealed functional 32, 33, 34 and structural 33, 34, 35 alterations in brain regions implicated within the emotion processing and regulation network, including vlPFC/dlPFC, anterior cingulate cortex or temporal gyrus, limbic brain regions (e.g., amygdala), insula, and striatum. In line with previous work, it was further demonstrated that the neural phenotype of CD varies depending on the level of CU traits or presence of comorbid disorders 35, 36, 37. For example, CD adolescents with high CU traits show hyporeactivity to emotional stimuli as compared to CD adolescents with low CU traits 15, 32, 37, 38. Most research on CD to date has been conducted in male adolescents only 9, 32; substantially less is known about CD in female adolescents (39).
Here we aimed to bridge this gap in the literature by investigating the neural basis of emotion regulation in female adolescents with CD. In line with the mediation hypothesis of cognitive emotion regulation derived from normative data in typically developing (TD) control adolescents 27, 28, 29, 40, the known neural phenotype of CD 32, 33, 34, and reports of emotion processing and regulation deficits in CD (9), we expected to observe atypical neural activation during effortful emotion regulation in female adolescents with CD compared with in TD individuals. This would be reflected by the following hypotheses: 1) hypoactivation of prefrontal and cognitive control regions (e.g., dlPFC/vlPFC, anterior middle cingulate, superior temporal gyrus, and angular gyrus) in individuals with CD compared with in TD individuals; 2) deficient modulation of affect-related regions (amygdala, ventral striatum, insula), which would be demonstrated by continuing heightened activation of these regions despite attempts to regulate emotions; and/ or 3) no need to initiate emotion regulation because of initial differences in emotional reactivity and/or processing (i.e., reduced reactivity in female adolescents with CD and high CU traits).
Methods and Materials
Participants and Measures
All participants included were recruited as part of the Neurobiology and Treatment of Female Adolescent Conduct Disorder study (9) and were tested at 2 sites (Universities of Basel and Frankfurt). A total of 59 female adolescents 15 to 18 years of age with either a clinical diagnosis of CD (n = 30; average age = 16.28 years) or without a current clinical diagnosis (TD: n = 29; average age = 16.74 years) were included. We initially scanned 65 female participants 15 to18 years of age. In a first step, we excluded 5 participants to match groups for age, sex, site, and pubertal status, which may all impact the neural correlates of reappraisal 40, 41, 42 [pubertal status was measured through the Pubertal Development Scale (43)]. One TD participant was additionally excluded owing to neuroanatomical abnormalities. CD and common psychiatric disorders were assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (44). Of the participants in the CD group, 26 met the diagnostic criteria for current CD (≥3 CD symptoms; 14 of them had comorbid oppositional defiant disorder [ODD]), 3 had 2 current CD symptoms while meeting full ODD criteria, and 1 participant had 1 CD symptom plus current ODD. Analyses were repeated including just those participants who met full CD criteria (n = 26) and including only the larger of the 2 sites (24 CD, 17 TD from Basel) to exclude diagnosis-dependent or nonlinear effects of site on our functional magnetic resonance imaging (fMRI) results (Supplemental Figure S2). For all participants, exclusion criteria included IQ < 70 [Wechsler Intelligence Scale for Children-IV/Wechsler Adult Intelligence Scale-IV (45)], autism spectrum disorder, psychosis, any neurological or genetic disorder, and standard MRI exclusion criteria. Finally, all participants were asked to complete the self-report Youth Psychopathic Traits Inventory (46) to assess psychopathic and CU traits and the parent-report Child Behavior Checklist (47) (owing to time constraints, Child Behavior Checklist data were only available for 17 participants with CD and 27 TD participants). Local ethics committees reviewed and approved the study at each site (Ethics Committees of Northwest and Central Switzerland in Basel and the Medical Faculty of Goethe University Frankfurt). Adolescents and their parents or caregivers provided informed assent and consent, respectively.
fMRI Task
All participants completed an age-appropriate adaptation of an fMRI emotion-regulation task 3, 20, 21, 48 (see the task design and training protocol in the Supplement). Each experimental trial lasted 20,000 ms and started with a 2500-ms instruction cue, indicating whether the subjects will have to look at a neutral or negative picture or regulate emotions evoked by a negative emotional picture (i.e., decrease the emotions). Cognitive emotion regulation required the participants to actively reappraise the experienced negative emotion to reduce the intensity of their emotional state. More specifically, they were asked to reinterpret the situations and scenes observed to experience more positive feelings [see also 3, 20, 21, 48]. The instruction cue was followed by a negative or neutral image presented for 10,000 ms. Subsequently, in-scanner self-report ratings of the strength of the negative affect (on a Likert scale of 1 = lowest to 4 = highest; presented for 5000 ms) were collected, before a relaxation period of 2500 ms preceded the next trial. This design allows modeling of 3 experimental conditions: 1) look neutral (nonemotional), 2) look negative (no regulation), and 3) decrease negative (emotion regulation). Participants completed 48 trials in total (16 in each of the 3 conditions). The experiment was divided across 2 runs (∼8 minutes each).
Image Acquisition
Prior to data collection, both sites underwent site-qualification procedures and preassessments to ensure comparability of fMRI data [see protocols in the Supplement and in 49, 50]. Per run, 196 images were acquired with a 41-slice echo planar imaging interleaved sequence on a Siemens Prisma 3T scanner (Berlin, Germany) at the Basel site and a Siemens Trio 3T MR at the Frankfurt site. Image acquisition at both sites included the following specifications: repetition time = 2500 ms; echo time = 30 ms; flip angle = 83°; field of view = 192 mm; voxel size = 3 × 3 × 2 mm. The first 4 functional volumes were discarded from later analysis to account for T1-weighted equilibration effects.
fMRI Analysis
All fMRI data were analyzed using SPM12 (University College London, London, UK; http://www.fil.ion.ucl.ac.uk/ spm/doc/manual.pdf) running under MATLAB2018b (The MathWorks, Inc., Natick, MA). To account for movement artifacts, all images were first realigned and unwarped with reference to the first image. Next, each participant’s structural scan was used for the coregistration and segmentation functions prior to normalization into standard space (McConnell Brain Imaging Center ICBM 152 template). Finally, all images were smoothed using a 6-mm full width at half maximum isotropic kernel. Using the ART imaging toolbox (NeuroImaging Tools and Resources Collaboratory; https://www.nitrc.org/projects/artifact_detect/), 7 separate regressors accounting for motion and variations in mean signal intensity were added to the first-level model. Additional regressors omitting images with visible motion artifacts (51) were used to remove artifactual time points. Regressors of interest were created using a boxcar function for each experimental condition (look neutral, look negative, decrease negative), and contrasts of interest included emotional reactivity (look negative > look neutral) and emotion regulation (activation by emotion regulation: decrease negative > look negative; modulation by emotion regulation: decrease negative < look negative). Affect rating and relaxation periods were not included in the neuroimaging analyses.
At the second level, differences in blood oxygen level–dependent signal intensity change in response to contrasts of interest were assessed using random-effects group analyses. General linear models were constructed using site and IQ as covariates of no interest. Contrasts for emotional reactivity and emotion regulation were entered in 1-sample t tests assessing within-group activation as well as 2-sample t tests to compare CD and TD groups. Statistical significance is reported based on a cluster-building threshold of p < .001 and a small-volume familywise error correction for a priori–defined regions of interest (ROIs) (p < .05; peak-level inference). This small-volume correction was achieved by creating an individual mask including all affective and prefrontal ROIs previously implicated in emotion regulation [affective: automatic anatomical labeling-based bilateral insula/amygdala and a 10-mm-spherical ROI for ventral striatum; cognitive: 10-mm-spherical ROIs for bilateral dlPFC/vlPFC, anterior cingulate, and angular and left middle temporal gyrus; according to 27, 28, 29; further details in the Supplement]. To inform about areas not previously implicated in emotion regulation, regions surviving whole-brain familywise error correction (p < .05, cluster-building threshold p < .001; cluster-level inference) are additionally reported.
Post Hoc Analyses: ROI and Functional Connectivity
For post hoc assessments and displaying purposes, we further extracted mean parameter estimates in a priori–defined affective and cognitive ROIs (as described above and in the Supplement) for emotion regulation (decrease negative vs. look negative). To assess emotional reactivity, mean parameter estimates for emotional reactivity (look negative − look neutral) were extracted for affective ROIs. Two-sample t tests implemented in SPSS, version 25.0 (IBM Corp., Armonk, NY) and corrected for multiple comparisons were used to assess post hoc assessments of group differences within a priori–defined ROIs.
Modulation of subcortical areas by cortical regions during cognitive emotion regulation has been demonstrated by some, but not all previous neuroimaging studies 21, 30, 52. Thereby, prefrontal-amygdala connections are considered particularly important 52, 53. We followed up on this evidence by first directly testing correlations between activity in the amygdala and dlPFC (defined as independent anatomical ROIs) during emotion regulation. Second, we tested for additional regions that showed task-dependent functional correlations (coactivations) with neural activation in left angular gyrus and left dlPFC during emotion regulation using a weighted task-dependent and seed-based connectivity approach through the CONN toolbox [NeuroImaging Tools and Resources Collaboratory; https://web.conn-toolbox.org/ (54)]. The condition time series convolved with a canonical hemodynamic response function served as weights within the analysis. Temporal correlations between activation in left angular gyrus and left dlPFC seeds to all other brain voxels were computed using a general linear model approach (further details in the Supplement).
Self-reported Emotion Intensity and Emotion Regulation Success
Self-reports of in-scanner affect ratings on a 1 to 4 Likert scale directly after each trial were obtained via button press to assess group differences on a behavioral level: ratings for each condition (emotional reactivity [look negative − look neutral]) and emotion-regulation success (look negative − decrease negative condition). Differences between conditions within each group were evaluated using paired-samples t tests, whereas independent-samples t tests were used to compare the CD and TD groups.
Results
Descriptive Statistics
Female adolescents with CD had significantly lower total and verbal IQ than TD female adolescents, but there were no group differences in age, performance IQ, or pubertal status. Adolescents with CD, compared with TD adolescents, scored significantly higher in psychopathic and CU traits and externalizing and/or internalizing symptoms (Table 1; see Supplemental Table S1 for site distributions and effects).
Table 1.
Group Characteristics
| CD, Mean ± SD or n | TD, Mean ± SD or n | CD/TD, n | p, Sig. 2-tailed | |
|---|---|---|---|---|
| Age, Years | 16.28 ± 0.85 | 16.74 ± 0.99 | 30/29 | .061 |
| Handedness (Right/Left/Ambidextrous) | 22/2/3 | 21/8/0 | 27/29 | .893 |
| Pubertal Statusa | 4.2 | 4.4 | 29/29 | .133 |
| Age of Onset (Childhood/Adolescence), Years | 10/19 | — | 29/— | |
| IQ | ||||
| Performance IQ | 103.67 ± 13.64 | 105.34 ± 11.25 | 30/29 | .609 |
| Verbal IQ | 95.67 ± 16.70 | 106.72 ± 12.91 | 30/29 | .006 |
| Total IQ | 99.90 ± 12.95 | 106.24 ± 9.64 | 30/29 | .038 |
| Comorbidities (DSM-5) | ||||
| Attention-deficit/hyperactivity disorder | 7 | 0 | 29/26 | |
| Major depressive disorder | 5 | 0 | 28/26 | |
| Generalized anxiety disorder | 0 | 0 | 29/26 | |
| Posttraumatic stress disorder | 2 | 0 | 30/26 | |
| Alcohol dependence | 1 | 0 | 28/26 | |
| Substance dependence | 5 | 0 | 28/26 | |
| YPI | ||||
| Grandiose-manipulative | 39.24 ± 10.43 | 32.97 ± 8.90 | 29/29 | .017 |
| Callous-unemotional | 30.10 ± 6.97 | 24.52 ± 5.16 | 29/29 | .001 |
| Impulsive-irresponsible | 39.93 ± 7.71 | 30.93 ± 6.48 | 29/29 | <.001 |
| Total score | 109.28 ± 20.31 | 88.41 ± 15.30 | 29/29 | <.001 |
| CBCL | ||||
| Internalizing subscale | 65.47 ± 10.28 | 49.3 ± 10.62 | 17/27 | <.001 |
| Externalizing subscale | 71.12 ± 5.85 | 47.0 ± 7.84 | 17/27 | <.001 |
| Total score | 69.65 ± 6.97 | 48.00 ± 10.16 | 17/27 | <.001 |
Unless otherwise indicated, t tests nonsignificant at threshold p = .05. For IQ, standard scores are reported; for Youth Psychopathic Traits Inventory (YPI) and Child Behavior Checklist (CBCL), mean scores are reported.
CD, conduct disorder; Sig., significance; TD, typically developing.
Pubertal status was measured using the Pubertal Development Scale.
Self-report of Emotional Reactivity and Emotion Regulation Success
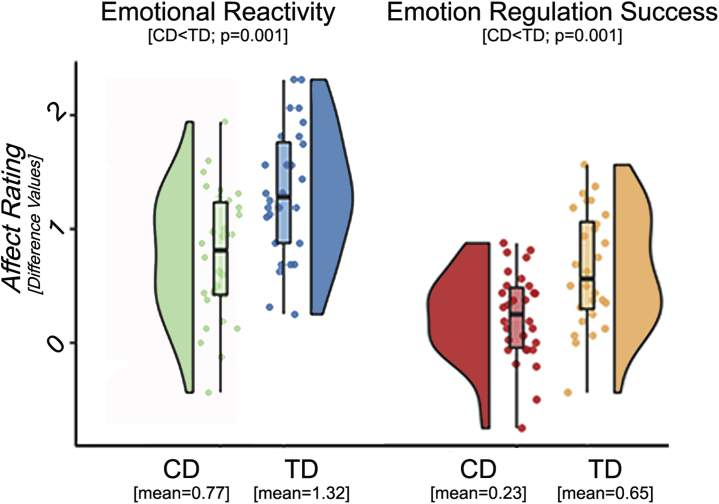
Relative to TD adolescents, adolescents with CD reported significantly lower scores for in-scanner emotional reactivity and emotion-regulation success (both p ≤ .001) (Figure 1). However, both adolescents with CD and TD adolescents displayed significant within-group emotional reactivity and regulation success (mean scores and detailed group statistics in Table 2).
Figure 1.
In-scanner affect ratings. Behavioral in-scanner reports as displayed using “raincloud” plots, which combine boxplots, raw jittered data, and a split-half “violin” [see (74)], revealed significantly lower ratings for female adolescents with conduct disorder (CD) compared with typically developing (TD) control adolescents in respect to emotional reactivity (difference for look negative − look neutral) and emotion regulation success (difference for look negative − decrease negative).
Table 2.
In-Scanner Affect Ratings
| Look Neutral | Look Negative | Decrease Negative | Emotion Intensity (Within Group; p Paired t / r) | Emotion-Regulation Success (Within Group; p Paired t / r) | |
|---|---|---|---|---|---|
| CD | 1.454 | 2.224 | 1.993 | 0.770 (<0.001 / .5961) | 0.231 (0.003 / .1839) |
| TD | 1.333 | 2.649 | 2.003 | 1.316 (<0.001 / .8036) | 0.646 (<0.001 / .5052) |
| CD vs. TD, p 2-Tailed | .221 (r = .1602) | .010 (r = −.3301) | .944 (r = −.0092) | .001 (r = −.4337) | .001 (r = −.4197) |
CD, conduct disorder; TD, typically developing.
fMRI Results
Within-Group (1-Sample t Tests)
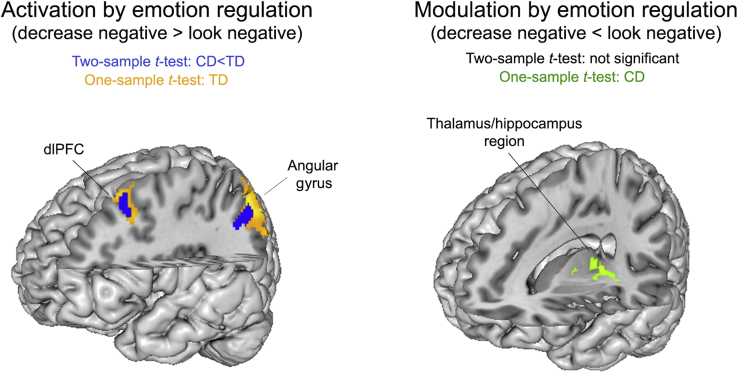
The neural correlates of emotion regulation (activation by emotion regulation: decrease negative > look negative) in TD adolescents corresponded to a network of brain regions previously linked to cognitive reappraisal 27, 28, 29, 40 and were reflected in significant activation of left angular gyrus and dlPFC (superior/middle frontal gyrus). No modulation by emotion regulation (decrease negative < look negative) of affect-associated regions was observed in the TD group. Adolescents with CD showed no significant activation increases during effortful emotion regulation, but they did show a significant decrease in activation in inferior orbitofrontal cortex, hippocampus/thalamus, and occipital cortex (Figure 2, Supplemental Table S2).
Figure 2.
Neural correlates of emotion regulation in and group differences between adolescents with conduct disorder (CD) and typically developing (TD) adolescents. Statistical parametric maps reflecting the neural correlates of emotion regulation. Activation by emotion regulation: (Left) Clusters that were significantly activated in the TD group are shown in orange-yellow; regions that were less active in participants with CD than in TD participants are shown in blue (based on a cluster-building p < .001 and small-volume familywise error correction of p < .05). (Right) Regions that were modulated during emotion regulation in the CD group are shown in green (based on an exploratory whole-brain search with a cluster-building p < .001 and familywise error–corrected p < .05). dlPFC, dorsolateral prefrontal cortex.
Between-Group (2-Sample t Tests)
Group comparisons revealed significantly lower activation during emotion regulation (decrease negative > look negative) in adolescents with CD, compared with TD adolescents, in left dlPFC (inferior/middle frontal gyrus) and angular gyrus (Figure 2, Table 3). There were no significant differences in activation between adolescents with CD and TD adolescents for emotional reactivity (look negative > look neutral). Our findings remained unchanged when analyses were repeated in an IQ-matched subsample or when restricted to include 1 site per diagnosis only (see Supplemental Figure S2 and the Supplement).
Table 3.
Peak Activation Reports for Activation and Modulation by Emotion Regulation
| Brain Region |
t | PFWE | Cluster Size (k) | MNI Coordinates |
||||
|---|---|---|---|---|---|---|---|---|
| Lobe | Area | Side | x | y | z | |||
| Small-Volume Correction | ||||||||
| TD: Activation by ER (Decrease Neg > Look Neg) | ||||||||
| Parietal | Angular gyrus, superior/inferior parietal lobe | L | 5.57 | .006 | 327 | −46 | −56 | 40 |
| Frontal | Superior/middle frontal gyrus (dlPFC) | L | 4.71 | .038 | 231 | −30 | 18 | 42 |
| TD: Modulation by ER (Look Neg > Decrease Neg) | NS | |||||||
| CD: Activation by ER (Decrease Neg > Look Neg) | NS | |||||||
| CD: Modulation by ER (Look Neg > Decrease Neg) | NS | |||||||
| TD > CD: Activation by ER (Decrease Neg > Look Neg) | ||||||||
| Parietal | Angular gyrus, mid occipital/inferior parietal lobe | L | 4.85 | .009 | 129 | −30 | 18 | 40 |
| Frontal | Inferior/middle frontal gyrus (dlPFC) | L | 4.55 | .023 | 118 | −40 | −58 | 36 |
| TD > CD: Modulation by ER (Look Neg > Decrease Neg) | NS | |||||||
| Whole-Brain Correction | ||||||||
| TD: Activation by ER (Decrease Neg > Look Neg) | ||||||||
| Parietal | Angular gyrus, superior/inferior parietal, middle occipital lobe | L | 5.57 | <.001 | 972 | −46 | −56 | 40 |
| Frontal | Middle/superior frontal lobe | L | 4.71 | .028 | 385 | −30 | 18 | 42 |
| TD: Modulation by ER (Look Neg > Decrease Neg) | NS | |||||||
| CD: Activation by ER (Decrease Neg > Look Neg) | NS | |||||||
| CD: Modulation by ER (Look Neg > Decrease Neg) | ||||||||
| Frontal | Olfactory, anterior cingulate, caudate, putamen, OFC | L/R | 6.57 | .012 | 180 | −18 | 30 | −6 |
| Temporal | Thalamus, hippocampus, lingual, precuneus, parahippocampus | L/R | 6.56 | <.001 | 412 | −6 | −24 | 6 |
| Occipital | Fusiform gyrus, inferior/middle occipital gyrus, lingual, calcarine | R | 4.96 | .003 | 238 | 30 | −84 | −10 |
| Occipital | Middle, inferior, superior occipital lobe | L | 4.57 | .008 | 195 | −22 | −98 | 8 |
| TD > CD: Activation by ER (Decrease Neg > Look Neg) | ||||||||
| Parietal | Angular, inferior parietal, mid occipital lobe | L | 4.64 | .046 | 231 | −38 | −58 | 34 |
| TD > CD: Modulation by ER (Look Neg > Decrease Neg) | NS | |||||||
CD, conduct disorder; dlPFC, dorsolateral prefrontal cortex; ER, emotion regulation; FWE, familywise error; L, left; MNI, Montreal Neurological Institute; Neg, negative; NS, not significant; OFC, orbitofrontal cortex; R, right; TD, typically developing.
Post Hoc ROI Results
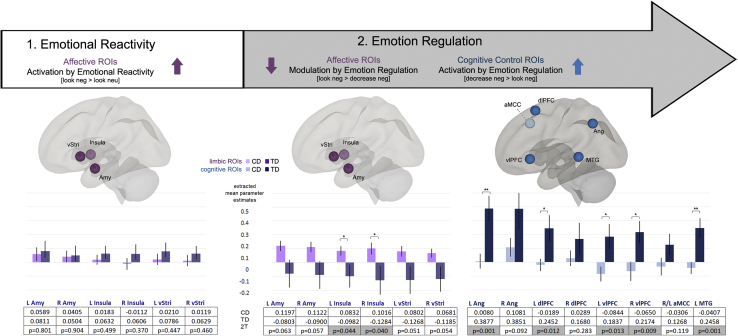
To assess putative models of cognitive reappraisal as previously suggested in healthy adolescents and adults 27, 28, 29 and for consequent follow-up analyses (e.g., assessment for CU traits–related effects), we extracted mean parameter estimates from the relevant ROIs. We expected emotional reactivity–related activation in amygdala, striatum, and insula regions, which in turn may be altered in CD. No significant group differences in emotional reactivity were observed in the ROIs. We expected the neural correlates of emotion regulation to differ in adolescents with CD compared with TD individuals. In line with the mediation hypothesis of emotion regulation 28, 30, amygdala, striatum, and insula ROIs were hypothesized to decrease in activation (reflecting modulation), while cognitive ROIs were expected to be activated in TD adolescents, but less so in CD adolescents. Between-group findings were assessed using 2-sample t tests adjusted for multiple comparisons (Bonferroni correction). Familywise adjusted α levels were achieved by dividing p < .05 by the number of tests conducted for modulation by emotion regulation (adjusted p < .008) or activated activation by emotion regulation (adjusted p < .006). We found that adolescents with CD showed lower left angular and middle temporal gyrus activation than did TD adolescents during emotion regulation, following correction for multiple comparisons (Figure 3, Supplemental Table S2).
Figure 3.
Post hoc display of neural activation in regions of interest (ROIs) and group differences in activation. Graphical display reflecting processes preceding emotion regulation (emotional reactivity) and actual emotion regulation (activation of cognitive ROIs [blue] or modulation of affective ROIs [lilac] during emotion regulation). Extracted mean parameter estimates for ROIs are reported for adolescents with conduct disorder (CD) (lilac/blue: light colors) and typically developing (TD) adolescents (lilac/blue: dark colors). Two-sample t tests corrected for multiple comparisons indicate hypoactivation in left angular and middle temporal gyrus in adolescents with CD compared with TD adolescents. *Uncorrected p < .05; **p corrected for multiple comparisons. Amy, amygdala; aMCC, anterior middle cingulate gyrus; Ang, angular gyrus; dlPFC, dorsolateral prefrontal cortex; L, left; MTG, middle temporal gyrus; neg, negative; neu, neutral; R, right; vlPFC, ventrolateral prefrontal cortex; vStri, ventral striatum.
Post Hoc Analyses Assessing Functional Connectivity During Emotion Regulation
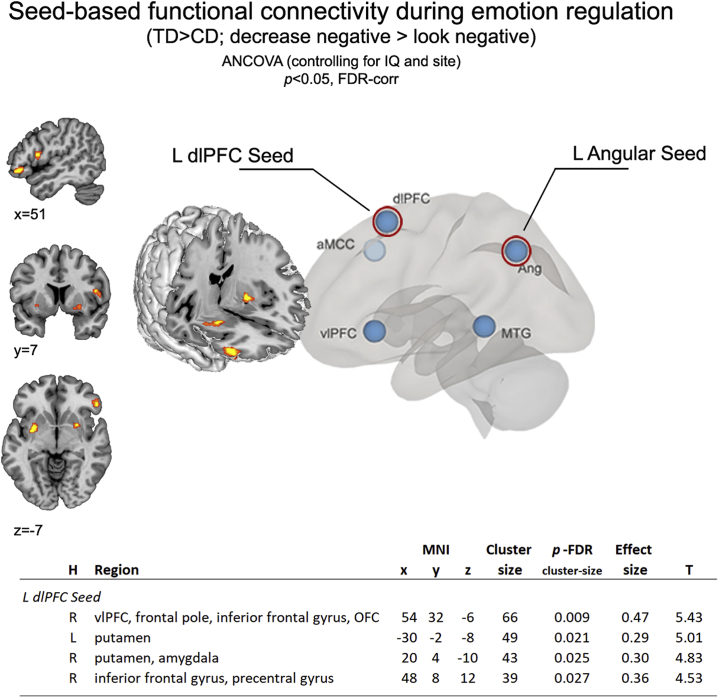
Correlational analyses (controlling for site and IQ) between extracted neural activation parameters from left dlPFC and amygdala ROIs during emotion regulation (decrease > look negative) were significant in TD individuals (p < .001; r = .747), but not in individuals with CD (p = .170; r = .257), giving rise to significant group differences in left dlPFC–amygdala connectivity (p < .010). Additionally, task-dependent and seed-based functional connectivity analyses revealed that areas of coactivation based on the left dlPFC differed between TD adolescents and adolescents with CD during reappraisal (2-tailed significance, cluster-building p < .001 and clusterwise false discovery rate correction of p < .05) (Figure 4). Follow-up analyses of this group effect revealed that individuals with CD, compared with TD individuals, showed lower connectivity between the left dlPFC seed and bilateral putamen, right orbitofrontal cortex/vlPFC, and amygdala. No group differences were observed for left angular gyrus.
Figure 4.
Reduced functional connectivity in adolescents with conduct disorder (CD) compared with typically developing (TD) adolescents during emotion regulation. Connectivity differences (CD < TD) were demonstrated by group assessments in coactivation patterns for left (L) dorsolateral prefrontal cortex (dlPFC) with bilateral putamen, right (R) orbitofrontal cortex (OFC)/ventrolateral prefrontal cortex (vlPFC) and amygdala during cognitive reappraisal (decrease negative > look negative). aMCC, anterior middle cingulate gyrus; ANCOVA, analysis of covariance; Ang, angular gyrus; FDR-corr, false discovery rate–corrected; H, hemisphere; MNI, Montreal Neurological Institute; MTG, middle temporal gyrus.
Influence of CU Traits on Behavior and Neural Activation
We assessed whether CU traits or the presence of comorbidities may have impacted the observed neural and behavioral group differences reported.
To assess CU traits and neural activity, the scaled mean parameter estimates from independent ROIs for areas of interest (i.e., left dlPFC and angular gyrus) were entered as individual dependent variables into 2 stepwise multiple regression models assessing changes in R2, with group status (TD, CD), covariates (site and IQ), CU traits, and comorbidities (attention-deficit/hyperactivity disorder, major depressive disorder, posttraumatic stress disorder, alcohol dependence, substance dependence) as independent variables. Our findings revealed that group differences in left angular gyrus and dlPFC (CD < TD) were unrelated to CU traits. Likewise, covariates and comorbidities were not significant factors in the model.
To assess CU traits and in-scanner affect ratings, we used stepwise multiple regression models to determine whether the variance in behavioral findings (emotional reactivity and emotion-regulation success as predictors) was explained by the independent variables—group status (TD, CD), covariates (site, IQ), CU traits, and comorbidities. The only significant predictor of variance in emotion-regulation success was group status (p = .002; 18.2%), while variance in emotional reactivity was explained by group status (p < .001; 21.5%), with an additional 9.8% of the variance explained by CU traits (p = .009). Covariates and comorbidities were not significant factors in the model. Follow-up partial correlational analysis, accounting for site and IQ, within the CD and TD groups individually demonstrated a negative relationship between emotional reactivity and CU traits in adolescents with CD (r = −.390, p = .044), but not in TD adolescents (r = −.269, p = .184) (Supplemental Figure S4).
Discussion
This is the first study to examine neural activity during effortful emotion regulation in female adolescents with CD. In line with our main hypothesis, our results indicate that female adolescents with CD, compared with TD adolescents, displayed atypical neural activation during emotion regulation, as indicated by reduced left dlPFC and angular gyrus activation. This was supported by behavioral findings of deficient emotion regulation in female adolescents with CD. Additionally, post hoc functional connectivity analyses revealed weaker connectivity between the left dlPFC and the right amygdala, vlPFC/orbitofrontal cortex, and bilateral putamen in the CD group versus the TD group. CU traits were negatively correlated with emotional reactivity as measured behaviorally, but unlike in previous studies 18, 19, 55, they were unrelated to the neural correlates of emotion regulation or reactivity.
Corresponding to hypothesis 1 and in line with prior evidence 3, 21, 28, our data indicate an involvement of left hemisphere angular gyrus and dlPFC during emotion regulation in TD adolescents, which is reduced in adolescents with CD. Functional neuroimaging studies in healthy control adolescents have suggested that the dlPFC is central to emotion regulation 28, 30, 52 and is associated with tasks requiring cognitive control [e.g., working memory, dual-task performance, or response inhibition 56, 57]. The mediation hypothesis of emotion regulation suggests that the dlPFC achieves negative affect reduction by modulating subcortical regions such as the amygdala (30). Successful coupling between prefrontal and subcortical brain regions has been associated with emotion-regulation success (53), while reduced prefrontal-subcortical connectivity has been associated with increased symptoms in psychiatric populations [e.g., posttraumatic stress disorder/anxiety (58)]. However, depending on the strategy used, PFC activity can be related to both positive and negative appraisal processes and amygdala decreases and increases, respectively (30). Overall, functional and structural alterations of the dlPFC are common in adolescents with CD/ODD 34, 39, 59. Our findings of reduced dlPFC activation during emotion regulation in CD may thus reflect a more generic disruption in PFC functioning in CD. Some of the behavioral characteristics seen in individuals with CD may be explained by deficient recruitment of cognitive control regions (i.e., dlPFC and angular gyrus). Reduced prefrontal activity in CD has previously also been associated with implicit forms of emotion regulation 60, 61.
Contrary to hypothesis 2 and prior evidence 14, 20, 28, 39, 62, no significant neuronal evidence of downregulation/modulation of affect-related areas was observed during reappraisal. On a first view, our data may therefore be less supportive of the mediation hypothesis of emotion regulation because emotion regulation was only associated with prefrontal and angular gyrus activation, without accompanying modulation of subcortical structures. However, the developmental and clinical sample investigated renders interpretation challenging. Developmental research has indicated that a reduced amygdala response following emotion regulation may become apparent only in late adolescence (40). The expected inverse prefrontal-subcortical coupling could still be developing with age 40, 63. Furthermore, differences in brain age (i.e., delayed developmental trajectories) are characteristic of psychiatric disorders (64), complicating interpretations. Finally, potentially coexisting positive and negative dlPFC-amygdala correlations could cancel each other out (30). Transient subcortical modulatory effects within the amygdala may have been missed in the present paradigm using a relatively long blocked time window for the modeled regressors of interest. It remains to be investigated whether shorter, event-related designs enable detection of more transient subcortical signals (65). Overall, the neural differences identified here could reflect fundamental differences in emotion regulation (consistent across the life span) between individuals with CD or TD individuals and/or transient effects resulting from delayed brain development in CD that may eventually normalize.
While some previous evidence supports a prefrontal-subcortical mediation theory [e.g., 20, 21, 28, 66], the precise nature of such relations, particularly in clinical populations, warrants further investigation. Using post hoc assessments of functional connectivity, we observed positive connectivity between the left dlPFC and bilateral putamen, amygdala, and vlPFC during reappraisal in TD individuals, which was reduced in the CD group. Putamen and nucleus accumbens conjointly constitute the dorsal striatum, which is in close anatomical and functional association with the insula (67). Functionally, the putamen has been implicated during tasks of emotion and reward processing, learning, or working memory (68). Overall, coactivation findings of dlPFC with vlPFC/prefrontal brain regions, amygdala, and striatum are in line with meta-analytic evidence on emotion regulation (28).
The within-group analyses of behavioral affect ratings collected in the scanner suggest that both TD adolescents and adolescents with CD showed intact emotional reactivity (their affect ratings were higher to negative than neutral images) and were able to successfully apply emotion-regulation strategies (affect ratings of negative images were lower in the decrease condition than in the look condition). However, adolescents with CD were significantly less successful in emotion regulation and reported lower emotional reactivity overall, which corresponds to behavioral deficits reported for CD 8, 9, 15, 69, 70. Hypothesis 3 was therefore only partly supported: there were behavioral differences for both emotion regulation and emotional reactivity (CD < TD), but there were only neural differences between the groups in respect to reappraisal and not emotional reactivity, contrary to previous reports 14, 39, 71.
Because of the intricate nature of the task design and population studied, there are several limitations that should be noted. Behavioral evidence of reduced emotional reactivity could suggest that despite reported reappraisal success, there was less need for or potential to show emotion regulation in the CD group. While prior studies suggest stability, reliability, and predictive value of in-scanner self-reports 30, 72, and while we carefully trained all participants, we cannot fully exclude the possibility that participants with CD were less motivated to engage in effortful reappraisal or that their reports were influenced by reporting biases. Finally, it is possible that differences in the choice of emotion-regulation strategies (e.g., increased use of suppression in CD) impacted the present findings. The neural correlates of emotion regulation are known to depend on the strategy used [e.g., PFC activation during suppression and reappraisal, but decreased amygdala and insula response for reappraisal vs. increased response in the same areas during suppression (73)]. Additional noteworthy limitations are the presence of comorbid disorders, particularly substance and alcohol abuse (information on potential substance consumption in the days prior to testing was incomplete) and the absence of a male comparison group. Accumulating evidence suggests that the neural correlates of CD may be sex specific 49, 59. It remains to be investigated whether male adolescents with CD show similar distinct neural alterations (if any) during emotion regulation.
The present findings of atypical left angular gyrus and dlPFC activation during emotion regulation in female adolescents with CD and reduced functional connectivity between the left dlPFC and cortical (vlPFC/orbitofrontal cortex, putamen), and subcortical (amygdala) regions emphasize the importance of addressing emotion regulation when treating CD. Future studies may investigate whether targeted interventions, such as cognitive behavioral therapy, enhance emotion-regulation skills in adolescents with CD and/or ameliorate the alterations in prefrontal and parietal activation observed here.
Acknowledgments and Disclosures
This study was funded by the European Commission under the 7th Framework Health Programme (Grant No. 602407). This work was supported by the Jacobs Foundation (Grant No. 2016 1217 13 [to NMR]) and the University Hospital of Basel (Junior Researchers Research Fund [to NMR]). Additional funding was provided by The Medical Research Council (Grant No. MR/K022474/1 [to GF]) and by the Swiss National Science Foundation (Grant No. 105314M_150282 [to CS]).
All authors have approved the manuscript.
We thank the adolescents, families, and institutions that took part and all the members of the Neurobiology and Treatment of Female Adolescent Conduct Disorder study consortium who contributed to this research.
Preliminary data from this study were presented at the Swiss Society for Biological Psychiatry meeting in Geneva, Switzerland, in 2015; the European Society for Child and Adolescent Psychiatry meeting in Geneva, Switzerland, in 2017; and the Bench to Bedside Symposium of the Neuroscience Network in Basel, Switzerland, in 2018.
CF receives royalties from books on autism spectrum disorder, attention-deficit/hyperactivity disorder, and major depressive disorder and is a consultant for Roche and Desitin. GF is an editor of the journal European Child and Adolescent Psychiatry. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2019.05.003.
Supplementary Material
References
- 1.Guyer A.E., Silk J.S., Nelson E.E. The neurobiology of the emotional adolescent: From the inside out. Neurosci Biobehav Rev. 2016;70:74–85. doi: 10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulkes L., Blakemore S.J. Studying individual differences in human adolescent brain development. Nat Neurosci. 2018;21:315–323. doi: 10.1038/s41593-018-0078-4. [DOI] [PubMed] [Google Scholar]
- 3.Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross J.J. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 5.Shulman E.P., Smith A.R., Silva K., Icenogle G., Duell N., Chein J. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppes G., Suri G., Gross J.J. Emotion regulation and psychopathology. Annu Rev Clin Psychol. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- 7.Cisler J.M., Olatunji B.O., Feldner M.T., Forsyth J.P. Emotion regulation and the anxiety disorders: An integrative review. J Psychopathol Behav Assess. 2010;32:68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke J.D., Hipwell A.E., Loeber R. Dimensions of oppositional defiant disorder as predictors of depression and conduct disorder in preadolescent girls. J Am Acad Child Adolesc Psychiatry. 2010;49:484–492. doi: 10.1097/00004583-201005000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freitag C. Neurobiology and treatment of adolescent female conduct disorder: FemNAT-CD consortium: A new European cooperation. Eur Child Adolesc Psychiatry. 2014;23:723–724. doi: 10.1007/s00787-014-0536-9. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 11.Nock M.K., Kazdin A.E., Hiripi E., Kessler R.C. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychol Med. 2006;36:699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frick P.J., Morris A.S. Temperament and developmental pathways to conduct problems. J Clin Child Adolesc Psychol. 2004;33:54–68. doi: 10.1207/S15374424JCCP3301_6. [DOI] [PubMed] [Google Scholar]
- 13.Herpertz S.C., Mueller B., Qunaibi M., Lichterfeld C., Konrad K., Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. Am J Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- 14.Sterzer P., Stadler C., Krebs A., Kleinschmidt A., Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Frick P.J., Viding E. Antisocial behavior from a developmental psychopathology perspective. Dev Psychopathol. 2009;21:1111–1131. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- 16.Pardini D., Stepp S., Hipwell A., Stouthamer-Loeber M., Loeber R. The clinical utility of the proposed DSM-5 callous-unemotional subtype of conduct disorder in young girls. J Am Acad Child Adolesc Psychiatry. 2012;51:62–73.e4. doi: 10.1016/j.jaac.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanti K.A., Demetriou C.A., Kimonis E.R. Variants of callous-unemotional conduct problems in a community sample of adolescents. J Youth Adolesc. 2013;42:964–979. doi: 10.1007/s10964-013-9958-9. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood P.L., Sebastian C.L., McCrory E.J., Hyde Z.H., Gu X., De Brito S.A. Association of callous traits with reduced neural response to others' pain in children with conduct problems. Curr Biol. 2013;23:901–905. doi: 10.1016/j.cub.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalska K.J., Zeffiro T.A., Decety J. Brain response to viewing others being harmed in children with conduct disorder symptoms. J Child Psychol Psychiatry. 2016;57:510–519. doi: 10.1111/jcpp.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 21.Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Miller A.B., McLaughlin K.A., Busso D.S., Brueck S., Peverill M., Sheridan M.A. Neural correlates of emotion regulation and adolescent suicidal ideation. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:125–132. doi: 10.1016/j.bpsc.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J Am Acad Child Adolesc Psychiatry. 2015;54:753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erk S., Mikschl A., Stier S., Ciaramidaro A., Gapp V., Weber B. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck A.T. The current state of cognitive therapy: A 40-year retrospective. Arch Gen Psychiatry. 2005;62:953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- 26.Gratz K.L., Weiss N.H., Tull M.T. Examining emotion regulation as an outcome, mechanism, or target of psychological treatments. Curr Opin Psychol. 2015;3:85–90. doi: 10.1016/j.copsyc.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation: An ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braunstein L.M., Gross J.J., Ochsner K.N. Explicit and implicit emotion regulation: A multi-level framework. Soc Cogn Affect Neurosci. 2017;12:1545–1557. doi: 10.1093/scan/nsx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Neural mechanisms of emotion regulation: Evidence for two independent prefrontal-subcortical pathways. Neuron. 2008;59:1037. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dore B.P., Rodrik O., Boccagno C., Hubbard A., Weber J., Stanley B. Negative autobiographical memory in depression reflects elevated amygdala-hippocampal reactivity and hippocampally associated emotion regulation. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:358–366. doi: 10.1016/j.bpsc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Alegria A.A., Radua J., Rubia K. Meta-analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry. 2016;173:1119–1130. doi: 10.1176/appi.ajp.2016.15081089. [DOI] [PubMed] [Google Scholar]
- 33.Noordermeer S.D., Luman M., Oosterlaan J. A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychol Rev. 2016;26:44–72. doi: 10.1007/s11065-015-9315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raschle N.M., Menks W.M., Fehlbaum L.V., Tshomba E., Stadler C. Structural and functional alterations in right dorsomedial prefrontal and left insular cortex co-localize in adolescents with aggressive behaviour: An ALE meta-analysis. PLoS One. 2015;10:e0136553. doi: 10.1371/journal.pone.0136553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers J.C., De Brito S.A. Cortical and subcortical gray matter volume in youths with conduct problems: A meta-analysis. JAMA Psychiatry. 2016;73:64–72. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- 36.Frick P.J., Cornell A.H., Bodin S.D., Dane H.E., Barry C.T., Loney B.R. Callous-unemotional traits and developmental pathways to severe conduct problems. Dev Psychol. 2003;39:246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- 37.Viding E., Sebastian C.L., Dadds M.R., Lockwood P.L., Cecil C.A., De Brito S.A. Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. Am J Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- 38.Blair R.J. The neurobiology of psychopathic traits in youths. Nat Rev Neurosci. 2013;14:786–799. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairchild G., Hagan C.C., Passamonti L., Walsh N.D., Goodyer I.M., Calder A.J. Atypical neural responses during face processing in female adolescents with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2014;53:677–687.e5. doi: 10.1016/j.jaac.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R.E. vlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb Cortex. 2017;27:3502–3514. doi: 10.1093/cercor/bhw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noftle E.E., Fleeson W. Age differences in big five behavior averages and variabilities across the adult life span: Moving beyond retrospective, global summary accounts of personality. Psychol Aging. 2010;25:95. doi: 10.1037/a0018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McRae K., Ochsner K.N., Mauss I.B., Gabrieli J.J., Gross J.J. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Process Intergroup Relat. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial -reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Petermann F., Wechsler D. Verlag Hans Huber, Hogrefe, Testverlag; Göttingen, Germany: 2008. HAWIK-IV: Hamburg-Wechsler-Intelligenztest für Kinder-IV. [Google Scholar]
- 46.Andershed H.A., Kerr M., Stattin H., Levander S. Psychopathic traits in non-referred youths: A new assessment tool. In: Blaauw E., Sheridan L., editors. Psychopaths: Current international perspectives. Elsevier; The Hague: 2002. pp. 131–158. [Google Scholar]
- 47.Achenbach T.M. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. [Google Scholar]
- 48.McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smaragdi A., Cornwell H., Toschi N., Riccelli R., Gonzalez-Madruga K., Wells A. Sex differences in the relationship between conduct disorder and cortical structure in adolescents. J Am Acad Child Adolesc Psychiatry. 2017;56:703–712. doi: 10.1016/j.jaac.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Raschle N.M., Menks W.M., Fehlbaum L.V., Steppan M., Smaragdi A., Gonzalez-Madruga K. Callous-unemotional traits and brain structure: Sex-specific effects in anterior insula of typically-developing youths. Neuroimage Clin. 2018;17:856–864. doi: 10.1016/j.nicl.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raschle N.M., Zuk J., Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci U S A. 2012;109:2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 55.Lozier L.M., Cardinale E.M., VanMeter J.W., Marsh A.A. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridderinkhof K.R., Van Den Wildenberg W.P., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Curtis C.E., D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 58.Lanius R.A., Williamson P.C., Densmore M., Boksman K., Neufeld R.W., Gati J.S. The nature of traumatic memories: A 4-T FMRI functional connectivity analysis. Am J Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- 59.Fairchild G., Hagan C.C., Walsh N.D., Passamonti L., Calder A.J., Goodyer I.M. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fehlbaum L.V., Raschle N.M., Menks W.M., Prätzlich M., Flemming E., Wyss L. Altered neuronal responses during an affective Stroop task in adolescents with conduct disorder. Front Psychol. 2018;9:1961. doi: 10.3389/fpsyg.2018.01961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang S., Nolan Z.T., White S.F., Williams W.C., Sinclair S., Blair R. Dual neurocircuitry dysfunctions in disruptive behavior disorders: Emotional responding and response inhibition. Psychol Med. 2016;46:1485–1496. doi: 10.1017/S0033291716000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urry H.L., van Reekum C.M., Johnstone T., Kalin N.H., Thurow M.E., Schaefer H.S. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spielberg J.M., Forbes E.E., Ladouceur C.D., Worthman C.M., Olino T.M., Ryan N.D. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc Cogn Affect Neurosci. 2015;10:408–415. doi: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaufmann T., van der Meer D., Doan N.T., Schwarz E., Lund M.J., Agartz I. 2018. Genetics of brain age suggest an overlap with common brain disorders [published online ahead print Apr 17] bioRxiv. [Google Scholar]
- 65.Somerville L.H., Wagner D.D., Wig G.S., Moran J.M., Whalen P.J., Kelley W.M. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2012;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Ghaziri J., Tucholka A., Girard G., Boucher O., Houde J.-C., Descoteaux M. Subcortical structural connectivity of insular subregions. Sci Rep. 2018;8:8596. doi: 10.1038/s41598-018-26995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Packard M.G., Knowlton B.J. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 69.Blair R.J., Leibenluft E., Pine D.S. Conduct disorder and callous-unemotional traits in youth. N Engl J Med. 2014;371:2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blair R., Veroude K., Buitelaar J. Neuro-cognitive system dysfunction and symptom sets: a review of fMRI studies in youth with conduct problems. Neurosci Biobehav Rev. 2018;91:69–90. doi: 10.1016/j.neubiorev.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 71.Passamonti L., Fairchild G., Goodyer I.M., Hurford G., Hagan C.C., Rowe J.B. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross J.J., Muñoz R.F. Emotion regulation and mental health. Clin Psychol Sci Pract. 1995;2:151–164. [Google Scholar]
- 73.Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen M., Poggiali D., Whitaker K., Marshall T.R., Kievit R. Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Res. 2019;4:63. doi: 10.12688/wellcomeopenres.15191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.