Abstract
A comprehensive understanding of common diseases of backyard poultry flocks is important to providing poultry health information to flock owners, veterinarians, and animal health officials. We collected autopsy reports over a 3-y period (2015–2017) from diagnostic laboratories in 8 states in the United States; 2,509 reports were collected, involving autopsies of 2,687 birds. The primary cause of mortality was categorized as infectious, noninfectious, neoplasia or lymphoproliferative disease, or undetermined. Neoplasia or lymphoproliferative disease was the most common primary diagnosis and involved 42% of the total birds autopsied; 63% of these cases were diagnosed as Marek’s disease or leukosis/sarcoma. Bacterial, parasitic, and viral organisms were commonly detected, involving 42%, 28%, and 7% of the birds autopsied, respectively, with 2 or more organisms detected in 69% of birds. Our findings demonstrate the importance of educating flock owners about disease prevention and biosecurity practices. The detection of zoonotic bacteria including paratyphoid salmonellae, Campylobacter spp., Listeria monocytogenes, and Mycobacterium avium, and the detection of lead and other heavy metals, indicate public health risks to flock owners and consumers of backyard flock egg and meat products.
Keywords: backyard flock, chickens, poultry disease, poultry mortality, zoonoses
Introduction
The commercial poultry industry has successfully reduced or eliminated many previously common and problematic infectious and noninfectious diseases of poultry.23,30 The continuing rise in backyard poultry ownership in the United States has increased concern that many of these diseases may re-emerge within backyard poultry populations, creating a threat of increased prevalence of these diseases nationwide.9 Many studies have demonstrated that backyard poultry owners lack knowledge of biosecurity practices and are unwilling or unable to seek veterinary care for flock illnesses, further exacerbating the threat of disease within these populations (U.S. Department of Agriculture [USDA]. Poultry 2004. Part I: reference of health and management of backyard/small production flocks in the United States, 2004. Report N432.0805. Available from: https://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry04/Poultry04_dr_PartI.pdf; USDA. Poultry 2010. Reference of the health and management of chicken flocks in urban settings in four U.S. cities, 2010. Report 592.0511. Available from: https://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry10/Poultry10_dr_Urban_Chicken.pdf).38 In addition, as backyard flock ownership has grown, so too have zoonotic disease outbreaks affecting backyard poultry owners.20 Increasing numbers of veterinarians are seeking information about poultry medicine and providing individual bird and flock health care in suburban and urban flock environments. Additional resources for veterinarians detailing common diseases of backyard flock populations are needed.6
Disease surveys of backyard flocks have described specific noninfectious and infectious diseases within this population.9,18,35 Noninfectious causes of mortality, such as management-related, nutritional, and metabolic diseases in backyard flocks may occur as a result of widely varied breeding, housing, and husbandry practices. New flock owners may not be aware of general flock husbandry as well as changing and specific dietary needs of newly acquired laying hens or meat chickens. Most backyard flock owners have little knowledge of poultry rearing prior to obtaining birds for their flock.12 Additionally, most veterinarians receive only minimal poultry medicine and husbandry education during veterinary school and may not feel comfortable providing veterinary services for backyard flocks.6 These limitations can make raising backyard flocks a challenge to owners and may result in the manifestation of preventable diseases within these flocks.9,33
Extensive research and management strategies have been implemented over time in the commercial poultry sector to reduce and eliminate infectious diseases within their flocks. Although infectious diseases are still common in commercial poultry, significant efforts are made by producers to prevent and control these diseases.14,21,23,30 In contrast, many infectious diseases are common in backyard flocks and may spread unchecked.8,28,29 Incidence of some of these diseases could be increased as backyard flocks expand in numbers and flock owners move birds between flocks and to and from poultry events, with suboptimal or absent biosecurity practices.6,39 Backyard poultry can also be the source of several important zoonotic pathogens, including paratyphoid salmonellae, Listeria monocytogenes, Campylobacter spp., and Escherichia coli, which can be clinically undetectable in poultry but cause severe disease in humans.2,7,13,32
Virally induced neoplastic diseases, including Marek’s disease (MD) and leukosis/sarcoma group diseases (L/S), occur worldwide; MD is the most commonly reported cause of mortality in backyard poultry.8,27 MD is primarily controlled in commercial operations through the use of rigorous vaccination protocols.11,16 For backyard flocks, many owners are not aware of the necessity of vaccination, and the available MD vaccine for small flocks is limited, impractical for use, and poorly implemented, which impairs disease control within this population.10,12
Previous studies of postmortem findings in backyard poultry populations have evaluated autopsy submissions to one veterinary diagnostic laboratory or to a network of laboratories in one state or region.8,28 Here we expand this information nationwide, to 8 states across the United States, to gain insights into the most common causes of mortality and further understanding of backyard flock programs implemented by various state agencies. This information will provide valuable, more generalizable data on backyard poultry diseases to veterinarians seeking to provide care to backyard flocks, commercial poultry industry professionals, and public health agencies.
Materials and methods
We included in our study veterinary diagnostic laboratories from California, Colorado, Georgia, Hawaii, Iowa, Pennsylvania, South Carolina, and Texas, representing different regions of the United States. Autopsy reports were submitted from a single laboratory from each state with the exception of California (4 networked laboratories) and Pennsylvania (2 networked laboratories). Each laboratory was asked to provide up to 1,000 consecutive backyard poultry autopsy reports per year for each of 3 y from 2015 to 2017. California provided 3,000 reports and was the only state to process >1,000 backyard poultry submissions per year. The next highest submitting state was Colorado with 307 reports over the 3-y study period. In order to not skew the data given the large number of California reports and maintain a proportionate sample across the 4-laboratory system and across years, a subset of 1,547 California autopsy reports was included in the study (547 reports from 2015; 501 reports from 2016; 499 reports from 2017).
Participants were asked to include flock location information to the county or postal (ZIP) code level, and to include any clinical history documented with the case. All identifying submitter information was redacted from reports included in the study. Data on sex, age, breed, and flock sizes were collected if available in the submitted record. Findings from each report were reviewed by a veterinarian knowledgeable in diseases of poultry, and the primary cause of mortality was categorized as neoplastic, infectious, noninfectious, or undetermined, based on the case coordinator’s primary diagnosis. For birds with more than one described disease contributing to mortality, all diseases were further categorized. In cases of neoplasia or lymphoproliferative disease, locations in the body were noted, as well as information on the determination of whether the disease was potentially virally induced. The infectious diseases were categorized into bacterial, viral, parasitic, or fungal causes of mortality. We counted all infectious agents that potentially contributed to mortality or are associated with zoonotic transmission. Noninfectious diseases were categorized as nutritional, management, or environmental; developmental; toxic; traumatic; or other noninfectious causes of mortality. Cases in which the cause of mortality could not be determined were classified as undetermined. Cases in which the determined cause of mortality fit into none of the assigned categories were classified as miscellaneous. Microsoft Excel (2010; Microsoft, Redmond, WA) was used to categorize and enumerate the disease conditions contributing to cause of death in poultry cases.
In order to gather information on backyard poultry programs in the participating states, each submitter was surveyed to collect information on management, size and funding of their state’s program, including the field services and testing provided to backyard flock owners. The survey included a standardized set of questions and was administered by phone.
Results
During the 3-y study period, 2,509 accession reports involving 2,687 autopsied birds were submitted by the 12 participating laboratories from 8 U.S. states. The number of total reports used per state for our study ranged from 22 (Hawaii) to 1,547 (California; Table 1). The vast majority of submissions were chickens (n = 2,582; 96%). Other birds included turkeys (n = 54), ducks (n = 43), and geese (n = 8). The majority of birds were female; males represented only 14.8% of submissions (n = 397). Birds were 4 d to 11 y old. Breed information was often not provided by owners. Of the breeds provided, the most frequently submitted breeds were Rhode Island Red, Orpington, Silkie, Ameraucana, Plymouth Rock, Leghorn, Wyandotte, and Maran. The top 3 breeds were the same across all states.
Table 1.
Number of backyard poultry accessions and birds submitted for categorization between 2015 and 2017, by U.S. state.
| State | Accessions | Birds |
|---|---|---|
| California | 1,547 | 1,646 |
| Colorado | 313 | 357 |
| Georgia | 208 | 210 |
| Hawaii | 22 | 22 |
| Iowa | 96 | 97 |
| Pennsylvania | 194 | 224 |
| South Caroline | 105 | 105 |
| Texas | 24 | 26 |
| Total | 2,509 | 2,687 |
All of the autopsy reports for the 2,687 birds submitted included gross autopsy findings, 2,477 (92.2%) included histopathologic findings, and 2,233 (83.1%) included associated testing reports. All autopsied birds were first categorized into a primary category of mortality (Fig. 1): neoplasia or lymphoproliferative disease (42.1%, n = 1,131), infectious (36.2%, n = 970), noninfectious (17.3%, n = 463), and undetermined (4.6%, n = 123). All diseases contributing to mortality described in the reports were classified into disease categories (Fig. 2, Table 2). In birds in which more than one cause of mortality was reported (69.1% of birds autopsied), all major diagnoses were categorized and are described in this results section; thus, the total number of diseases described exceeds the total number of birds autopsied. Diagnoses that were described in multiple cases are included in the results section below; diagnoses involving only one or a low number of birds were not typically included in our results section.
Figure 1.
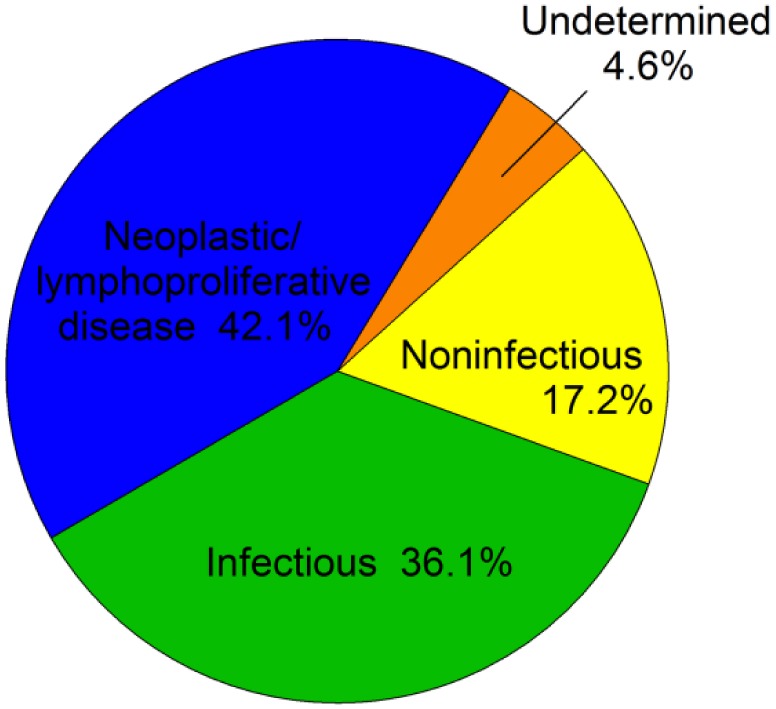
Primary causes of mortality in backyard poultry submitted to 12 veterinary diagnostic laboratories in 8 states, 2015–2017.
Figure 2.
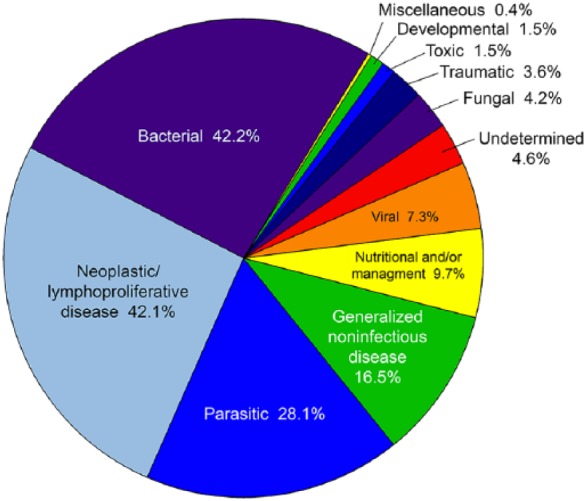
All causes of mortality of backyard poultry, by disease category. Percentages exceed 100% because of concurrent disease in multiple categories (69.1% of birds autopsied).
Table 2.
Causes of backyard bird mortality (n = 2,687) in 8 states during 2015–2017.
| No. of birds | |
|---|---|
| Bacterial | 1,135 |
| Neoplastic/lymphoproliferative | 1,131 |
| Parasitic | 755 |
| Generalized noninfectious | 444 |
| Nutritional/management/environmental | 261 |
| Viral | 195 |
| Undetermined | 123 |
| Fungal | 114 |
| Traumatic | 96 |
| Toxic | 41 |
| Developmental | 40 |
Concurrent disease was diagnosed in 69.1% of birds autopsied; thus, total numbers within the diagnosis categories exceeds number of birds autopsied per year.
Neoplastic or lymphoproliferative disease
Neoplasia, including lymphoma or lymphoproliferative disease (LPD), was the most common cause of mortality, documented in 1,131 (42.1%) backyard birds. Such cases were categorized as viral etiology or non-viral in origin. Virally induced neoplasms or LPD, including MD, L/S group diseases, and reticuloendotheliosis (RE), were described as the presumed cause of mortality for 717 birds, or 63.4% of the neoplasia diagnoses. There were 582 cases of presumptive MD, 99 cases of presumptive L/S-type neoplasia, and 1 case of presumptive RE. Two cases involved variability in the lymphomas and were presumed to have both MD and L/S involvement. Thirty-three of the cases were reported as LPD with a suspected viral etiology and were not attributed to a specific disease by the case coordinator. Of the birds with presumptive virally induced neoplasia/LPD, 50.2% of the cases (n = 360) were reported in birds <1 y old, which includes 320 cases of presumptive MD.
There were 414 cases of neoplasia not attributed to a viral origin. The most common non-virally induced neoplasms were ovarian adenocarcinoma and carcinomatosis (314 cases). Of these, case coordinators diagnosed 10 birds with an additional type of non-viral neoplasia and 10 with presumptive virally induced neoplasia. Of the 125 remaining neoplasia diagnoses, tumors included adenocarcinoma (n = 43), lymphoma presumed to be non-viral in origin (n = 20), leiomyosarcoma or leiomyoma of the reproductive tract or gastrointestinal tract (n = 17), squamous cell carcinoma of the oropharynx (n = 14), hemangiosarcoma (n = 6), astrocytoma (n = 4), teratoma (n = 4), osteosarcoma (n = 3), seminoma (n = 3), nephroblastoma (n = 2), and 9 single cases of neoplasia.
Infectious causes of mortality
Bacterial diseases
Of the 1,135 (42.2%) total birds with bacterial disease, E. coli was detected in 34.0% of the birds (n = 386), followed by detection of Mycoplasma gallisepticum and/or Mycoplasma synoviae in 296 (26.1%) birds. In 311 (27.4%) of the cases in which a bacterial etiology was suspected, the bacterial agent could not be identified because of carcass autolysis, lack of authorization to perform additional testing, or previous antimicrobial agent administration. Other bacterial infections detected included 74 cases of Gallibacterium anatis; 40 cases of Pasteurella multocida, of which 38 isolates were associated with fowl cholera infection; 38 cases of Avibacterium paragallinarum, of which 33 isolated infections were associated with respiratory infectious coryza, 4 were associated with reproductive infections, and 1 case was associated with septicemia; 25 cases of Clostridium perfringens, of which 22 cases were associated with necrotic enteritis in conjunction with coccidiosis (Eimeria spp.), 2 cases were associated with hepatitis and 1 case was associated with septicemia; 34 cases of Staphylococcus spp.; 38 cases of Enterococcus faecalis; 8 cases of Streptococcus spp.; 9 cases of Pseudomonas aeruginosa; and 4 cases of Riemerella anatipestifer. In 187 cases, there was >1 bacterial agent identified. Among bacterial diagnoses that have zoonotic potential, 27 cases of paratyphoid Salmonella enterica, 6 cases of Mycobacterium avium, 4 cases of Listeria monocytogenes, and 5 cases of Campylobacter jejuni infection were identified. Twelve of the paratyphoid Salmonella enterica infections were attributed as the cause of mortality, 7 as a result of septicemia, and 5 causing enteritis, typhlitis, or coelomitis. An additional 15 cases were detected on general Salmonella surveillance and were not associated with clinical signs. Of the isolates serotyped, Enteritidis, Give, Manhattan, Mbandaka, Muenchen, Newport, Senftenberg, and Typhimurium were identified.
Of the total number of bacterial cases, associated reproductive disease was diagnosed in 342 (30.1%) birds. Reproductive lesions included coelomitis (peritonitis), salpingitis, vent prolapse, and egg-laying abnormalities. The most commonly isolated bacteria in these birds was E. coli (n = 200).
Viral diseases
Viral agents were detected in 195 (7.3%) birds, with a single viral agent detected in 184 birds and 2 different concurrent viruses detected in 11 birds. Infectious bronchitis virus (species Avian coronavirus) was the most commonly detected virus (n = 81), followed by infectious laryngotracheitis virus (n = 39 birds; species Gallid alphaherpesvirus 1), avian poxviruses (n = 40; genus Avipoxvirus), very virulent infectious bursal disease virus (n = 5), fowl aviadenovirus (n = 3), chicken proventricular necrosis virus (n = 3), and avian encephalomyelitis virus (n = 3; species Tremovirus A). Duck enteritis virus (species Anatid alphaherpesvirus 1) was detected in 2 Muscovy ducks from the same county in California. Virally induced neoplastic disease cases are reported in the neoplasia section.
Parasitic diseases
Internal parasites were attributed as a primary cause of mortality in 69 (2.6%) birds and were the most common secondary finding (n = 686; 25.5%) of all case reports. Coccidiosis was the most common cause of parasitic disease–related mortality (40 of 69 primary cases) and was detected in 297 birds, 129 of which occurred in birds ≤4 mo old. In 210 birds, there was more than one type of parasite detected. Heavy mixed infestations of multiple external and internal parasites such as hematophagous mites, Knemidocoptes mutans leg mites, ascarid nematodes, cestodes, and Heterakis sp. cecal worms were detected in 7 birds. Histomoniasis was detected in 14 turkeys and 7 chickens. In 4 birds, heavy infestations with ascarid nematodes (n = 3) or tapeworms (n = 1) resulted in intestinal blockage. Additional parasites detected included Trichomonas gallinae (n = 29) and Cryptosporidium spp. (n = 4). Two cases of Baylisascaris spp. resulting in cerebral larval migrans were detected in 2 chickens from Colorado and California, respectively.
Fungal diseases
Of the 114 (4.2%) diagnosed fungal infections, there were 54 respiratory infections; 46 gastrointestinal infections, of which 43 were diagnosed as fungal ingluvitis; 12 systemic fungal infections; and 1 case each of probable fungal hepatitis and favus. Forty-four of the fungal infections were not identified to genus. Candida albicans was the most commonly identified fungus (n = 38), followed by Aspergillus fumigatus (n = 29), Mucor sp. (n = 2) and other zygomycetes (n = 3), and Penicillium spp. (n = 1). Some of these detections were mixed fungal infections.
Noninfectious causes of mortality
Nutritional and management diseases
Nutrition- and management-related diseases were attributed to 261 (9.7%) of the bird mortalities, with both nutritional- and management-related problems affecting 247 (94.6%) of these birds. Nutritional deficiencies, excesses, and imbalances were detected in 64 birds. Dehydration, salt toxicosis, or other evidence of lack of water access accounted for mortality in 51 birds. Starvation was identified in 43 birds. Gastrointestinal impactions, usually with fibrous plant material, were identified in 47 birds. Obesity with hepatic lipidosis affected 38 birds. Management-related problems that resulted in mortality included temperature-related problems such as frostbite and heat stroke (n = 6), evidence of overcrowding or lighting issues (n = 2), air quality problems (n = 4), and foot issues related to wet litter (n = 2). Three birds were diagnosed with multiple nutrition- and management-related issues including heavy parasitism, nutritional deficiencies, poor body condition, and infectious disease.
Generalized noninfectious diseases
Generalized noninfectious diseases were grouped into a single category and attributed as the cause of 444 (16.5%) bird mortalities. Some birds were diagnosed with multiple systemic diseases. The most common disease detected was hemorrhagic liver syndrome (HLS),40 affecting 131 birds. Diseases of the cardiovascular system (n = 78) included cardiomyopathy and congestive heart failure (n = 40), pulmonary hypertension syndrome (ascites syndrome; n = 26), atherosclerosis (n = 11), and aortic rupture (n = 1). There were 62 cases of visceral (renal) and/or articular gout and 8 cases of noninfectious hepatopathy of undetermined etiology. Primary reproductive diseases, including coelomitis, salpingitis, and egg-laying abnormalities, with no bacterial involvement given lack of detection or lack of testing, were identified as a primary cause of mortality in 40 birds and a contributing cause of mortality in 57 birds. There were 40 cases of neurologic disease, including 30 birds with suspected bacterial, viral, traumatic, nutritional, or developmental etiology (most were presumptive diagnoses without confirmation) and 10 with no gross or histologic findings leading to a proposed etiology. Primary gastrointestinal (GI) involvement was reported in 12 submitted cases and included GI intussusception, torsion, or volvulus (n = 5) and GI impaction with a non-food foreign body (n = 3).
Toxic diseases
Toxic causes of mortality included a wide variety of sources. There were 41 (1.5%) mortalities caused by toxic exposures. The most commonly diagnosed toxicity was ingestion of lead-containing materials, often in the form of ammunition (n = 4). The highest lead level detected was 21 ppm, detected in 2 birds that had ingested lead ammunition. In another 18 birds, lead was detected at levels below the toxic threshold and reported as a secondary finding, but these are still at a concerning level for potential human exposure. Two birds died of lead, iron, and zinc toxicities, one from ammunition ingestion and one from ingestion of a wide variety of screws, nuts, washers, nails, and other metal. Four birds died of confirmed or presumptive heavy metal toxicities including iron and/or zinc. Rodenticide ingestion killed 5 birds in one case and 1 bird in a second. There were 5 cases of botulism, each involving multiple birds per the owner history, and 5 mycotoxin cases. Other toxicities included 1 case of insecticide ingestion, 1 instance of rattlesnake envenomation, 2 cases of oxalate exposure, 1 medication overdose, and 1 case in which the bird was diagnosed with ionophore toxicity. In 9 reports of toxic exposure, the toxic agent was not identified.
Developmental diseases
Developmental abnormalities affected 40 (1.5%) birds. In 6 birds, unilateral kidney agenesis or hypoplasia accounted for increased risk of compensatory kidney dysfunction leading to mortality. Persistent cystic right oviducts that either acted as coelomic space-occupying compressive lesions or became infected contributed to 17 mortalities. There were 6 cases of GI abnormalities, including cloacal agenesis in 1 bird. There were also 3 cases of various musculoskeletal malformations, 3 cardiovascular malformations, and 3 brain or spinal cord abnormalities.
Undetermined causes of mortality
There were 123 (4.6%) autopsy reports with an undetermined cause of mortality. In 21 of those cases, there were no clinical signs noted in history, no gross or histologic findings, and no abnormalities detected on additional testing. The remainder of the cases had findings or history reported, but case coordinators were unable to determine a specific etiology.
State backyard poultry programs and services
All states that participated in the study provide backyard poultry services, and 4 indicated that they had an official program focusing on backyard flocks. Programs were started as early as the 1980s and as recently as 2018. California defined backyard poultry as flocks <1,000 birds. All other states defined backyard flocks as any flock that was not classified as a commercial producer. Program activities were funded through state departments of agriculture, with the exception of 2 states that used USDA cooperative agreement funding, 1 that obtained partial funding through university extension services, and 1 funded through the state health administration. Services provided included packages of free or reduced cost autopsy and/or infectious disease testing (n = 2) and free autopsy/histology only (n = 1). Three states provided some level of field services, either sample collection and courier services, or sick flock consultation and testing. All states but one provided free avian influenza virus (AIV) and Newcastle disease virus (NDV) testing for birds submitted to the laboratory or through backyard flock and poultry show surveillance.
Discussion
The number of backyard flock autopsies performed during the 3-y study period by the participating laboratories varied widely, from 22 in Hawaii to 3,938 in California. All of the states conducted autopsies in conjunction with histopathology on all or the majority of their cases. All but one state routinely conducted avian influenza testing on backyard birds submitted for autopsy. There were differences in the number of additional tests run, with some states providing additional testing at no or low cost to flock owners and some states rarely running additional tests, given lack of owner interest in paying for additional testing. Commonly added tests included bacterial culture, parasitology, and viral respiratory pathogen testing.
Concurrent disease was common in backyard flocks, and 69.1% of the case reports had more than one possible cause of mortality listed on the report. Detection of more than one significant disease makes the ultimate determination of cause of mortality difficult. We counted all major diseases that could be attributed as the cause of mortality of the bird in the overall disease numbers. Thus, the total number of diagnosed disease conditions that we report exceeds the number of birds autopsied. For example, coccidiosis was a secondary finding on 249 of the case reports. Although coccidiosis was not the primary disease condition, this disease contributed to overall disease burden in the bird and potentially to the death of the bird. Additionally, some of the diagnoses, such as starvation, were likely not the primary cause of mortality but were the only diagnosis made given that the primary disease issue was not apparent.
Neoplasia and lymphoproliferative disease were the most common causes of mortality reported. Neoplasia and lymphoproliferative diseases in poultry are often viral in origin, as the result of transmissible infection with MDV (species Gallid herpesvirus 2), avian leukosis/sarcoma (retroviruses), or reticuloendotheliosis (retroviruses). Similar to prior reports,8,28,31 MD was the most common cause of mortality in all states studied, with a total of 582 birds (22%) diagnosed with MD, demonstrating the widespread nature of this disease among backyard poultry flocks. A diagnosis of MD is typically based on autopsy and histopathology findings, along with other criteria such as age (more common in birds <6 mo old) and clinical presentation, although it can be highly challenging to differentiate MD, L/S, and RE.36 Evaluation of all of the case reports submitted for our study for birds <1 y old showed that 37.3% died of probable virally induced neoplasia, primarily MD, with the highest number of mortalities at 3–6 mo old. In commercial poultry, MD has been controlled through extensive breed selection and use of in ovo vaccination.15,19 However, this vaccine is only administered at some hatcheries producing birds for backyard flocks and, when used, is often administered at a 1- or 2-d-old, instead of in ovo, delaying development of immunity.37 When hatcheries provide an option to purchase MDV-vaccinated birds, this is typically done at an additional cost to the owner, decreasing owner compliance. In addition, many of the backyard flock hatcheries only have the option to vaccinate with serotype 3 (HVT) MDV, which provides less protection against MD versus available commercial hatchery vaccinations. Further focus on educating backyard flock owners about the importance of purchasing only MDV-vaccinated chicks, and vaccinating any chicks they raise from hatch, may help in controlling this disease in backyard flocks.
Retrovirus-induced leukosis/sarcoma diseases were presumptively diagnosed in 99 birds (3.7%). Retrovirus-induced RE is uncommonly detected in the United States26 and was only detected in 1 case in our study as a presumptive diagnosis. Commercial vaccines against L/S and RE are not available, limiting ability to control the diseases in backyard chickens.36
Infectious causes of mortality were most commonly attributed to bacterial agents, followed by viruses. Considering that most neoplasms and lymphoproliferative diseases are virally induced, the viral infectious disease category would be the most common cause of mortality had these diseases not been separated into their own category. The viral and bacterial organisms detected in the cases described in our study cause a wide variety of localized and systemic disease conditions that can result in flock production losses and mortality. The detection of such a variety of infectious organisms highlights the need for backyard flocks to improve biosecurity practices to decrease organism transmission between flocks. Four important zoonotic agents: paratyphoid salmonellae, M. avium, L. monocytogenes, and C. jejuni, were detected in 42 cases (1.6%), highlighting the importance of educating backyard flock owners about prevention of zoonotic disease transmission via proper bird handling and processing and consumption of eggs and meat.32 Given that zoonotic infectious agent testing was not conducted in the vast majority of cases, the prevalence of zoonotic diseases is likely higher than the number of cases detected as part of our study.
The most common parasitic disease diagnosed was coccidiosis, which was primarily detected in birds <4 mo old. Control of coccidiosis is a challenge for backyard flocks given that the flocks are floor-raised adn thus have continual exposure to Eimeria spp. oocysts from contaminated environmental areas, and owners may not know how to utilize prophylactic or therapeutic medications.17 There are also small flock owners who elect not to use medications because they are trying to raise birds in an organic environment. Increased utilization of coccidiosis vaccination programs in backyard flocks could be helpful in disease control. Additional parasitic disease findings included severe nematode infestations and histomoniasis, the latter being a significant problem for flocks given lack of treatment options. Vaccines being evaluated for use in control of histomoniasis would be highly beneficial for backyard flock owners as well as commercial flocks.5,25,34
Most fungal infections are opportunistic and can be attributed to poor management of the flock environment. Evidence of fungal infection in flocks may signify issues with environmental and equipment sanitation and moldy feed and litter.
Of the noninfectious categories, nutritional and management issues were the most common causes of mortality. The majority of these mortality issues can be attributed to lack of poultry rearing and husbandry knowledge by backyard flock owners. The nutritional diseases were related to deficiencies, excesses, or imbalances, and included vitamin deficiencies, calcium and phosphorus deficiencies or imbalances, and salt toxicosis. Hepatic lipidosis in obese birds can be the result of overfeeding of high-energy diets, which may be an issue in backyard flocks when owners do not select the correct feed for production type and/or choose to supplement the diet with human food. Chickens raised in backyard flocks have more access to potential foreign bodies, and ingestion of these foreign bodies resulted in a number of mortalities. General management issues, such as lack of protection from predators, exposure to extreme cold or heat, poor air quality, and wet litter all contributed to mortalities. Starvation and cannibalism were also common findings but were likely not the original cause of disease in the bird but were instead secondary findings related to inappetence, recumbency, and immobility resulting from illness, with the exception of starve-out cases in newly hatched poultry.
Most environmental toxins are also related to management issues. Lead was the most commonly detected heavy metal, as the result of consumption of lead-containing items such as ammunition. Two laboratories routinely performed lead surveillance testing, which increased the number of lead detections. Given that exposure to lead via consumption of eggs is a significant human health threat, this finding is concerning, and flock owners, primarily pregnant women and children who are consuming eggs, should be advised of this risk and of routine testing options.3,24,39 Most of the additional toxin-related mortalities were preventable, including rodenticide, insecticide, and ionophore exposures, and medication overdoses. One case of botulism involved the mortality of 14 chickens that had access to pecking on a backyard compost pile, which poses a newly described threat for backyard flock owners.
The most common systemic diseases diagnosed were gout and cardiovascular disease. Visceral gout is attributed to renal failure as a sequela of a number of different issues including excess dietary calcium, infectious diseases targeting the kidneys, such as infectious bronchitis virus, and water deprivation. Articular gout is a sporadic disease and may be related to excess dietary protein or hereditary defects. Flock owners have some ability to prevent gout through appropriate feeding, prevention of disease introduction, and providing continual access to clean water. Many of the diseases of the cardiovascular system are attributed to production issues related to fast growth of heavy birds, which overwhelms the cardiovascular system.22,41 In some cases, these conditions can also be controlled through appropriate feeding for production type. Many flock owners do not understand nutritional needs for poultry, including the different needs of layers versus broilers, which can lead to inappropriate nutrition. Additionally, many flock owners have health issues with backyard flocks because of high altitude or continuing to raise broiler birds past slaughter weight.
Backyard flocks have been one of the major focus areas for surveillance of foreign poultry diseases, such as highly pathogenic AIV (HPAIV) and virulent NDV (vNDV). In the H5 HPAI outbreak in the United States in 2014–2015 (USDA. Final report for the 2014–2015 outbreak of highly pathogenic avian influenza (HPAI) in the United States. Available from: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai/2015-hpai-final-report.pdf), backyard flocks were among the first affected poultry, and subsequent studies have noted that minor gallinaceous species in these mixed flocks may have incubated, amplified, and adapted the disease to their chicken flock mates.4 All states but one in the study regularly conducted AIV surveillance on poultry submitted for autopsy, and all test results were negative. Two large-scale outbreaks of vND in California, Nevada, Arizona, and Texas in 2002–2003 and California in 2018–2019 primarily affected backyard flocks and involved the depopulation of tens of thousands of backyard birds.1 Seven states also conducted surveillance for NDV on some of the autopsies submitted based on case presentation and available funding. This surveillance is important for early detection of introduction of HPAI or vNDV into commercial poultry.
Extensive research and management strategies have been implemented over time in the commercial poultry sector to reduce and eliminate disease. A number of factors have interfered with the reduction of disease in backyard flocks, including commingling of different species and breeds in flocks; interaction of backyard flocks with wild birds and other wildlife; extensive movement of backyard birds between hatcheries, other backyard flocks, and poultry events; poor husbandry, biosecurity, and disease prevention measures by flock owners; and lack of veterinary resources and often disinterest in paying for costs associated with veterinary care (USDA, Poultry 2004: part 1; USDA, Poultry 2010).38 Spread of infectious diseases within backyard flocks are particularly concerning, given that these flocks may serve as a source of infectious disease transmission to commercial flocks. Backyard poultry programs that provide veterinary consultation, disease diagnostic consultation, and educational opportunities for flock owners may help improve overall health of backyard flocks, along with increasing knowledge of backyard flock husbandry and disease prevention within flock owners, and backyard flock medicine within the veterinary community. Poultry medicine resources provided to practicing veterinarians will be a useful way to improve veterinary care to the backyard poultry community. Resources that can be provided at low cost or free of charge will likely increase utilization of these services. Further research into common diseases of backyard flocks and evaluation of control measures within these flocks will benefit not only backyard flocks but also commercial operations.
The most commonly diagnosed diseases (MD and E. coli septicemia) in our study are also the top 2 diagnosed causes of mortality in 3 postmortem studies of backyard poultry.8,28,31 In contrast to previous postmortem studies of backyard poultry that focused on one region, we evaluated disease conditions detected throughout the United States, which provides a broader and more inclusive reference to veterinarians treating backyard poultry in the United States, as well as infectious disease surveillance authorities and the commercial poultry sector. Additionally, our study provided general information on the variety of backyard poultry program services available in different states.
Acknowledgments
We thank all of the veterinary pathologists and diagnosticians who conducted the autopsies and wrote the reports used in our study. We also thank poultry associations who participated in this work, including the Iowa Poultry Association. We thank Travis Heskett, Heather Reider, and Jenna Oxenhandler for technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Partial funding was provided by the USDA, cooperative agreement AP18VSSPRS00CO23.
ORCID iD: Kristy L. Pabilonia  https://orcid.org/0000-0001-7741-8497
https://orcid.org/0000-0001-7741-8497
References
- 1. Alexander DJ. Ecology and epidemiology of Newcastle disease. In: Capua I, Alexander DJ, eds. Avian Influenza and Newcastle Disease: A Field and Laboratory Manual. Milan: Springer Milan, 2009:19–26. [Google Scholar]
- 2. Anderson J, et al. The prevalence and genetic diversity of Campylobacter spp. in domestic ‘backyard’ poultry in Canterbury, New Zealand. Zoonoses Public Health 2012;59:52–60. [DOI] [PubMed] [Google Scholar]
- 3. Bautista AC, et al. Lead exposure from backyard chicken eggs: a public health risk? J Med Toxicol 2014;10:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertran K, et al. Pathobiology of clade 2.3.4.4 H5Nx high-pathogenicity avian influenza virus infections in minor gallinaceous poultry supports early backyard flock introductions in the western United States in 2014–2015. J Virol 2017;91:e00960-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark S, Kimminau E. Critical review: future control of blackhead disease (histomoniasis) in poultry. Avian Dis 2017;61:8. [DOI] [PubMed] [Google Scholar]
- 6. Crespo R, et al. Pet poultry training for veterinary practitioners. J Vet Med Educ 2010;37:383–387. [DOI] [PubMed] [Google Scholar]
- 7. Crespo R, et al. Outbreak of Listeria monocytogenes in an urban poultry flock. BMC Vet Res 2013;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crespo R, Senties-Cue G. Postmortem survey of disease conditions in backyard poultry. J Exotic Pet Med 2015;24:156–163. [Google Scholar]
- 9. Derksen T, et al. Biosecurity assessment and seroprevalence of respiratory diseases in backyard poultry flocks located close to and far from commercial premises. Avian Dis 2017;62:1–5. [DOI] [PubMed] [Google Scholar]
- 10. Duff JP. Marek’s disease vaccination of backyard, hobby and small-producer poultry flocks. Vet Rec 1998;142:731–732. [PubMed] [Google Scholar]
- 11. Dunn JR, Gimeno IM. Current status of Marek’s disease in the United States and worldwide based on a questionnaire survey. Avian Dis 2013;57(2 Suppl):483–490. [DOI] [PubMed] [Google Scholar]
- 12. Elkhoraibi C, et al. Backyard chickens in the United States: a survey of flock owners. Poultry Sci 2014;93:2920–2931. [DOI] [PubMed] [Google Scholar]
- 13. Gaffga NH, et al. Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N Engl J Med 2012;366:2065–2073. [DOI] [PubMed] [Google Scholar]
- 14. García M. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet Microbiol 2017;206:157–162. [DOI] [PubMed] [Google Scholar]
- 15. Gimeno I, et al. Novel criteria for the diagnosis of Marek’s disease virus-induced lymphomas. Avian Pathol 2005;34:332–340. [DOI] [PubMed] [Google Scholar]
- 16. Gimeno IM. Marek’s disease vaccines: a solution for today but a worry for tomorrow? Vaccine 2008;26:C31–C41. [DOI] [PubMed] [Google Scholar]
- 17. Godwin RM, Morgan JA. A molecular survey of Eimeria in chickens across Australia. Vet Parasitol 2015;214:16–21. [DOI] [PubMed] [Google Scholar]
- 18. Haesendonck R, et al. High seroprevalence of respiratory pathogens in hobby poultry. Avian Dis 2014;58:623–627. [DOI] [PubMed] [Google Scholar]
- 19. Haq K, et al. Immunity to Marek’s disease: where are we now? Developmental Comp Immunol 2013;41:439–446. [DOI] [PubMed] [Google Scholar]
- 20. Hardy MC, et al. Notes from the field: environmental investigation of a multistate salmonellosis outbreak linked to live backyard poultry from a mail-order hatchery—Michigan, 2018. MMWR Morb Mortal Wkly Rep 2019;67:1430–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan B. Vaccination against infectious bronchitis virus: a continuous challenge. Vet Microbiol 2017;206:137–143. [DOI] [PubMed] [Google Scholar]
- 22. Julian RJ. Physiological, management and environmental triggers of the ascites syndrome: a review. Avian Pathol 2000;29:519–527. [DOI] [PubMed] [Google Scholar]
- 23. Kennedy DA, et al. Industry-wide surveillance of Marek’s disease virus on commercial poultry farms. Avian Dis 2017;61:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leibler JH, et al. Lead exposure to children from consumption of backyard chicken eggs. Environmental Res 2018;167:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liebhart D, et al. Histomonosis in poultry: previous and current strategies for prevention and therapy. Avian Pathol 2017;46:1–18. [DOI] [PubMed] [Google Scholar]
- 26. Mays JK, et al. Characterization of reticuloendotheliosis virus isolates obtained from broiler breeders, turkeys, and prairie chickens located in various geographical regions in the United States. Avian Pathol 2010;39:383–389. [DOI] [PubMed] [Google Scholar]
- 27. Mete A, et al. Marek’s disease in backyard chickens, a study of pathologic findings and viral loads in tumorous and nontumorous birds. Avian Dis 2016;60:826–836. [DOI] [PubMed] [Google Scholar]
- 28. Mete A, et al. Causes of mortality in backyard chickens in northern California: 2007–2011. Avian Dis 2013;57:311–315. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen JC, et al. Phylogenetic relationships among virulent Newcastle disease virus isolates from the 2002–2003 outbreak in California and other recent outbreaks in North America. J Clin Microbiol 2004;42:2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peebles ED. In ovo applications in poultry: a review. Poultry Sci 2018;97:2322–2338. [DOI] [PubMed] [Google Scholar]
- 31. Pohjola L, et al. Questionnaire study and postmortem findings in backyard chicken flocks in Finland. Acta Vet Scand 2015;57:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pohjola L, et al. Zoonotic public health hazards in backyard chickens. Zoonoses Public Health 2016;63:420–430. [DOI] [PubMed] [Google Scholar]
- 33. Pollock SL, et al. Raising chickens in city backyards: the public health role. J Community Health 2012;37:734–742. [DOI] [PubMed] [Google Scholar]
- 34. Regmi PR, et al. Regulatory considerations for the approval of drugs against histomoniasis (blackhead disease) in turkeys, chickens, and game birds in the United States. Avian Dis 2016;60:725–730. [DOI] [PubMed] [Google Scholar]
- 35. Sary K, et al. Esophagitis and pharyngitis associated with avian infectious laryngotracheitis in backyard chickens: two cases. Avian Dis 2017;61:255–260. [DOI] [PubMed] [Google Scholar]
- 36. Schat KA, Nair V. Neoplastic diseases. In: Swayne DE, ed. Diseases of Poultry. 13th ed. Ames, IA: Wiley-Blackwell, 2013:515–673. [Google Scholar]
- 37. Schijns VEJC, et al. Practical aspects of poultry vaccination. In: Schat KA, et al., eds. Avian Immunology. 2nd ed. San Diego, CA: Elsevier, 2014:345–356. [Google Scholar]
- 38. Smith E, et al. Epidemiologic characterization of Colorado backyard bird flocks. Avian Dis 2012;56:263–271. [DOI] [PubMed] [Google Scholar]
- 39. Sobhakumari A, et al. Lead contamination in backyard chicken layer flocks in California. J Vet Diagn Invest 2018;31. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trott KA, et al. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet Pathol 2014;51:787–795. [DOI] [PubMed] [Google Scholar]
- 41. Wideman RF, et al. Pulmonary arterial hypertension (ascites syndrome) in broilers: a review. Poultry Sci 2013;92:64–83. [DOI] [PubMed] [Google Scholar]


