Abstract
Glycated hemoglobin A1c (HbA1c) is widely used for monitoring and diagnosing human diabetes mellitus, but is rarely used in veterinary clinics. The goal of our study was to validate the commercial HbA1c testing system SD A1cCare analyzer (Bionote, Gyeoggi-do, South Korea) for use in dogs. Dogs were recruited with owner’s consent. Diabetic status was determined based on clinical signs, fasting hyperglycemia, and glycosuria. Intra-assay precision and linearity were evaluated with EDTA, heparin, or citrate as anticoagulants, and had excellent precision with mean coefficients of variation (CVs) of 2.47%, 2.26%, and 1.92%, respectively. Diluted anticoagulated blood samples showed excellent linear relationships with R2 of 0.991, 0.996, and 0.994, respectively. Inter-assay precision revealed that the mean CV of the normal control was 2.18% and that of the high control was 2.01% (30 repeats). Observed total error of a normal control was 7.81%, and 6.12% for the high control. HbA1c level measured before and after removal of plasma and replacement by saline showed minimal interference by lipid contents (p = 0.929). The HbA1c concentrations of diabetic dogs were significantly higher than those of non-diabetic dogs (p < 0.001). HbA1c value >6.2% indicated canine diabetes through a classification and regression tree model. In most cases, fructosamine and HbA1c were highly correlated (r = 0.674, p < 0.001). The HbA1c testing system could be a valuable testing system to evaluate canine diabetes mellitus, providing an alternative in-house option for use by veterinary clinicians.
Keywords: diabetes mellitus, dogs, fructosamine, glucose, glycated hemoglobin, HbA1c
Introduction
Diabetes mellitus (DM) is a relatively common disease in dogs. It is characterized by clinical signs of polyuria, polydipsia, and polyphagia, and is defined by persistent hyperglycemia and glucosuria.7 The status of canine DM is diagnosed and monitored by measuring blood glucose concentrations. However, blood glucose concentrations tend to vary depending on the time of sample collection and other physiologic conditions, such as fasting and stress.6,17 Therefore, glycated protein markers that can indicate long-term glycemic levels have been utilized in human and veterinary medicine for the diagnosis and monitoring of DM.12,15,38
Glucose binds to several proteins in circulation, which creates glycated proteins including hemoglobin A1c (HbA1c) and fructosamine.38 HbA1c is formed by the irreversible chemical binding of glucose molecules to the amino-terminal group of hemoglobin.31 Considering the half-life of red blood cells, the concentration of HbA1c reflects the average plasma glucose concentration over the previous 2–3 mo.22,26 Fructosamine is formed by glycation of total serum proteins.19 Because albumin is the most abundant protein in plasma and has a half-life of ~8 d,11,14 fructosamine concentrations generally reflect the plasma glucose concentration over the previous 1–2 wk.5,19–22,38
In human medicine, evaluation of HbA1c concentration has been universally adopted to diagnose DM and assess glycemic control in DM patients, whereas fructosamine levels are rarely used.1,37 In contrast, fructosamine testing has been widely used to monitor canine DM patients. However, fructosamine testing reagents and equipment are unavailable in some countries.38 In addition, fructosamine levels are affected by lipemia and concurrent diseases that disrupt serum protein turnover.10,33,34
Given the molecular and structural similarities between canine and human hemoglobin, antibodies developed for human hemoglobin can recognize their canine counterpart.3 Some studies have suggested that HbA1c testing could be applicable to canine DM patients.8,19,20,26,27 Because HbA1c testing is infrequently used, and is widely unavailable,8,30 clinical utility of HbA1c testing is limited in veterinary medicine; however, the commercial point-of-care (POC) human HbA1c testing system, SD A1cCare analyzer (Bionote, Gyeoggi-do, South Korea), is a rapid test, thus clinically convenient, and requires a minimal amount of blood and minimal handling.3,15 We evaluated the potential utility of the SD A1cCare analyzer in the diagnosis of DM in dogs.
Materials and methods
Study design
We performed our study in 3 phases. In phase I, we aimed to partially validate the HbA1c assay. In phase II, we assessed the discriminatory value of HbA1c levels in different groups. In phase III, we compared HbA1c and fructosamine results. All animal experimentation was performed in accordance with guidelines for the care and use of animals approved by the Institutional Animal Care and Use Committee of Seoul National University (SNU-180205-4).
Patient selection
In phase I, we enrolled 7 healthy dogs owned by veterinarians of Seoul National University Veterinary Medical Teaching Hospital (SNU-VMTH; Seoul, South Korea) and 15 SNU-VMTH client-owned DM dogs. Owner consent was obtained before enrollment. The patients were used to evaluate HbA1c intra-assay precision and effects of storage (3 healthy dogs and 4 DM dogs), assay linearity (3 DM dogs), and lipemic interference (4 DM dogs with lipemia, 4 DM dogs without lipemia, and 4 healthy dogs).
The phase II population was different from phase I. For the phase II study, to evaluate the potential value of HbA1c as a screening test, 3 groups of dogs were recruited: healthy, non-diabetic sick, and diabetic. Owners of healthy dogs consisted of staff and students of SNU-VMTH and voluntary participants. Healthy dogs were defined based on the absence of remarkable findings in history, clinical signs, physical examination, complete blood count (CBC), electrolyte, and serum biochemistry tests including total protein, albumin, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, total bilirubin, glucose, calcium, and phosphorus. The non-diabetic sick and diabetic dogs were canine patients that visited the SNU-VMTH and the Haemaru Referral Animal Hospital (HRAH; Seongnam, South Korea). Eligible diabetic dogs were enrolled sequentially. Client-owned sick dogs without DM were enrolled as non-diabetic sick dogs. Naïve diabetic dogs were enrolled based on relevant clinical findings including polyuria and polydipsia, hyperglycemia, and glycosuria.9 All dogs were enrolled with owner consent.
Diabetic dogs that visited the SNU-VMTH and the HRAH for DM monitoring were enrolled in the phase III study. All DM dogs received appropriate treatment based on published guidelines.36 The DM population included 3 phase II DM dogs who received treatment after diagnosis. The healthy dog population was identical to phase II.
Sample collection
All blood samples were collected from the jugular vein. In phase I, to assess intra-assay precision and effects of sample storage, ~3 mL of blood was collected from each dog, and ~1 mL was aliquoted into EDTA, lithium heparin, and sodium citrate tubes, respectively.
To analyze linearity, ~6.5-mL blood samples were collected from each dog. Blood samples were distributed into EDTA, heparin, and citrate tubes, 1 mL each. To obtain excess serum for dilutions, ~3.5 mL of blood was transferred to serum separator tubes (Vacutainer SST II Advance tube; Becton Dickinson, Tokyo, Japan) for centrifugation and serum harvesting.
For the lipemic interference evaluation, ~4.5 mL of blood was collected from each dog; ~1 mL of blood was transferred to EDTA tubes, and ~3.5 mL into serum separator tubes.
In phases II and III, ~4.5-mL blood samples were collected; ~1 mL was transferred to EDTA tubes, and ~3.5 mL was transferred to serum separator tubes. HbA1c from all of the EDTA-anticoagulated samples was measured immediately after collection.
Serum separator tube samples were allowed to clot at room temperature for 30 min followed by centrifugation (1,300 × g) for 10 min. After centrifugation, sera were collected.
Measurement of HbA1c percentage
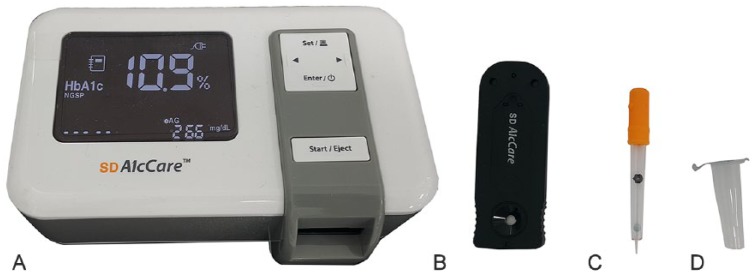
A human immunochromatographic HbA1c testing system, the SD A1cCare analyzer, was used to measure HbA1c levels. Values are reported as a percentage of total hemoglobin. The corresponding SD A1cCare test kit (Bionote) includes a test cartridge, a tube containing a lysis buffer, and a dropper containing a latex tablet (Fig. 1). The percentage of HbA1c was measured according to the manufacturer’s recommendations. Briefly, ~5 μL of whole blood was added to the buffer-containing tube. The blood was mixed thoroughly with the buffer solution by pipetting with the dropper. The buffer lysed the erythrocytes and released glycated hemoglobin and other contents. The latex tablet inside the dropper dissolved and released latex microparticles that were conjugated to the specific antibody against HbA1c. The mixture containing the resulting complex was then transferred to a test cartridge that was inserted into the commercial analyzer. The instrument measures one sample at a time. Three minutes after pushing the analyzer’s start button, the percentage of HbA1c results appears on the screen. The test was performed blindly, and results were recorded along with each dog’s clinical information.
Figure 1.
The SD A1cCare analyzer and SD A1cCare test kit: A. SD A1cCare analyzer; B. test cartridge; C. dropper; D. buffer tube.
Phase I: partial HbA1c assay validation
To validate HbA1c percentages measured by the commercial analyzer, we evaluated intra-assay precision, linearity, inter-assay precision, observed total error (TEobs), and interference. In order to assess intra-assay precision, we tested 7 EDTA, 7 heparin, and 7 citrate anticoagulated samples from 7 dogs 5 times each on the same day. To assess the effect of storage time, we stored EDTA, heparin, or citrate blood samples at 4°C for up to 7 d. To evaluate linearity, we measured HbA1c levels in diluted samples (0:100, 10:90, 20:80, 30:70, 40:60, 50:50, 60:40, 70:30, 80:20, 90:10, and 100:0) with EDTA, heparin, or citrate as anticoagulants. Serum obtained from each dog was used to dilute the samples. Results were analyzed by regression analysis (R Foundation, MA). To evaluate inter-assay precision, normal quality control (QC) material and high QC material (Bionote) were measured 30 times over 5 d. Bias was calculated using the measured mean of QC materials and reported mean provided by the manufacturer, according to the formula: bias% = | meanmanufacturer – meanmeasured | / meanmanufacturer × 100%.16 TEobs was calculated using coefficient of variation (CV) of inter-assay precision and bias according to the formula: TEobs = 2CV + bias%.16
Blood samples from 4 DM dogs with lipemia, 4 DM dogs without lipemia, and 4 healthy dogs were used to assess the effect of lipid values on HbA1c level. Milky white and turbid samples with triglyceride concentration >4.52 mmol/L were classified as lipemic. Triglyceride and total cholesterol concentrations were measured for serum samples (7180 automatic analyzer; Hitachi, Tokyo, Japan). HbA1c levels were measured first using EDTA samples of 12 dogs, followed by centrifugation (VS-5000 multi-purpose refrigerated centrifuge; Vision Scientific, Daejeon, South Korea) at 1,660 × g for 10 min. The plasma was carefully removed from EDTA-anticoagulated samples, and replaced with an equal volume of 0.9% NaCl. After mixing thoroughly, HbA1c levels were re-measured.
Phase II: evaluation of HbA1c levels in clinical samples
In the phase II patient signalment, clinical history, hematocrit (HCT), HbA1c, and glucose levels were recorded. CBC was determined (ADVIA 2120i; Siemens, Berlin, Germany). Serum biochemistry tests including glucose concentrations were measured (7180 automatic analyzer; Hitachi). Descriptive statistics evaluated patient characteristics of each dataset. A 2-tailed Student t-test was used to compare blood glucose and HbA1c levels between diabetic and non-diabetic dogs (SPSS 23.0 software; IBM, New York, NY). To determine if HCT levels affected the HbA1c level, bivariate correlation analysis was performed. To investigate diagnostic utility, the concentrations of blood glucose and the HbA1c of heathy and DM dogs were further analyzed (R program; R Foundation). Predictive models were constructed from the data. Associations between DM and HbA1c level were examined by using the classification and regression trees (CART) method, which is based on a machine-learning algorithm.32
Phase III: comparison of HbA1c to fructosamine
Fructosamine concentrations were measured (Catalyst DX chemistry analyzer; IDEXX, Westbrook, ME). We assessed the agreement between fructosamine and HbA1c by bivariate correlation analysis and Passing–Bablok analysis. Statistical analyses for the phase III study were performed (SPSS 23.0 software, IBM; R program, R Foundation).
Results
Phase I: partial HbA1c assay validation
The repeated measurement of canine samples treated with EDTA, heparin, or citrate as anticoagulants showed great intra-assay precision with a mean CV of 2.47%, 2.26%, and 1.92%, respectively (Supplementary Table 1). The CV ranges were 1.36–4.46%, 1.14–2.68%, and 0.99–3.26%, respectively (Supplementary Table 1). The HbA1c level in canine whole blood was minimally affected by the type of anticoagulant, with an overall CV of 2.22%. In the inter-assay precision analysis, the mean CV of the normal control was 2.18%, and that of the high control 2.01% (30 repeats). TEobs of the normal control was 7.81%, and that of the high control 6.12%. Regression analyses on EDTA-, heparin-, and citrate-anticoagulant–containing diluted blood samples showed excellent linear relationships (R2 = 0.991, 0.996, and 0.994, respectively; Supplementary Fig. 1). The HbA1c concentrations were highly reproducible up to 3 d of storage with CVs of 1.95%, 1.79%, and 2.04% in EDTA-, heparin-, and citrate-containing tubes, respectively. However, variability in the results slightly increased after 7 d of storage with CVs of 2.90%, 3.40%, and 3.37%, respectively. The HbA1c level measured before and after removal of plasma and replacement by saline showed minimal interference by lipid contents (Supplementary Table 2). The average differences between HbA1c level before and after replacement of plasma with saline were 0.1% in lipemic DM dogs, 0.1% in non-lipemic DM dogs, and 0.05% in non-lipemic healthy dogs. There was no significant difference between HbA1c level before and after replacement (p = 0.929).
Phase II: evaluation of HbA1c levels in clinical samples
The phase II study included 147 dogs: 36 healthy, 87 non-diabetic sick, and 24 naïve diabetic dogs. The healthy dog group was comprised of 17 neutered males, 4 intact males, 3 neutered females, and 12 intact females. The non-diabetic sick dog group comprised 30 neutered males, 12 intact males, 23 neutered females, and 22 intact females. The diabetic dog group comprised of newly diagnosed diabetic dogs included 17 neutered males, 2 intact males, 3 neutered females, and 2 intact females. The ages of the healthy dogs was 5 mo to 14 y (mean: 5 y; median: 4 y). The age range of non-diabetic sick dogs was 1–18 y (mean: 10 y; median: 11 y), and that of diabetic dogs was 3–16 y (mean: 11 y; median: 11 y).
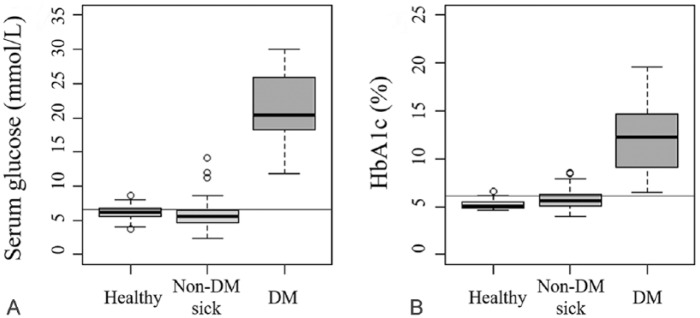
Glucose levels (reference interval [RI]: 3.3–6.7 mmol/L) and HbA1c levels clearly differentiated the diabetic and non-diabetic groups. Diabetic dogs had a significantly higher glucose level (median: 20.8 mmol/L; range: 11.8–30.0 mmol/L) than non-diabetic sick dogs (median: 5.5 mmol/L; range: 2.3–14.1 mmol/L; p < 0.001). Diabetic dogs also had higher glucose levels than healthy dogs (median: 6.1 mmol/L; range: 3.7–8.6 mmol/L; p < 0.001). The HbA1c level was significantly higher in the diabetic dogs (median: 11.6%; range: 6.5–19.6%) than that in both the non-diabetic sick (median: 5.6%; range: 4.0–8.6%) and healthy (median: 5.1%; range: 4.6–6.6%) dogs (p < 0.001). A box plot was used to visually compare groups (Fig. 2). The diagnostic threshold level of HbA1c was 6.2% (see CART analysis below). The data obtained from phase II were subjected to CART analysis, which uses a machine-learning algorithm to construct a prediction model. The classification tree results showed that an HbA1c level >6.2% indicated a high possibility of DM. Bivariate correlation to determine the effect of HCT on HbA1c results revealed no significant correlation between the HCT and HbA1c levels (p = 0.595; Supplementary Fig. 2).
Figure 2.
A. Comparison of serum glucose levels of healthy, non-diabetic sick, and diabetic dogs. The horizontal line represents the upper limit of the glucose reference interval, 6.7 mmol/L. B. Comparison of the HbA1c levels of healthy, non-diabetic sick, and diabetic dogs. The horizontal line represents a cutoff value of 6.2%.
Phase III: comparison of HbA1c to fructosamine
The phase III study was composed of 90 dogs including 54 treated DM dogs and 36 healthy dogs. The diabetic dog group consisted of 36 neutered males, 3 intact males, 9 neutered females, and 6 intact females. The ages of the diabetic dogs was 2–15 y (mean: 10 y; median: 11 y). Bivariate correlation analysis evaluated the degree of correlation between the HbA1c and fructosamine levels (RI: 225–375 μmol/L; Supplementary Table 3; Fig. 3). HbA1c and fructosamine were significantly correlated in the study population over a wide range of glycemic control (r = 0.674, p < 0.001). Passing–Bablok regression analysis also revealed that HbA1c and fructosamine were significantly correlated (r = 0.674, p < 0.001). The regression line of Passing–Bablok regression analysis had an intercept of 2.042 (p = 0.018), and slope of 0.018 (Supplementary Fig. 3).
Figure 3.

Distribution of HbA1c and fructosamine in diabetic and healthy dogs. Circle (o) indicates diabetic dogs, and x indicates healthy dogs. Cutoff values of 6.2% for HbA1c and 375 μmol/L for fructosamine are indicated.
Discussion
Our results indicate that the human SD A1cCare analyzer HbA1c testing system could be used to evaluate HbA1c in canine patients. The HbA1c levels were significantly higher in diabetic patients than in their non-diabetic counterparts. HbA1c had good agreement with fructosamine levels. Our results suggest a potential utility of HbA1c testing in monitoring glycemic control in dogs.
HbA1c showed excellent intra- and inter-assay precision. A limitation of our study is that potential matrix effect could not be evaluated because QC materials were used. The results obtained using QC materials vary because of matrix-related effects, and clinical samples are required to evaluate a possible matrix effect.23 In our study, other laboratory validation results, including linearity and lipemic interference, suggest that the HbA1c testing system provides reliable and reproducible results.
In most cases, HbA1c and glucose levels had good agreement. Both HbA1c and glucose levels of DM dogs were significantly higher than those of non-diabetic dogs. Therefore, both HbA1c and glucose levels appear to have discriminatory value in distinguishing DM. However, these 2 values were not completely concordant; several dogs had normal HbA1c levels but elevated glucose levels, which may be attributable to stress. Contrary to glucose levels that fluctuate within a day, HbA1c levels reflect the degree of glucose exposure over time.2 The HbA1c measurement procedure does not require fasting or timed samples. In addition, HbA1c levels are relatively stable, and unaffected by acute perturbation, such as stress or illness.18,27,28,39
We used a CART method, which employs a machine-learning algorithm, to create a useful flow chart for assessment of canine DM patients. Unlike traditional analytical methods, CART analysis utilizes a tree-building technique. Therefore, CART analysis is considered ideal for generating clinical decision rules.25,32 The CART-predicted outcomes indicate the class to which the data belong and their expected numbers. The CART model results showed that HbA1c testing could be used to diagnose canine DM reliably and with precision, with a proposed diagnostic threshold of 6.2%.
When using the HbA1c assay, several factors should be considered.29 For example, clinical conditions that alter red blood cell turnover may lead to spurious HbA1c results.3 In our study, the effect of HCT level on the HbA1c level was not significant. However, considering the lack of study population members with significant anemia (only 1 patient had a HCT <0.20 L/L), the result may be inconclusive, warranting further study. Although DM diagnosis using HbA1c in non-anemic or non-polycythemic patients may be reliable, care should be taken when a patient has severe anemia, polycythemia, or has a history of blood transfusion.4,13
Phase III of the study demonstrated that fructosamine and HbA1c levels were highly correlated. In veterinary medicine, fructosamine testing has been widely used because that assay’s RI and relevant clinical data are well established.19 However, clinicians should exercise caution when interpreting fructosamine levels of patients with an abnormal rate of serum protein turnover, such as subjects with hyperthyroidism, protein-losing enteropathy, nephrotic syndrome, or liver failure.15,24,34,40
In human medicine, HbA1c is more commonly used for diabetic monitoring than fructosamine, because diabetic care is rarely adjusted within a 1–2 wk period.35 Moreover, fructosamine testing may only be available in commercial reference laboratories, increasing the difficulty in adjusting a patient’s treatment schedule efficiently.13 Fructosamine testing requires a serum sample, whereas HbA1c testing requires only 5 µL of whole blood. Measurement of HbA1c is much faster and simpler than fructosamine testing because it does not require additional sample preparation. In addition, DM patients are prone to develop hyperlipidemia, which may lead to a falsely decreased fructosamine level.2,34 Unlike fructosamine, HbA1c level was not influenced by serum lipid level in our study. To validate clinical utility of HbA1c testing in monitoring canine DM, further studies are warranted, including thorough follow-up analysis after variably prolonged insulin treatment.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638719832071 for Evaluation of a human glycated hemoglobin test in canine diabetes mellitus by Na-Yon Kim, Jaehoon An, Jae-Kyung Jeong, Sumin Ji, Sung-Hyun Hwang, Hong-Seok Lee, Myung-Chul Kim, Hyun-Wook Kim, Sungho Won and Yongbaek Kim in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Haet-Sal Shin and Eugene Jung for their valuable technical assistance, and all of the owners whose dogs were enrolled in this study.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplementary material: Supplementary material for this article is available online.
ORCID iD: Yongbaek Kim  https://orcid.org/0000-0003-1633-9247
https://orcid.org/0000-0003-1633-9247
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81–S90. [DOI] [PubMed] [Google Scholar]
- 2. Bennett N. Monitoring techniques for diabetes mellitus in the dog and the cat. Clin Tech Small Anim Pract 2002;17:65–69. [DOI] [PubMed] [Google Scholar]
- 3. Catchpole B, et al. Evaluation of a disposable device for the measurement of haemoglobin A1c in dogs. Vet Rec 2008;162:47–49. [DOI] [PubMed] [Google Scholar]
- 4. Coban E, et al. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol 2004;112:126–128. [DOI] [PubMed] [Google Scholar]
- 5. Coppo J, Coppo NB. Serum fructosamine: a reference interval for a heterogeneous canine population. Vet Res Commun 1997;21:471–476. [DOI] [PubMed] [Google Scholar]
- 6. Csoltova E, et al. Behavioral and physiological reactions in dogs to a veterinary examination: owner-dog interactions improve canine well-being. Physiol Behav 2017;177:270–281. [DOI] [PubMed] [Google Scholar]
- 7. Davison L, et al. Study of 253 dogs in the United Kingdom with diabetes mellitus. Vet Rec 2005;156:467–471. [DOI] [PubMed] [Google Scholar]
- 8. Davison L, et al. Evaluation of two point-of-care analysers for measurement of fructosamine or haemoglobin A1c in dogs. J Small Anim Pract 2002;43:526–532. [DOI] [PubMed] [Google Scholar]
- 9. Davison L, et al. Anti-insulin antibodies in diabetic dogs before and after treatment with different insulin preparations. J Vet Intern Med 2008;22:1317–1325. [DOI] [PubMed] [Google Scholar]
- 10. Desjarlais F, et al. Technical and clinical evaluation of fructosamine determination in serum. Clin Biochem 1989;22:329–335. [DOI] [PubMed] [Google Scholar]
- 11. Dixon FJ, et al. Half-lives of homologous serum albumins in several species. Exp Biol Med 1953;83:287–288. [DOI] [PubMed] [Google Scholar]
- 12. Elliott DA, et al. Glycosylated hemoglobin concentrations in the blood of healthy dogs and dogs with naturally developing diabetes mellitus, pancreatic beta-cell neoplasia, hyperadrenocorticism, and anemia. J Am Vet Med Assoc 1997;211:723–727. [PubMed] [Google Scholar]
- 13. English E, et al. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia 2015;58:1409–1421. [DOI] [PubMed] [Google Scholar]
- 14. Furth ED, et al. The distribution, metabolic fate and radiation dosimetry of 131I labeled macroaggregated albumin. J Nucl Med 1965;6:506–518. [Google Scholar]
- 15. Goemans AF, et al. Validation and determination of a reference interval for canine HbA1c using an immunoturbidimetric assay. Vet Clin Pathol 2017;46:227–237. [DOI] [PubMed] [Google Scholar]
- 16. Harr KE, et al. ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Pathol 2013;42:424–436. [DOI] [PubMed] [Google Scholar]
- 17. Hegstad-Davies RL, et al. Breed-specific reference intervals for assessing thyroid function in seven dog breeds. J Vet Diagn Invest 2015;27:716–727. [DOI] [PubMed] [Google Scholar]
- 18. Hooghuis H, et al. Ion-exchange microchromatography and thiobarbituric acid colorimetry for the measurement of canine glycated hemoglobins. Vet Clin Pathol 1994;23:110–116. [DOI] [PubMed] [Google Scholar]
- 19. Jensen AL. Glycated blood proteins in canine diabetes mellitus. Vet Rec 1995;137:401–405. [DOI] [PubMed] [Google Scholar]
- 20. Jensen AL, Aaes H. Reference interval and critical difference for canine serum fructosamine concentration. Vet Res Commun 1992;16:317–325. [DOI] [PubMed] [Google Scholar]
- 21. Kaneko J, et al. Evaluation of serum fructosamine concentration as an index of blood glucose control in cats with diabetes mellitus. Am J Vet Res 1992;53:1797–1801. [PubMed] [Google Scholar]
- 22. Kawamoto M, et al. Relation of fructosamine to serum protein, albumin, and glucose concentrations in healthy and diabetic dogs. Am J Vet Res 1992;53:851–855. [PubMed] [Google Scholar]
- 23. Koerbin G, et al. Bias assessment of general chemistry analytes using commutable samples. Clin Biochem Rev 2014;35:203. [PMC free article] [PubMed] [Google Scholar]
- 24. Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta 2013;418:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lord C, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry 2012;69:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loste A, et al. Fructosamine and glycated hemoglobin in the assessment of glycaemic control in dogs. Vet Res 2001;32:55–62. [DOI] [PubMed] [Google Scholar]
- 27. Marca M, et al. Effect of acute hyperglycaemia on the serum fructosamine and blood glycated haemoglobin concentrations in canine samples. Vet Res Commun 2000;24:11–16. [DOI] [PubMed] [Google Scholar]
- 28. Marca M, et al. Blood glycated hemoglobin evaluation in sick dogs. Can J Vet Res 2000;64:141. [PMC free article] [PubMed] [Google Scholar]
- 29. Nathan DM. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oikonomidis I, et al. Validation, reference intervals and overlap performance of a new commercially available automated capillary electrophoresis assay for the determination of the major fraction of glycated haemoglobin (HbA1c) in dogs. Vet J 2018;234:48–54. [DOI] [PubMed] [Google Scholar]
- 31. Peterson KP, et al. What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem 1998;44:1951–1958. [PubMed] [Google Scholar]
- 32. Quinlan JR. Induction of decision trees. Mach Learn 1986;1:81–106. [Google Scholar]
- 33. Reusch C, et al. Serum fructosamine concentrations in dogs with hypothyroidism. Vet Res Commun 2002;26:531–536. [DOI] [PubMed] [Google Scholar]
- 34. Reusch C, Haberer B. Evaluation of fructosamine in dogs and cats with hypo- or hyperproteinaemia, azotaemia, hyperlipidaemia and hyperbilirubinaemia. Vet Rec 2001;148:370–376. [DOI] [PubMed] [Google Scholar]
- 35. Rosediani M, et al. Correlation between fasting plasma glucose, post prandial glucose and glycated haemoglobin and fructosamine. Med J Malaysia 2006;61:67–71. [PubMed] [Google Scholar]
- 36. Rucinsky R, et al. AAHA diabetes management guidelines for dogs and cats. J Am Anim Hosp Assoc 2010;46:215–224. [DOI] [PubMed] [Google Scholar]
- 37. Sacks DB. Hemoglobin variants and hemoglobin A1c analysis: problem solved? Clin Chem 2003;49:1245–1247. [DOI] [PubMed] [Google Scholar]
- 38. Sako T, et al. Diagnostic significance of serum glycated albumin in diabetic dogs. J Vet Diagn Invest 2008;20:634–638. [DOI] [PubMed] [Google Scholar]
- 39. Sato KK, et al. Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes the Kansai healthcare study. Diabetes Care 2009;32:644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1C, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr 2012;25:141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638719832071 for Evaluation of a human glycated hemoglobin test in canine diabetes mellitus by Na-Yon Kim, Jaehoon An, Jae-Kyung Jeong, Sumin Ji, Sung-Hyun Hwang, Hong-Seok Lee, Myung-Chul Kim, Hyun-Wook Kim, Sungho Won and Yongbaek Kim in Journal of Veterinary Diagnostic Investigation