Abstract
Objectives
The aim of this study was to perform a systematic review of clinical practice guidelines to identify nonpharmacologic recommendations for osteoporosis treatment.
Methods
A systematic review of literature following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)-statement methodology for clinical practice guidelines was conducted; PROSPERO CRD42019138548. Assessment of selected clinical practice guidelines with the AGREE (Appraisal of Guidelines for Research & Evaluation)-II methodological quality instrument was performed, and those graded over 60 points were selected for recommendations extraction and evidence analysis.
Results
Only 6 clinical practice guidelines fulfilled criteria, 69 nonpharmacological recommendations were extracted: 13 from American Association of Clinical Endocrinologists and American College of Endocrinology guideline, 16 from Malaysian Osteoporosis Society guideline, 15 from the Ministry of Health in Mexico guideline, 14 from Royal Australian College of General Practitioners guideline, 7 from Sociedad Española de Investigación Ósea y del Metabolismo Mineral guideline, and 7 from National Osteoporosis Guideline Group guideline. Percentage by theme showed that the highest number of recommendations were 12 (17.1%) for vitamin D, 11 (15.7%) for a combination of calcium and vitamin D, and 11 (15.7%) for exercise.
Conclusions
These recommendations address integrating interventions to modify lifestyle, mainly calcium and vitamin D intake, and exercise. Other recommendations include maintaining adequate protein intake, identification and treatment of risk factors for falls, and limiting the consumption of coffee, alcohol and tobacco. Considerations on prescription must be taken.
Keywords: Osteoporosis, Nonpharmacologic treatment, Clinical practice guideline, Recommendations
1. Introduction
Given the overwhelming amount of information currently published, at least 75 trials and 11 systematic reviews every day [1,2], it is difficult for the clinician to keep up with evidence production to integrate it in his daily practice. Clinical practice guidelines (CPG) are intended to assist clinicians in decision making through recommendations that synthetize the best available evidence [3].
A frequent problem that the clinician faces is selecting guidelines with adequate methodology. The quality of a clinical guideline is defined as “the confidence that potential biases in the development of a guide have been adequately managed and that the recommendations have internal and external validity, and are feasible for clinical practice” [4,5]. In Mexico, Centro Nacional de Excelencia Tecnológica en Salud (National Center of Health Technology Excellence, CENETEC) develops CPGs; those available for the treatment of osteoporosis are not updated (https://cenetec-difusión.com/GPC-sns/).
The goal of osteoporosis treatment is the prevention of fragility fracture [6]. This should be achieved through a multidisciplinary treatment that includes pharmacological and nonpharmacological interventions. There is lack of knowledge and implementation of recommendations for nonpharmacological treatment.
The aim of this study was to perform a systematic review of CPG for osteoporosis with high methodological quality, to identify nonpharmacological interventions and favor its implementation in daily practice in Mexico.
2. Methods
The present document is derived from an osteoporosis CPG adaptation protocol, through the ADAPTE process by the Guidelines International Network [7] and considering GRADE-ADOLOPMENT model [8]. Guideline adaptation is the systematic approach to the endorsement and/or modification of a guideline(s) produced in one cultural and organizational setting for application in a different context. For this purpose, we developed a systematic review of CPG for diagnosis and treatment of osteoporosis. The proposed question to assess nonpharmacological interventions through the ADAPTE process was: which nonpharmacological treatments should be considered in patients with osteoporosis?
2.1. Identification and selection of guidelines
A systematic literature review aimed to identify CPG for the treatment of osteoporosis was conducted (PROSPERO CRD42019138548). The language was limited to English and Spanish; the search strategy was designed using: MeSH (medical subject headings) terms (osteoporosis, guideline, and treatment), Boolean operators and advanced search in five different databases (PubMed, PEDro, Ovid, Embase, and ScienceDirect), publication dates from 2012 to June 18th, 2019 (see Appendix). Three reviewers (RCZ, AGL, AOGL) analyzed abstracts and retrieved full-text of documents identified as CPG. As this is an adaptation process, we decided to include the last update of CPG from CENETEC since it is published by the Mexican health system, even it is from 2009.
Classification of CPG was performed by two reviewers (RCZ, AOGL) based on the published methodology of each CPG applying the following categorization of the Association of the Scientific Medical Societies in Germany (AWMF) [9,10]: S1 expert recommendations contain treatment recommendations developed by informal consensus of a group of experts, S2 guidelines are created by formal consensus-finding and/or formal search for evidence, and S3 guidelines contain all of the elements of systematic development: representative committee, systematic review and synthesis of the evidence, structured consensus process. Only S3 CPG were included.
2.2. Clinical practice guidelines assessment
AGREE-II methodological quality assessment was performed by three independent reviewers for each guideline; agreement reliability was done through intraclass correlation coefficient, which was considered over 0.6 as substantial agreement. Platform https://www.agreetrust.org/ was used, it includes 23 items rated from 1 (strongly disagree) to 7 (strongly agree) each one. All items are grouped in 6 domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability and editorial independence. An overall guideline assessment and recommendations for use are included: yes, yes with modifications, no. CPG graded 60 points or more in the overall guideline assessment were selected for recommendations extraction. All these recommendations of non-pharmacological interventions were included for analysis, evidence level and recommendation strength.
2.3. Synthesis of recommendations
Analysis was performed using a descriptive textual synthesis directed to scope, context and consistency. Recommendations were selected based on the proposed question. Interventions were categorized by theme (e.g., calcium, vitamin D, exercise). Synthesis of recommendations was done by a panel of reviewers in a consensus process using RAND-UCLA method. It grades appropriateness in a 9-point scale based on assessment of benefit/harm risk assessment, where 9 represents that benefits overcome harms and 1 represents that harms overcome benefits. Each panelist was asked to assess and grade in individual way the extracted recommendations. Assessment was reviewed and synthetized by median calculation. Only recommendations with a median between 7 and 9 were considered appropriate according to the RAND-UCLA method; discussion was then performed to select and synthetize recommendations.
3. Results
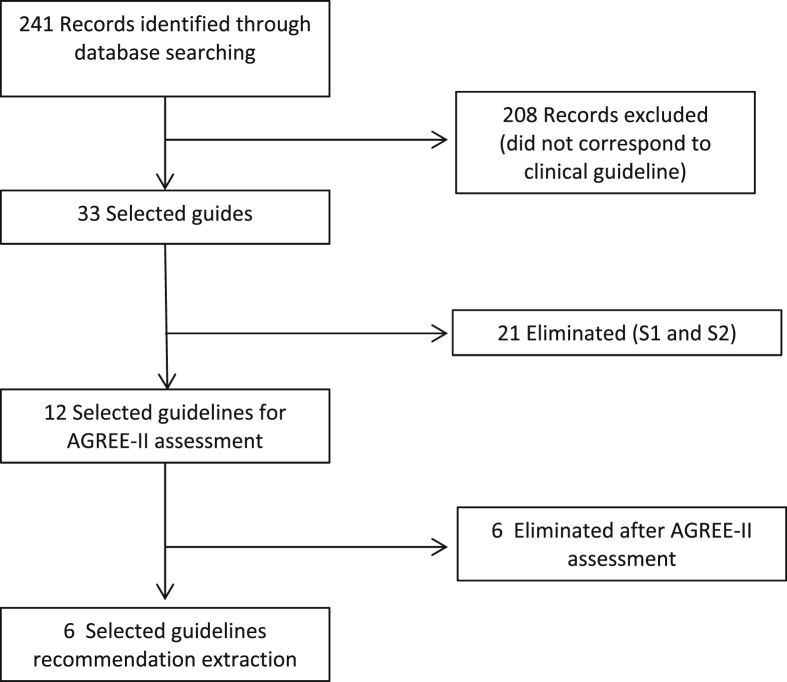
Two-hundred forty-one references were identified through databases search, and 208 were excluded that were not clinical guidelines (e.g., guidance, reviews of literature, randomized controlled trials). Of the 33 selected guidelines, 21 were eliminated as they were classified as S1 and S2 by the AWMF criteria [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]]. Of the remaining 12 [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]], 6 were eliminated after AGREE-II assessment (Fig. 1, Table 1). Intraclass correlation coefficient is shown in Table 2.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of guidelines selection process. AGREE, Appraisal of Guidelines for Research & Evaluation.
Table 1.
AGREE-II assessment of clinical guidelines.
| Domain | Description | Guidelines |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AACE [33] | Malaysian [34] | Philipinnes [43] | CENETEC [35] | CENETEC [32] | CENETEC [36] | RACGP [37] | Rheumatology [38] | SEIOMM [39] | NOGG [40] | NOGG [41] | AMMOM [42] | ||
| I | Source and purpose | 81 | 94 | 91 | 22 | 94 | 59 | 91 | 19 | 22 | 24 | 67 | 85 |
| II | Stakeholder involvement | 50 | 69 | 44 | 19 | 61 | 52 | 57 | 20 | 69 | 22 | 74 | 59 |
| III | Rigour of development | 74 | 60 | 61 | 22 | 67 | 49 | 69 | 27 | 69 | 24 | 63 | 51 |
| IV | Clarity of presentation | 80 | 85 | 80 | 22 | 72 | 65 | 93 | 37 | 70 | 20 | 85 | 43 |
| V | Applicability | 24 | 88 | 17 | 19 | 25 | 19 | 47 | 22 | 24 | 21 | 61 | 28 |
| VI | Editorial independence | 67 | 58 | 19 | 22 | 39 | 61 | 33 | 19 | 44 | 19 | 83 | 11 |
|
Overall guideline assessment |
67 |
61 |
50 |
22 |
72 |
44 |
67 |
33 |
61 |
22 |
78 |
50 |
|
| Would you recommend this guide for use? (3 = yes; 2 = yes with modifications; 1 = no) | |||||||||||||
| Reviewer 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Reviewer 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | |
| Reviewer 3 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | |
AGREE, Appraisal of Guidelines for Research & Evaluation; AACE, American Association of Clinical Endocrinologists; CENETEC, Centro Nacional de Excelencia Tecnológica en Salud; RACGP, Royal Australian College of General Practitioners; SEIOMM, Sociedad Española de Investigación Ósea y del Metabolismo Mineral; NOGG, National Osteoporosis Guideline Group; AMMOM, Asociaci ón Mexicana del Metabolismo Óseo y Mineral.
Table 2.
Reliability assessment: intraclass correlation coefficient (ICC).
| CPG | Cronbach alpha | ICCa | CI |
|---|---|---|---|
| AACE [33] | 0.775 | 0.780 | 0.56–0.89 |
| Malaysian [34] | 0.765 | 0.760 | 0.53–0.89 |
| CENETEC [32] | 0.769 | 0.685 | 0.33–0.86 |
| RACGP [37] | 0.752 | 0.753 | 0.52–0.88 |
| SEIOMM [39] | 0.749 | 0.674 | 0.33–0.85 |
| NOGG [41] | 0.648 | 0.637 | 0.30–0.83 |
CPG, Clinical practice guideline (N = 3 reviewers); CI, confidence interval; AACE, American Association of Clinical Endocrinologists; CENETEC, Centro Nacional de Excelencia Tecnológica en Salud; RACGP, Royal Australian College of General Practitioners; SEIOMM, Sociedad Española de Investigación Ósea y del Metabolismo Mineral; NOGG, National Osteoporosis Guideline Group.
F test: p = 0.001.
Characteristics of the selected guidelines were documented, including the classification used for level of evidence and grade of recommendation (Table 3). The CENETEC guideline [32] was included even though the evidence levels were not stated, since they had already been extracted from the original guidelines.
Table 3.
General information of selected guidelines.
| Year of publication | Name | Organisation | Levels of evidence grading system | Recommendations grading system |
|---|---|---|---|---|
| 2016 | Clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis | AACE/ACE | 2010 Mapping Evidence Levels to Recommended Grading | 2004 AACE Criteria for Grading Recommendations |
| 2012 | Clinical guidance on Management of Osteoporosis | Malaysian Osteoporosis Society | 1999 National Guidelines Clearinghouse | 2001 Modified SIGN |
| 2009 | Guía de práctica clínica para el diagnóstico y tratamiento de la osteoporosis en el adulto | CENETEC | The classification criteria for evidence level and recommendations grading were based on the original CPG: e.g., Shekelle et al.; US Preventive Services Tasks Force; SIGN 2003; Technology assessment report 2000; ICSI Health Care Guidelines Diagnosis and Treatment of Osteoporosis 2006. | |
| 2017 | Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age | RACGP and OA | 2009 Adapted from National Health and Medical Research Council | |
| 2008 (update 2014) | Guías de práctica clínica en la osteoporosis posmenopáusica, glucocorticoidea y del varón | SEIOMM | Oxford Center for Evidence Based Medicine | |
| 2017 | Clinical guideline for the prevention and treatment of osteoporosis | NOGG | 2017 National Osteoporosis Guideline Development Group | |
AACE/ACE, American Association of Clinical Endocrinologists/American College of Endocrinology; CENETEC, Centro Nacional de Excelencia Tecnológica en Salud; RACGP/OA, Royal Australian College of General Practitioners/Osteoporosis Australia; SEIOMM, Sociedad Española de Investigación Ósea y del Metabolismo Mineral; NOGG, National Osteoporosis Guideline Group; SIGN, Scottish Intercollegiate Guidelines Network; CPG, clinical practice guidelines; ICSI, Institute for Clinical Systems Improvement.
From the 6 selected clinical guidelines, 69 nonpharmacological recommendations were extracted: 13 from AACE/ACE guideline [33], 16 from Malaysian Osteoporosis Society guideline [34], 15 from CENETEC guideline [32], 14 from RACGP guideline [37], 7 from SEIOMM guideline [39], and 7 from NOGG guideline [41]. Only one recommendation was modified by consensus. Percentage by theme showed that the highest number of recommendations were for vitamin D with 12 (17.1%), exercise with 11 (17.1%), and a combination of calcium and vitamin D with 11 (15.7%) (Table 4).
Table 4.
Synthesis of number of recommendations with level of evidence and strength of recommendations, according to each topic, and based on grading systems mentioned in Table 3.
| Theme and guideline recommendation category | AACE [33] |
Malaysian [34] |
CENETEC [32] |
RACGP [37] |
SEIOMM [39] |
NOGG [41] |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NR | LE and/or SR reported | NR | LE and/or SR reported | NR | LE and/or SR reported | NR | LE and/or SR reported | NR | LE and/or SR reported | NR | LE and/or SR reported | |
| Protein intake | 2 | I IV |
3 | IIb; B III; B IV; C |
1 | C | 1 | Ib | ||||
| Calcium | 1 | II; B | 3 | Ia; A IIa; B Ia |
1 | A | 1 | † | 1 | Ia; B | ||
| Vitamin D | 4 | I; A II; B III; B IV; C |
4 | Ia; A IV Ia; A IIa |
2 | A A |
1 | † | 1 | Ia; A | ||
| Combination of calcium and vitamin D | 3 | Ia Ia Ib |
3 | A A B |
2 | C C |
2 | Ia,Ib, IIb; B |
1 | B | ||
| Vitamin K | 1 | 1; B | ||||||||||
| Other supplements (magnesium, copper, zinc, phosphorus, manganese, iron) | 1 | D | ||||||||||
| Hip protectors | 1 | I; B | 1 | C | 1 | Ia | ||||||
| Caffeine | 2 | III; B III |
2 | II; B †† |
||||||||
| Smoking | 1 | II; B | 1 | IIa; B | 1 | †† | 1 | C | 1 | † | ||
| Alcohol | 1 | II; B | 1 | IIa; B | 1 | †† | 1 | C | ||||
| Physical therapy | 1 | I; A | ||||||||||
| Exercise | 1 | II; B | 1 | IV; C | 3 | B B B |
5 | B C A A A |
1 | † | 1 | Ia; B |
| Risk falls | 1 | I; A | 2 | A A |
1 | † | 1 | A | ||||
| Education | 1 | D | ||||||||||
AACE, American Association of Clinical Endocrinologists; CENETEC, Centro Nacional de Excelencia Tecnológica en Salud; RACGP, Royal Australian College of General Practitioners; SEIOMM, Sociedad Española de Investigación Ósea y del Metabolismo Mineral; NOGG, National Osteoporosis Guideline Group; NR, number of recommendations; LE, level of evidence; SR, strength of recommendations.
†SEIOMM integrates one recommendation including calcium, vitamin D, smoking, exercise and risk falls. ††CENETEC integrates one recommendation including caffeine, smoking and alcohol.
3.1. Synthesis of recommendations of CPGs for practitioner (implications for rehabilitation)
-
R1.
Maintenance of an adequate protein intake is important for the preservation of musculoskeletal function in postmenopausal women and men over 50 years of age [33,34,37,41].
-
R2.
The use of protein supplements in patients with hip fracture minimizes bone loss, decreases the risk of infection, decreases the length of hospital stay and increases functional recovery. Recommended protein intake is 1.2 g/kg daily. Modified by consensus from CENETEC guide [33].
-
R3.
Within the population-based strategies, lifestyle modifications are recommended to achieve an adequate intake of calcium and sufficient intake of vitamin D (diet, sun exposure) [39,41].
-
R4.
The desirable blood levels of vitamin D are 30–50 ng/mL. In case of requiring supplementation, as low blood levels or patients with risk factors for low levels, a minimum dose of 400 IU is recommended [33]. In patients with greater risk of deficiency, such as the elderly and the chronically ill, doses between 800 and 2000 IU are recommended [32].
-
R5.
The recommended calcium intake is 1000–1200 mg daily, preferably through nutritional intake. If the diet does not contain enough, supplementation is required without exceeding 1200 mg daily. To favor its absorption, it is not recommended to exceed 500–600 mg per dose [[32], [33], [34],39,41].
-
R6.
Calcium supplementation is not recommended in older, non-institutionalized adults [37].
-
R7.
Supplementation with vitamin K, magnesium, cooper, zinc, phosphorus, iron, or essential fat acids is not recommended for the prevention or treatment of osteoporosis [32].
-
R8.
Reducing the intake of caffeine is recommended by limiting the consumption of no more than 4 cups of coffee per day [32,34].
- R9.
-
R10.
It is recommended to limit the consumption of alcohol to no more than 2 units per day [[32], [33], [34],37].
-
R11.
A validated multifactorial assessment of fall risk that evaluates the history of falls and risk of fragility fracture is recommended in patients over 75 years of age, to decide on whom interventions are indicated [33,37,39,41].
-
R12.
To reduce the risk of fragility fracture through the prevention of falls, exercise with weight, balance and resistance load is recommended, to improve mobility, strength and physical performance [33,37,39,41]. Special attention must be taken in patients with high risk of fragility fracture within institutions, through supervision of therapy (e.g., physical therapy) [33].
-
R13.
The use of hip protectors is recommended for institutionalized patients with a high risk of falls [34,37,41].
-
R14.
Provide postmenopausal women and men over 50 years with risk or diagnosis of osteoporosis access to education, psychosocial support and encourage them to seek support from appropriate sources according to individual needs [33].
4. Discussion
The adaptation of a clinical practice guide is an eclectic process that aims to make recommendations, in the form of syncretic postulates based on the best and most current evidence available, giving rise to a true practice based on evidence considering the contextual situation.
According to the AGREE methodology, the selection criterion of clinical practice guidelines was based solely on the overall rating. However, it is important to note that this rating is integrated by the appraiser’s global appreciation of the guide, without considering numerical rating of each domain.
Another section of the AGREE method includes the appraiser’s recommendations for the usage of each guide: recommended, recommended with modifications, not recommended; but these results are not considered in the overall rating neither included in the manual of the instrument. It is necessary to consider that all the reviewers recommended the use of the selected guides with modifications, and of the non-selected guides some were recommended for their use. Furthermore, based on the AGREE method these guides were not selected for extraction of recommendation.
CPG concentrate recommendations on pharmacological interventions. And when considered non-pharmacological interventions, they provide limited information through recommendations for these interventions. Reviewing the source of evidence used in the CPGs, we found that the majority of articles were published in past decades, and it is worth mentioning that there is more recent evidence, and some recommendations may have implications in clinical practice. Therefore, we took on the task of carrying out a review of the current literature of each of the recommendations extracted, which we suggest to consider.
Considerations of recommendations based on literature:
Recommendation 1. Maintenance of an adequate protein intake is important for the preservation of musculoskeletal function in postmenopausal woman and man over 50 years of age.
Recommendation 2. The use of protein supplements in patients with hip fracture minimizes bone loss, decreases the risk of infection, decreases the length of hospital stay and increases functional recovery. Recommended protein intake is 1.2 g/kg daily.
Malnutrition in elderly population is frequent, prevalence range from 14% to 40% in elderly subjects [44]. Protein supplementation in institutionalized patients with recent hip fracture improves subsequent clinical course by diminishing length of hospital stay and infection rate [45]. In general population, protein intake diminishes bone mineral loss and increases muscle strength, probably by improving insulin-like growth factor-1 levels [46].
The extracted recommendation on protein intake is 0.8 g/kg/day. There is evidence that suggest that the recommended intake protein for general elderly population is 1–1.2 g/kg/day, and is most effective on muscle and bone mass when combined with exercise [47]. For this purpose, the consensus considered to modify the original recommendation 0.8 g/kg/day because this is based on the Food and Nutrition Board of recommended dietary allowance, but it reflects the lowest end of the acceptable macronutrient distribution range [48].
Recommendation 3. Within the population-based strategies, lifestyle modifications are suggested to achieve an adequate intake of calcium and sufficient intake of vitamin D (diet, sun exposure).
Recommendation 4. The desirable blood levels of vitamin D are 30–50 ng/mL. In case of requiring supplementation, a minimum dose of 400 IU is recommended. In patients with greater risk of deficiency, such as the elderly and the chronically ill, doses between 800 and 2000 IU are recommended.
Recommendation 5. The recommended calcium intake is 1000–1200 mg daily, preferably through nutritional intake. If it is not completed, supplementation is required without exceeding this amount. To favor its absorption, it is not recommended to exceed 500–600 mg per dose.
Recommendation 6. Calcium supplementation is not recommended in older, noninstitutionalized adults.
Recommendation 7. Supplementation with vitamin K, magnesium, cooper, zinc, phosphorus, iron or essential fat acids is not recommended for the prevention or treatment of osteoporosis.
Apparently, calcium supplementation is not so innocuous. Calcium prescription should be based on a detailed analysis of calcium intake. The excessive calcium intake may lead to hypercalcemia. Patients who have an adequate calcium intake through diet have a reduced risk of kidney stone formation, in contrast with calcium supplementation consumers, who are at a higher risk. On the other hand, patients with type 2 diabetes are more prone to uric acid stone development [49].
Similarly, hypervitaminosis D is a rare cause of hypercalcemia. After long term (7 years) vitamin D supplementation, an increase of 17% for kidney stone risk formation was detected [50,51].
When prescribing calcium, the type of salt must be taken in consideration, as there are differences in cost, absorption mechanisms and associated risks.
Calcium citrate is the most expensive presentation, its absorption is not affected by concomitant food intake. It produces a lower saturation of calcium oxalate compared to calcium carbonate supplements, and for this reason it may be indicated in the presence of nephrolithiasis [52].
One of the most important pharmacological interaction that need to be considered is with protein pump inhibitors (PPI). Omeprazole 20 mg for 7 days significantly decreases the absorption of calcium carbonate under fasting conditions with elevated pH, although the effect of concomitant food intake is unknown [53]. Potential mechanisms underlying the association of PPI and the risk of fragility fracture may be related to effects of chronic acid suppression on calcium metabolism [54]:
-
(1)
Chronic hypergastrinemia induced by PPI therapy may lead to parathyroid hyperplasia primary hyperparathyroidism, resulting in increased loss of calcium from the bone trough parathormone (PTH) elevation.
-
(2)
Profound gastric acid suppression may reduce the bioavailability of calcium for intestinal absorption, which may lead to secondary hyperparathyroidism, and increased PTH.
The form of supplementation must be ionized. Under physiological conditions calcium is absorbed by 90% in the small intestine (duodenum); one of the main determinants of this process is the concentration of calcitriol (1,25-dihydroxyvitamin D3). Any condition that decreases ionized serum calcium triggers PTH increase. Ingested calcium requires hydrochloric acid to become Ca2+. The consumption of fasting calcium and patients with decreased gastric acid secretion have reduced absorption [54].
Calcium carbonate is poorly soluble, it needs to be converted to calcium chloride in the presence of hydrochloric acid. Calcium citrate, calcium lactate and calcium gluconate are more ionizable and soluble in the presence of a neutral pH. In patients with achlorhydria, calcium citrate is absorbed 10 times more than calcium carbonate [54].
Calcium salts are linked to abdominal distension, because they react with hydrochloric acid and produces carbonate dioxide release that clinically presents as meteorism and flatulence. Calcium citrate does not produce carbon dioxide, so it has not been linked to these phenomena. There is controversy about the possible cardiovascular risks due to calcium supplementation. Recent evidence has linked calcium supplements with increased risk of coronary artery calcification [55].
A general recommendation for daily calcium requirements is that, when possible, it should be achieved through an adequate intake of calcium enriched foods [56,57].
Recommendations issued by the US Preventive Services Task Force (USPSTF) establish that there is not enough evidence to evaluate the balance between the benefits and the risks of vitamin D and calcium supplementation, alone or in combination, for the prevention of fragility fracture in subjects in the community. The current evidence is insufficient to assess the balance between the benefits and risks of daily supplementation with doses greater than 400 UI of vitamin D or greater than 1000 mg of calcium in the primary prevention of fragility fracture in postmenopausal women living in community. Supplementation with 400 IU or less of vitamin D and 1000 mg or less of calcium is not recommended for the primary prevention of fragility fracture in postmenopausal women living in the community (Recommendation D). These recommendations do not apply to people with history of fragility fracture due to osteoporosis, high risk of falls or for the diagnosis of osteoporosis or vitamin D deficiency [57]. Our position is that the intake of calcium and levels of vitamin D and calcium should be assessed to determine the need for supplementation.
There is lack of evidence on the relation between vitamin K, magnesium, cooper, zinc, phosphorus, iron or essential fatty acids and osteoporosis; only considered by one CPG [32] in which supplementation was not recommended.
Recommendation 8. It is recommended to reduce the intake of caffeine by limiting the consumption of no more than 4 cups of coffee per day.
Evidence of observational studies has shown an association between caffeinated beverages consumption and fragility fracture [58]. Caffeine decreases intestinal calcium absorption and increases urinary calcium excretion. Although this effect could be related to the replacement of milk in the diet.
The effect of different drinks containing caffeine has been implicated as a cause of osteoporosis and fragility fracture [59]. Due to the information of observational studies in favor and against this association, there is controversy [60]. Some studies have suggested that the effect of coffee on bone health may be mediated by different mechanisms: deleterious effect on osteoblasts [61,62], increased urinary calcium excretion [[63], [64], [65]], decrease in the efficiency of intestinal absorption [66], osteoblasts apoptosis [61] and low calcium intake in coffee drinkers [67,68].
The cytotoxicity of caffeine has been linked to apoptosis [61,69]. Caspases [70] and Bcl-2 family [71] play an important role in apoptosis, they regulate mitochondrial membrane potential changes and the release of cytochrome C by modulating the permeability of the outer mitochondrial membrane.
Heavy caffeine intake increases the urinary excretion of calcium, whereas moderate coffee consumption (1–2 cups per day) does not have a significant impact on calcium imbalance in postmenopausal women [64].
At the same time, recent evidence suggests that coffee consumption can help reduce the risk of several diseases: type 2 diabetes, Parkinson disease, Alzheimer disease, cardiovascular disease and cancer [65].
Coffee may exert beneficial effects on bone health due to its high polyphenols composition, the impact may be especially prominent in men, who are resistant to caffeine-induced bone loss [72,73]. Recent evidence supports this assumption on younger [74] and older [75] males.
Recommendation 9. Smoking cessation is recommended.
There is evidence that tobacco smoking increases fragility fracture risk [76] and that current active smokers have higher risk than previous smokers [77]. But, there is a lack of evidence to determine whether smoking cessation will reduce fracture risk, and no clear mechanisms have been proposed.
Mechanisms linked to bone loss due to tobacco: decreases intestinal calcium absorption [78], decreases body weight in women [[78], [79], [80]], it can anticipate menopause age [81], promotes alterations of the blood supply of the femoral head [82], decreases vitamin D levels [83] with stronger association in decreased spinal bone mineral density (BMD) and affects estrogenic metabolism in women [84] and androgenic in men [85].
Even though these mechanisms have been linked to osteoporosis, it is difficult to analyze the impact of cigarette smoking because there are indirect effects involved, such as socioeconomic, physical and nutritional factors.
Recommendation 10. It is recommended to limit the consumption of alcohol to no more than 2 units per day.
Alcohol intake is associated with increased fracture risk, with multifactorial mechanism including a negative effect on bone formation, predisposition to falls, calcium deficiency and chronic liver disease, which causes vitamin D deficiency [86].
Although there is controversial evidence about alcohol consumption and its effects on bone, a recent study [87] pointed out that light consumption (2–3 times per week and from 1 to 2 or 5 to 6 drinks per event) is related to a higher concentration of BMD (total femur, femoral neck, femoral trochanter, and intertrochanteric region) compared to nondrinkers and heavy-drinkers. The latter showed a 1.7 times higher risk than light drinkers. The same author indicated that the interpretation of these results should be taken with caution, due to the complex nature of associations that may exist among alcohol consumption, BMD and socioeconomic conditions.
Recommendation 11. A validated multifactorial assessment of fall risk that evaluates the history of falls and risk of fragility fracture is recommended in patients over 75 years of age, to decide on whom interventions are indicated.
Recommendation 12. To reduce the risk of fragility fracture through the prevention of falls, exercise with weight, balance and resistance load is recommended, to improve mobility, strength and physical performance. Special attention must be taken in patients with high risk of fragility fracture within institutions, through supervision in the execution (e.g., physical therapy).
Evidence for exercise as osteogenic therapy is not sufficient. The benefits of exercise are related to reduction of fall risk. There are many conditions that shall be taken in consideration when prescribing exercise for osteoporosis, like comorbidities [88]. Besides, there is little information on CPG related to prescription of exercise by means of type, intensity, duration and frequency.
Most guidelines recommend the analysis of risk of falls, but its performance is not detailed. Exercise decreases risk of falls by improving resistance, force and balance, but there is controversy on its evidence. A recent Cochrane systematic review concluded that even there is little or no effect on other fall-related outcomes, multifactorial and multicomponent interventions, usually including exercise, may reduce the rate and risk of falls compared with usual care or attention control [89].
Recommendations for physical therapy include exercise training in resistance and balance after hip fracture to improve mobility, strength and physical performance. There is limited evidence for its benefits after vertebral and non-hip fragility fracture [37].
Recommendation 13. The use of hip protectors is recommended for institutionalized patients with a high risk of falls.
There is limited evidence that allows the recommendation for hip protectors other than institutionalized patients. However poor acceptance and adherence of patients it’s a barrier. As most treatments for osteoporosis compliance plays a key role [37,90].
Recommendation 14. Provide postmenopausal women and men over 50 years with risk or diagnosis of osteoporosis access to education, psychosocial support and encourage them to seek support from appropriate sources according to individual needs.
Education must be focus on awareness about the disease process, prevention of fragility fracture, pain management, rehabilitation techniques and fall prevention and the importance of therapy compliance. There is little information on how to achieve and assess conduct modifications to ensure adequate therapy adherence and compliance.
4.1. Limitations on this study
Even though CPGs included were selected for high methodological quality, we found that many of the recommendations were based on observational studies and low-quality clinical trials, and most of the original information was not updated. We consider this was the most important limitation, and motivated us to search for updated information that could support or question the recommendations.
5. Conclusions
These recommendations focus on integrating interventions to modify lifestyle, maintain adequate protein intake, adequate serum levels of calcium and vitamin D, exercise, identification and treatment of fall risk factors and limit the consumption of coffee, alcohol and tobacco. However, there is little information on the possible adverse effects that the interventions may have. Current recommendations on non-pharmacological treatment options require revision.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors thank Gladys Faba Beaumont and Peter Tugwell for their support on style correction and review of this document. ORCID. Roberto Coronado-Zarco: 0000-0002-8268-9686. Andrea Olascoaga-Gómez de León: 0000-0003-0949-9103.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.afos.2019.09.005.
Appendix. Search strategy
| Database | Term | Reference citations |
|---|---|---|
| PubMed | “Osteoporosis" [Mesh] AND “Guideline" [Publication Type] AND ((“2012/01/01" [PDAT]: “2018/12/31" [PDAT]) AND (English [lang] OR Spanish [lang])) | 77 |
| PEDro | Osteoporosis guideline treatment | 5 |
| Ovid MEDLINE(R) | limit 1 to (yr = ”2012-current” and practice guideline) | 56 |
| Embase | Osteoporosis guideline | 43 |
| Science Direct | Osteoporosis AND guideline | 60 |
| Total | 241 |
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bastian H., Glasziou P., Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook S, Lamont T, Cook R. NIHR’s research signals in the BMJ. BMJ;364:l513. [DOI] [PubMed]
- 3.IOM (Institute of Medicine) The National Academies Press; Washington, DC: 2011. Clinical Practice Guidelines we can trust. [Google Scholar]
- 4.Siering U, Eikermann M, Hausner E, Hoffmann-Eßer W, Neugebauer EA. Apprisal tools for clinical practice guidelines: a systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwers M., Kho M.E., Browman G.P., Cluzeau F., Feder G., Fervers B. on behalf of the AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2010;182:E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dionyssiotis Y., Skarantavos G., Papagelopoulos P. Modern rehabilitation in osteoporosis, falls and fractures. Clin Med Insights Arthritis Musculoskelet Disord. 2014;7:33–40. doi: 10.4137/CMAMD.S14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The ADAPTE Collaboration . Guideline International Network; 2010. The ADAPTE process: resource toolkit for guideline adaptation. Version 2.0 [Internet]https://g-i-n.net/document-store/working-groups-documents/adaptation/adapte-resource-toolkit-guideline-adaptation-2-0.pdf [cited 2018 Jun 10] Available from: [Google Scholar]
- 8.Schunemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–110. doi: 10.1016/j.jclinepi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Semlitsch T., Blank W.A., Kopp I.A., Siering U., Siebenhofer A. Evaluating guidelines: a review of key quality criteria. Dtsch Arztebl Int. 2015;112:471–478. doi: 10.3238/arztebl.2015.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nothacker M.J., Muche-Borowski C., Kopp I.B. Guidelines in the register of the Association of scientific medical societies in Germany – a quality improvement Campaign. Geburtshilfe Frauenheilkd. 2014;74:260–266. doi: 10.1055/s-0034-1368227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Body JJ, Bergmann P, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S. Evidence-based guidelines for the pharmacological treatment of postmenopausal osteoporosis: a consensus document by the Belgian Bone Club. Osteoporos Int. 2010;21:1657–1680. doi: 10.1007/s00198-010-1223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boonen S, Body JJ, Boutsen Y, Devogelaer JP, Goemaere S, Kaufman JM. Evidence-based guidelines for the treatment of postmenopausal osteoporosis: a consensus document of the Belgian Bone Club. Osteoporos Int. 2005;16:239–254. doi: 10.1007/s00198-004-1812-1. [DOI] [PubMed] [Google Scholar]
- 14.Makras P., Vaiopoulos G., Lyritis G.P. 2011 guidelines for the diagnosis and treatment of osteoporosis in Greece. J Musculoskelet Neuronal Interact. 2012;12:38–42. [PubMed] [Google Scholar]
- 15.Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D. Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas. 2009;62:105–108. doi: 10.1016/j.maturitas.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal women (amended) London: National Institute for Health and Clinical Excellence (NICE) Technology Apprisal Guidence. 2011;160 [Google Scholar]
- 17.Waugh N, Royle P, Scotland G, Henderson R, Hollick R, McNamee P. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. Health Technol Assess. 2011;15(Suppl 1):51–59. doi: 10.3310/hta15suppl1/06. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Practice Guidelines for Osteoporosis [Internet] https://www.moh.gov.sg/content/dam/moh_web/HPP/Doctors/cpg_medical/withdrawn/cpg_Osteoporosis.pdf [cited 2018 Jun 10]. Available from:
- 19.Taiwanese guidelines for the prevention and treatment of osteoporosis. [Internet]. Taiwanese Osteoporosis Association; 2013 [cited 2018 Jun 10]. Available from: https://www.iofbonehealth.org/sites/default/files/PDFs/National%20Guidelines/Taiwanese_guidelines_prevention_treatment_osteoporosis.pdf.
- 20.Lau E.M.C., Sambrook P., Seeman E. Guidelines for diagnosing, prevention and treatment of osteoporosis in Asia. APLAR J Rheumatol. 2006;9:24–26. [Google Scholar]
- 21.Schurman L, Bagur A, Hermberg HC, Messina OD, Negri AL, Sánchez A. Guías 2012 para el diagnóstico, la prevención y el tratamiento de la osteoporosis [2012 Guidelines for diagnosis, prevention and treatment ofosteoporosis] Medicina. 2013;73:55–74. ISSN 0025-7680. [PubMed] [Google Scholar]
- 22.Pereira RM, Freire de Carvalho J, Paula AP, Zerbini C, Domiciano DS, Gonçalves H. Guidelines for the prevention and treatment of glucorticoid-induced osteoporosis. Rev Braz Reumatol. 2012;52:580–593. [PubMed] [Google Scholar]
- 23.Guías de diagnóstico, prevención y tratamiento de osteoporosis . Sociedad Chilena de Reumatologia; Sociedad Chilena de Osteología y Metablismo Mineral; 2006. [Diagnosis, prevention and treatment guides for osteoporosis]https://www.iofbonehealth.org/sites/default/files/PDFs/National%20Guidelines/chile_guidelines-2006.pdf [cited 2018 Jun 10] Available from: [Google Scholar]
- 24.Consenso Iberoamericano de Osteoporosis, SIBOMM Osteoporosis: prevención, diagnóstico y tratamiento . Sociedad Iberoamericana de Osteología y Metabolismo Mineral; 2009. [Osteoporosis: prevention, diagnosis and treatment]https://www.iofbonehealth.org/sites/default/files/PDFs/National%20Guidelines/Consenso-Iberoamerican0-de-Osteoporosis-SIBOMM2009.pdf [cited 2018 Jun 10] Available from: [Google Scholar]
- 25.Maalouf G, Gannagé-Yared MH, Ezzedine J, Larijani B, Badawi S, Rached A. Middle East and North Africa consensus on osteoporosis. J Musculoskelet Neuronal Interact. 2007;7:131–143. [PubMed] [Google Scholar]
- 26.Chakhtoura M, Leslie WD, McClung M, Cheung AM, Fuleihan GE. FRAX-based Lebanese osteoporosis treatment guidelines: rationale for a hybrid model. Osteoporos Int. 2017;28:127–137. doi: 10.1007/s00198-016-3766-5. [DOI] [PubMed] [Google Scholar]
- 27.Fuleihan G-H, Baddoura R, Awada H, Okais AA. First update of the lebanese guidelines for osteoporosis assessment and treatment. J Med Liban. 2007;55:176–191. [PubMed] [Google Scholar]
- 28.Fuleihan G-H, Baddoura R, Awada H, Okais J, Rizk P, McClung M. Lebanese guidelines on osteoporosis assessment and treatment: who to test? What measures to use? When to treat? J Clin Densitom. 2005;8:148–163. doi: 10.1385/jcd:8:2:148. [DOI] [PubMed] [Google Scholar]
- 29.Hough S, Amod A, Ascott-Evans BH, Brown SL, Cassim B, Davey M. South African clinical guideline for the diagnosis and management of osteoporosis. National Osteoporosis Foundation of South Africa (NOFSA). A summary of the guidelines was published in Journal of Endocrinology, metabolism and diabetes South Africa (JEMDSA) J Endocrinol Metab Diabetes S Afr. 2010;15:107–108. [Google Scholar]
- 30.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. For the Scientific Advisory Council of Osteoporosis Canada. CMAJ (Can Med Assoc J) 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NAMS continuing medical education activity Management of osteoporosis in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17:25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 32.Guía de práctica clínica para el diagnóstico y tratamiento de la osteoporosis en el adulto . Secretaría de Salud, CENETEC; 2009. [Clinical practice guideline for diagnosis and treatment of osteoporosis in adult]http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/083_GPC_OsteoporosisAdulto/GPC_CenetecOsteoporosis020909.pdf IMSS-083-08. [cited 2018 Jun 14] Available from: [Google Scholar]
- 33.Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL. American association of clinical Endocrinologists and American collage of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2016;22(Suppl 4):1–42. doi: 10.4158/EP161435.GL. [DOI] [PubMed] [Google Scholar]
- 34.Clinical Guidance on Management of Osteoporosis . Medical Development Division, Ministry of Health Malaysia; 2012. Malaysian Osteoporosis Society (Persatuan Osteoporosis Malaysia). Endorsed by the Malaysian Health Technology Assessment Section (MaHTAS)https://www.iofbonehealth.org/sites/default/files/PDFs/National%20Guidelines/Malaysia_CG_Mgmt_Osteoporosis_2012-0912-final.pdf [Google Scholar]
- 35.Guía de práctica clínica para el diagnóstico y tratamiento de la osteoporosis en mujeres posmenopáusicas . Secretaría de Salud, CENETEC. IMSS-673-18; 2018. [Diagnosis and treatment of osteoporosis in postmenopausal women. Evidence and recommendations guideline: Clinical practice guideline]http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/IMSS_673_13_Osteoporosisenpostmenopausia/673GRR.pdf [cited 2018 Jun 14] Available from: [Google Scholar]
- 36.Diagnóstico y tratamiento de la perimenopausia y postmenopausia. Guía de práctica clínica [Diagnosis and treatment of perimenopause and posmenopause. Clinical practice guideline]. Secretaría de Salud, CENETEC. S-19-08. Update 2013. [cited 2018 Jun 14] Available from: http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/019_GPC_ClimatyMenop/SS_019_08_GRR.pdf
- 37.The Royal Australian College of General Practitioners and Osteoporosis Australia . Second ed. RACGP; East Melbourne, Vic: 2017. Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age. [Google Scholar]
- 38.Rossini M, Adami S, Bertoldo F, Diacinti D, Gatti D, Giannini S. Guidelines for the diagnosis, prevention and management of osteoporosis. SIOMMMS. Reumatismo. 2016;68:1–39. doi: 10.4081/reumatismo.2016.870. [DOI] [PubMed] [Google Scholar]
- 39.González-Macías J, Del Pino-Montes J, Olmos JM, Nogués X, en nombre de la Comisión de Redacción de las Guías de Osteoporosis de la SEIOMM Guías de práctica clínica en la osteoporosis posmenopáusica, glucocorticoidea y del varón. Sociedad Española de Investigación Ósea y del Metabolismo Mineral (3° versión actualizada 2014). [Clinical practice guideline for postmenopausal, glucocorticoid-induced and male osteoporosis. Spanish Society for Research on Bone and Mineral Metabolism (3rd update version 2014)] Rev Clin Esp. 2015;215:515–526. doi: 10.1016/j.rce.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Compston J. National Osteoporosis Guideline Group (NOGG); 2014. Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK; pp. 1–10. [Google Scholar]
- 41.Compston J., Cooper A., Cooper C., Gittoes N., Gregson C., Harvey N. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guía de práctica clínica sobre el diagnóstico y tratamiento de la osteoporosis en la mujer posmenopáusica Mexicana [Clinical practice guideline on the diagnosis and treatment of osteoporosis of posmenopausal Mexican women]. Asociación Mexicana de Metabolismo Óseo y Mineral A. C. AMMOM; 2014 [cited 2018 Jun 10] Available from:
- 43.Li-Yu J, Perez EC, Canete A, Bonifacio L, Llamado LQ, Martinez R. Consensus statements on osteoporosis diagnosis, prevention, and management in the Philippines. Osteoporosis Society of the Philippines Foundation, Inc. (OSPFI); Philippine Orthopedic Association (POA) clinical practice guidelines task force committee on osteoporosis. Int J Rheum Dis. 2011;14:223–238. doi: 10.1111/j.1756-185X.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaiser MJ, Bauer JM, Rämshc C, Uter W, Guigoz Y, Cederholm T. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734–1738. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 45.Myint MW, Wu J, Wong E, Chan SP, To TS, Chau MW. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: a single blind randomized controlled trial. Age Ageing. 2013;42:39–45. doi: 10.1093/ageing/afs078. [DOI] [PubMed] [Google Scholar]
- 46.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuated proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- 47.Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, Fuleihan EG. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. 2013;24:1555–1566. doi: 10.1007/s00198-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 48.Baum J.I., Kim I.Y., Wolfe R.R. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016 doi: 10.3390/nu8060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg AE, Patel CJ, Chertow GM, Leppert JT. Diabetic severity and risk of kidney stone disease. Eur Urol. 2014;65:242–247. doi: 10.1016/j.eururo.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manappallil RG, Shylendran S, Kakkattil A, Thomas AD. Multiple renal calculi due to hypercalcemia induced by over-the-counter vitamin D intoxication. BMJ Case Rep. 2018 doi: 10.1136/bcr-2018-225849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Letavernier E, Daudon M. Vitamin D, hypercalciuria and kidney stones. Nutrients. 2018;10:366. doi: 10.3390/nu10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating kidney stones in adults. Cochrane Database Syst Rev. 2015;10 doi: 10.1002/14651858.CD010057.pub2. CD010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tahir R., Patel P.N. Role of proton pump inhibitors in calcium absorption, bone resorption and risk of hip fracture. J Pharm Technol. 2007;23:275–280. [Google Scholar]
- 54.Yang Y.X. Chronic PPI therapy and calcium metabolism. Curr Gastroenterol Rep. 2012;14:473–479. doi: 10.1007/s11894-012-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson JJB, Kruszka B, Delaney JAC, He K, Burke GL, Alonso A. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progr4ession among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA) J Am Heart Assoc. 2016;5e doi: 10.1161/JAHA.116.003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tankeu AT, Agbor VN, Noubiap JJ. Calcium supplementation and cardiovascular risk: a rising concern. J Clin Hypertens (Greenwich) 2017;19:640–646. doi: 10.1111/jch.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US Preventive Services Task Force, Grossman D.C., Curry S.J., Owens D.K., Barry M.J., Caughey A.B. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendations Statement. J Am Med Assoc. 2018;319:1592–1599. doi: 10.1001/jama.2018.3185. [DOI] [PubMed] [Google Scholar]
- 58.Hallström H, Wolk A, Glynn A, Michaëlsson K. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos Int. 2006;17:1055–1064. doi: 10.1007/s00198-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 59.Samelson Ej, Hannan M.T. Epidemiology of osteoporosis. Curr Rheumatol Rep. 2006;8:76–83. doi: 10.1007/s11926-006-0030-6. [DOI] [PubMed] [Google Scholar]
- 60.Hallström H, Byberg L, Glynn A, Lemming EW, Wolk A, Michaëlsson K. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am J Epidemiol. 2013;178:898–909. doi: 10.1093/aje/kwt062. [DOI] [PubMed] [Google Scholar]
- 61.Lu P.Z., Lai Cy, Chan W.H. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci. 2008;9:698–718. doi: 10.3390/ijms9050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuang YH, Sun JS, Chen LT, Sun SC, Chen SC. Direct effects of caffeine on osteoblastic cells metabolism: the possible causal effect of caffeine on the formation of osteoporosis. J Orthop Surg Res. 2006;1:7. doi: 10.1186/1749-799X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massey LK, Opryszek AA. No effects of adaptation to dietary caffeine on calcium excretion in young women. Nutr Res. 1990;10:741–747. [Google Scholar]
- 64.Hasling C, Sondergaard K, Charles P, Mosekilde L. Calcium metabolism in postmenopausal osteoporotic women is determined by dietary calcium and coffee intake. J Nutr. 1992;122:1119–1126. doi: 10.1093/jn/122.5.1119. [DOI] [PubMed] [Google Scholar]
- 65.Yang P., Zhang X.Z., Zhang K., Tang Z. Association between frequency of coffee consumption and osteoporosis in Chinese postmenopausal women. Int J Clin Exp Med. 2015;8:15958–15966. [PMC free article] [PubMed] [Google Scholar]
- 66.Barger-Lux M.J., Heaney R.P. Caffeine and the calcium economy revisited. Osteoporos Int. 1995;5:97–102. doi: 10.1007/BF01623310. [DOI] [PubMed] [Google Scholar]
- 67.Barrett-Connor E, Chang JC, Edelstein SL. Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. J Am Med Assoc. 1994;271:280–283. doi: 10.1001/jama.1994.03510280042030. [DOI] [PubMed] [Google Scholar]
- 68.Harris SS, Dawson-Hughes B. Caffeine and bone loss in healthy postmenopausal women. Am J Clin Nutr. 1994;60:573–578. doi: 10.1093/ajcn/60.4.573. [DOI] [PubMed] [Google Scholar]
- 69.Fernández M.J., López A., Santa-María A. Apoptosis induced by different doses of caffeine on Chinese hamster ovary cells. J Appl Toxicol. 2003;23:221–224. doi: 10.1002/jat.910. [DOI] [PubMed] [Google Scholar]
- 70.Martins LM, Kottke T, Mesner PW, Basi GS, Sinha S, Frigon N., Jr Activation of multiple interleukin-1 beta converting enzyme homologues in cytosol and nuclei of HL-60 cells during etoposide-induced apoptosis. J Biol Chem. 1997;272:7421–7430. doi: 10.1074/jbc.272.11.7421. [DOI] [PubMed] [Google Scholar]
- 71.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 72.Yano K, Heilbrun LK, Wasnich RD, Hankin JH, Vogel JM. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr. 1985;42:877–888. doi: 10.1093/ajcn/42.5.877. [DOI] [PubMed] [Google Scholar]
- 73.Holbrook TL, Barrett-Connor E, Wingard DL. Dietary calcium and risk of hip fracture: 14 year prospective population study. Lancet. 1988;2:1046–1049. doi: 10.1016/s0140-6736(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 74.Choi M.K., Kim M.H. The association between coffee consumption and bone status in young adult males according to calcium intake level. Clin Nutr Res. 2016;5:180–189. doi: 10.7762/cnr.2016.5.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hallström H, Wolk A, Glynn A, Michaëlsson K, Byberg L. Coffee consumption and risk of fracture in the cohort of Swedish men (COSM) PLoS One. 2014;9 doi: 10.1371/journal.pone.0097770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giampietro PF, McCarty C, Mukesh B, McKiernan F, Wilson D, Shuldiner A. The role of cigarette smoking and statins in the development of postmenopausal osteoporosis: a pilot study utilizing the Marshfield Clinical Personalized Medicine Cohort. Osteoporos Int. 2010;21:467–477. doi: 10.1007/s00198-009-0981-3. [DOI] [PubMed] [Google Scholar]
- 77.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 78.Krall E.A., Dawson-Hughes B. Smoking and bone loss among postmenopausal women. J Bone Miner Res. 1991;6:331–337. doi: 10.1002/jbmr.5650060404. [DOI] [PubMed] [Google Scholar]
- 79.Jensen J., Christiansen C., Rodbro P. Cigarette smoking, serum estrogens and bone loss during hormone-replacement therapy early after menopause. N Engl J Med. 1985;313:973–975. doi: 10.1056/NEJM198510173131602. [DOI] [PubMed] [Google Scholar]
- 80.Slemenda CW, Hui S, Longcope C, Johnston CC., Jr Cigarette smoking, obesity, and bone mass. J Bone Miner Res. 1989;4:737–741. doi: 10.1002/jbmr.5650040513. [DOI] [PubMed] [Google Scholar]
- 81.Baron JA. Smoking and estrogen-related disease. Am J Epidemiol. 1984;119:9–22. doi: 10.1093/oxfordjournals.aje.a113730. [DOI] [PubMed] [Google Scholar]
- 82.Hirota Y, Hirohota T, Fukuda K, Mori M, Yanagawa H, Ohno Y. Association of alcohol intake, cigarette smoking and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137(5):530–538. doi: 10.1093/oxfordjournals.aje.a116706. [DOI] [PubMed] [Google Scholar]
- 83.Fung YK, Mendlik MG, Haven M.C., Akhter MP, Kimmel DB. Short-terms effects of nicotine on bone and calciotropic hormones in adult female rats. Pharmacol Toxicol. 1998;82:243–249. doi: 10.1111/j.1600-0773.1998.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 84.Thomas EJ, Edrige W, Weddell A, McGill A, McGarrigle HH. The impact of cigarette smoking on the plasma concentrations of gonadotrophins, ovarian steroids and androgens and upon the metabolism of oestrogens in the humane female. Hum Reprod. 1993;8:1187–1193. doi: 10.1093/oxfordjournals.humrep.a138226. [DOI] [PubMed] [Google Scholar]
- 85.Barbieri RL, York CM, Cherry ML, Ryan KJ. The effects of nicotine, cotinine and anabasine on rat adrenal 11 beta-hydroxilase and 21-hydroxilase. J Steroid Biocem. 1987;28:25–28. doi: 10.1016/0022-4731(87)90119-1. [DOI] [PubMed] [Google Scholar]
- 86.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 87.Jang HD, Hong JY, Han K, Lee JC, Shin BJ, Choi SW. Relationship between bone mineral density and alcohol intake: a nationwide health survey analysis of postmenopausal women. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Regil-González P, Olascoaga-Gómez de León A, Chávez-Arias DD, Nava-Bringas TI, Macías-Hernández SI, Cruz-Medina E. Appraisal of exercise recommendations for osteoporosis treatment of current guidelines: a systematic Review. Acta Univ. 2015;25:28–35. [Google Scholar]
- 89.Hopewell S, Adedire O, Copsey BJ, Boniface GJ, Sherrington C, Clemson L. Multifactorial and multicomponent interventions for preventing falls in older people living in community. Cochrane Database Syst Rev. 2018;7 doi: 10.1002/14651858.CD012221.pub2. CD012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santesso N., Carrasco-Labra A., Brignardello-Petersen R. Hip protector for preventing hip fractures in older people. Cochrane Database Syst Rev. 2014;3 doi: 10.1002/14651858.CD001255.pub5. CD001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.