Abstract
In the present study copper oxide nanoparticles (CuONPs) were synthesized via simple and eco-friendly green route using leaf extract of Enicostemma axillare (Lam.). Characterization of synthesized nanoparticles (NPs) was undertaken. The characteristic absorption peak of CuONPs was in range 264nm in UV–Vis spectrum. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) studies revealed the morphological and structural character of green NPs. The mean particle size was calculated to 30nm. Energy dispersive spectroscopy (EDS) showed high intense metallic peak of copper (Cu), oxygen (O) and low intense peaks of carbon (C), sulfur (S), phosphorus (P) elements due to the capping action of biomolecules of plant extract in CuONPs formation. The X-ray diffraction (XRD) pattern showed distinctive peaks corresponding to (200), (211) and (310) planes revealing the high crystalline nature of synthesized CuONPs with a primitive phase. Zeta potential and size distribution of synthesized green NPs was concluded by Dynamic light scattering (DLS) studies.
Keywords: Enicostemma axillare, Green synthesis, Nanoparticles, Copper sulfate, Characterization
Highlights
-
•
Plant extract mediated synthesis of CuONPs.
-
•
CuONPs characterization by UV–Vis spectroscopy, FE-SEM, EDS, TEM, XRD, and DLS techniques.
-
•
Simple and eco-friendly green route synthesis.
1. Introduction
NPs can be metallic or nonmetallic of the size range of 1–100 nm. NPs are attracting wide attention from varied disciplines of science due to their immense application in diverse field. NPs of Ag, ZnO and CuO are of prime importance due to their optical, electrical and thermo stability with various functionalities [1]. Synthesis of nanoparticles is by two technique namely top down and bottom up approaches [2]. These two approaches include three different methods of synthesis namely: chemical, physical and biological. The physical methods follows on top down approach and rest of two relies on bottom up approaches [3]. The bottom-up approach includes chemical synthesis [4], electrochemical synthesis [5], sonochemical synthesis [6,7], and polyol reduction [8] of nanoparticles. The chemical mediated synthesis of nanoparticles lead to toxicity to the environment due to the usage of toxic chemicals [9]. The biological methods can be carried out by microorganisms (bacteria, fungi, actinomycete, yeast, and viruses) [[10], [11], [12], [13], [14]] and plant mediated synthesis (plant extract from leaf, peel, flower, fruit and root) [[15], [16], [17], [18], [19], [20]]. The microorganism mediated synthesis of nanoparticles is a tedious process and often leads to contaminants. To overcome above mentioned drawback most of the researchers have focused towards plant mediated synthesis of NPs which can be also stated as green synthesis or eco-friendly synthesis. Enicostemma axillare is a perennial herb belonging to family Gentianaceae and is cosmopolitan in occurrence in India. The plant acts as a laxative, helps in curing fever, obesity, rheumatism, snake bite, skin disease, abdominal disorders and regulate blood sugar levels. The plant constituents have been reported for possessing antioxidant [21], hypolipidaemic, antiulcer [22], hypoglycemic [23], anti-inflammatory, hepatoprotective [24], and antimicrobial [25] properties. Different kind of active compounds are found in this plant such as steroids, catechins, sapogenin, saponins, flavonoids, triterpenoids, xanthones [26] and different amino acids like tryptophan, l-glutamic acid, serine, alanine, l-tyrosine, iso leucine, aspartic acid, l-proline, phenyl alanine, methionine, threonine, l-histidine monohydrochloride, l-arginine monohydrochloride, l-Glycine, 2-amino butyric acid, DOPA, and valine [27]. These active compounds may play important role in reduction of copper ions into copper nanoparticles. (see Table 1)
Table 1.
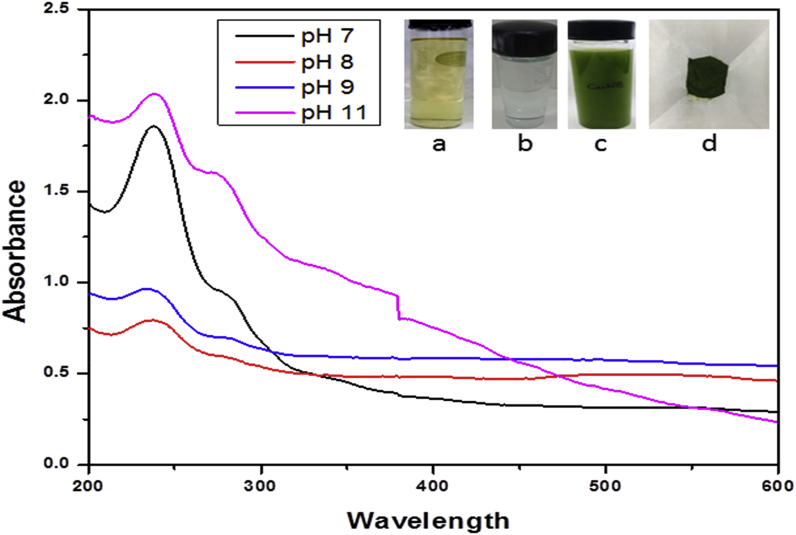
UV–Vis absorption spectrum of CuONPs at different pH values.
| pH values | Wavelength (nm) |
|---|---|
| 7 | 234 |
| 8 | 228 |
| 9 | 225 |
| 11 | 229 |
2. Materials and methods
2.1. Collection of plants
Leaf samples of plant Enicostemma axillare was collected from local area around Udaipur region. Collected plant materials was authenticated by University of Rajasthan; Jaipur, India (RUBL211634).The leaves were washed thrice with tap water followed by double distilled water to remove dust particles and shade dried for 1 h to remove moisture content.
2.2. Preparation of plant extract
Extract was prepared by mixing 10 gm of fresh leaves in 100ml deionized water and boiled at 80 °C temperature for 30 min in water bath. The extract was filtered with Whatman filter paper of retention size 11μm (HiMedia, Mumbai, India) and cellulose nitrate membrane by vacuum filtration unit. The filtrate was stored at 4 °C for further use.
3. Synthesis of copper oxide nanoparticles
The CuONPs was synthesized by dissemination of 50 ml of 5mM copper sulfate (Sigma-Aldrich, St.Louis, USA.) with 5ml of the prepared aqueous plant extracts in a 100 ml conical flask. The change in color from reddish brown to viscous green at pH 7.0 indicated the formation of CuONPs (as monitored by UV–Vis spectra of the solution (Fig. 1). The viscous precipitate was centrifuged at 10,000 rpm for 10mins and washed 3–4 times with autoclaved deionized water. The transparent solution was discarded and the viscous layer of CuONPs was collected. The pellet was obtained after drying the viscous layer in the oven at 450 to 500 C for 24 h.
Fig. 1.
UV–Visible absorption spectrum of CuONPs at different pH (a) plant extracts (b) CuSO4 solution (c) CuONPs (d) CuONPs powder.
4. Characterization of synthesized CuONPs
Synthesized CuONPs primarily characterized by UV–Visible spectroscopy (ELICO SL-159 UV–Visible spectrophotometer). The UV–Visible absorption spectrum was recorded using quartz cuvette with deionized water as a reference. The spectrometric reading was recorded at a scanning speed of 200 to 700 nm (Fig. 1). The mean particle size, polydispersity index (PDI) and zeta potential of CuONPs was measured using Malvern ZS-Nano analyzer (Malvern instrument Inc., London U.K). The analysis was carried out at the parameters of temperature of 25 °C. X-ray diffraction pattern of CuONPs was obtained using a powder diffractometer (X-ray diffractometer Ultima IV, Rigaku, Japan) with Kα radiation (λ= 1.54059 nm) in the 2θ range from 20o to 80o. Mean particle size concluded out by transmission electron microscopy experiments in an FEI Tecnai G2 20 transmission electron microscope, operating at 200 kV, with a resolution point of 2.04 nm. Scanning Electron Microscopy performed using JEOL SM-7600F, Japan model to record morphological characters of synthesized nanoparticles. Energy dispersive X-rays spectrometry was performed to analyze the elemental constituent of the nanoparticle using Oxford-EDS system.
5. Results and discussion
5.1. UV–visible absorption spectra of CuONPs
The leaf extract of Enicostemma axillare was added to the aqueous copper sulfate solution which changes color from reddish brown to dark green indicating the formation of CuONPs (Fig. 1). Further characterization was performed using UV–Vis spectrophotometry, which showed a distinct peak at 234nm similar to other studies on the green synthesis of CuONPs using plant extract [28]. UV–Visible absorption spectrum was performed between pH ranging from 7 to 11. The surface Plasmon absorbance of copper colloids was obtained for all pH except at pH 10. The Plasmon resonance is clearly visible for pH-7 at 234nm.This probably indicates very small particles at such low pH due to Surface Plasmon Resonance excitation of CuONPs. UV–Vis absorption spectra by different pH values mentioned in Table 1.
5.2. Morphological characterization of CuONPs
The morphological characterization of CuONPs was performed using FE-SEM and TEM analysis.
5.2.1. FE-SEM and EDS analysis
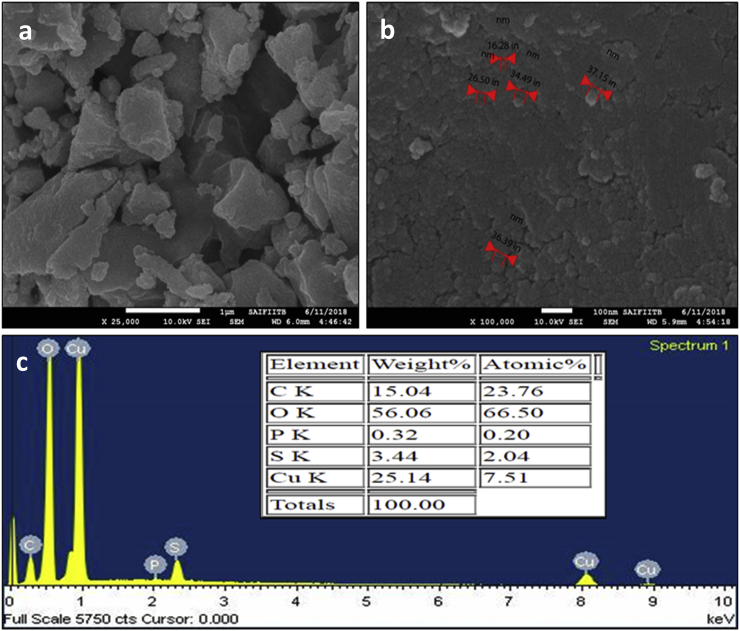
FE-SEM showed the nanoparticles are agglomerated in some amount due to sticky nature of the plant extract. The FE-SEM micrographs shows average size of 30nm of CuONPs indicating well established synthesized nanoparticles. The FE-SEM micrographs were taken at 1μm (low resolution) and 100 nm (high resolution) as depicted in the inset of (Fig. 2a-b). EDS analysis revealed the purity of synthesized CuONPs. Oxygen with copper in EDS spectrum indicate the copper in the form of oxide or dioxide. The weight compositions for copper (Cu) and oxygen (O) were 25.14% and 56.06%, respectively. The atomic compositions were then calculated as 7.51% and 66.50%, respectively (Fig. 2c). Carbon, Sulfur, Calcium, Potassium and Phosphorus were detected in small amount owing to interactions with extract during bioprocessing.
Fig. 2.
SEM analysis of CuONPs (a & b) CuONPs micrograph (c) EDS spectrum.
5.2.2. TEM analysis
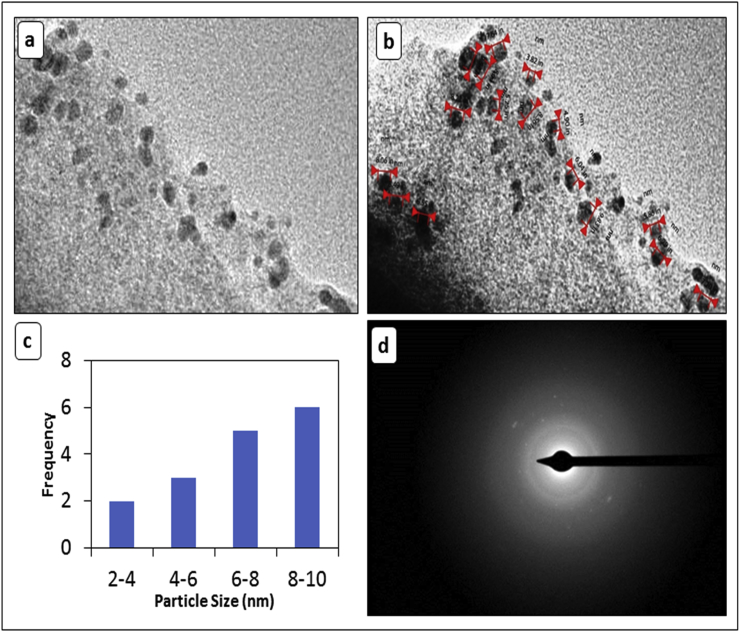
Crystalline nature of CuONPs was reported by TEM analysis which is in agglomerated cluster structure as depicted in the (Fig. 3a-b). The obtained CuONPs are quite uniform in size with average size of 6.44nm. Fig. 3c Shows the size distribution of NPs.The TEM images revealed that the small particle aggregates are coated with a thin organic layer, which acts as a capping organic agent. Presence of intermittent dots in Selected Area Electron Diffraction (SAED) on the concentric circles confirmed the crystalline nature of synthesized CuONPs as shown in the Fig. 3d. This may well explain that the nanoparticles show a very good dispersion inside the bio-reduced aqueous solution, even in the macroscopic scale.
Fig. 3.
TEM micrograph (a–b) CuONPs with different magnification (c) Size distribution (d) SAED image.
5.3. XRD analysis
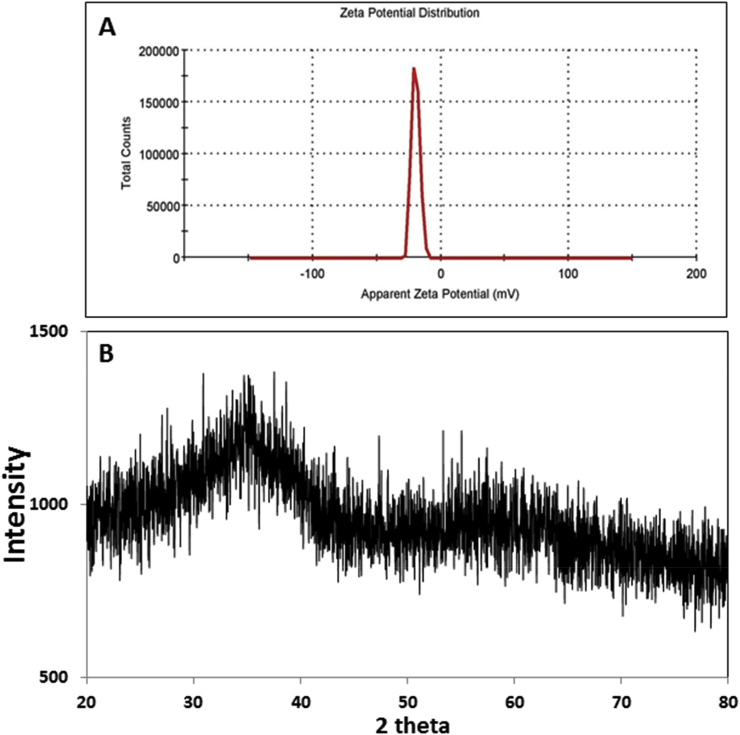
XRD pattern analysis revealed the crystalline nature of CuONPs as shown in Fig. 4b. The XRD spectrum showed various small distinct diffraction peaks at 37.46, 50.09 and 70.48. This represents (200), (211) and (310) of primitive structure of copper oxide nanoparticles respectively. XRD pattern of NPs was matched with a database of Joint Committee on Powder Diffraction Standards (JCPDS Card No. 05–0667, [31]). The mean grain size of CuONPs formed in the bioreduction process was measured using the Debye-Scherrer formula D= kλ/βcosθ, where D is the average crystalline size (Å), k is a constant 1, ‘λ’ is the wavelength of X-ray source (0.1541nm), β is the angular line full width at half maximum (FWHM) intensity in radians and ‘θ’ the Bragg’s angle [29,30]. The XRD pattern showed the average particle size 22.95nm.
Fig. 4.
(a) XRD spectrum of CuONPs (b) Zeta potential.
5.4. Zeta potential and size distribution measurements by dynamic light scattering (DLS)
Nano size distribution studied using DLS analysis revealed the negative charge of about -19.5mV. Higher negative zeta potential denoted the strong repulsion force between the particles indicating stability and quality. DLS is one of the most commonly used techniques to determine the size of nanoparticles. The size distribution ranged from 88 to 307nm. From the peak position, the Z-average diameter of synthesized CuONPs was found to be 470nm. From the particle size analysis the polydispersity index (PDI) was 0.782 (Fig. 4a).
6. Conclusion
In this article, CuONPs were successfully synthesized through green process using Enicostemma axillare leaf extract. The green synthesized CuONPs were characterized using various analytical techniques UV–Vis spectroscopy, SEM, TEM, XRD and DLS studies. The novelty of the study is that NPs were synthesized without any toxic reagent and expensive technique. The phyto-chemical compound present in the leaf extract helped in the formation of nanoparticles. SEM and XRD results showed that the synthesized NPs are in nano scale in size.
Declaration of competing interest
The Author declares no conflict of interest.
Acknowledgement
The authors gratefully acknowledge the DBT-JRF fellowship F.No. DBT/JRF/BET-16/I/2016/AL/63 New Delhi, India to Suresh Chand Mali and UGC-BSR meritorious fellowship F.No.25-1/2014-15 (BSR)/7–125/2007/(BSR), University Grant Commission to Shani Raj for financial support. The authors would like to express sincere thanks to the UGC-DAE, Indore and IIT SAIF, Bombay for TEM and SEM-EDS Analysis, respectively. The authors also thank to Prof. N. Laxmi Department of Physics, MLSU and Dr. Vinod Saharan, Nanotechnology laboratory, RCA, Udaipur, India for their help in XRD and DLS studies, respectively.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100699.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Gong P., Li H., He X., Wang K., Hu J., Tan W., Yang X. Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology. 2007;18:285604. [Google Scholar]
- 2.An J., Wang D., Luo Q., Yuan X. Antimicrobial active silver nanoparticles and silver/polystyrene core-shell nanoparticles prepared in room-temperature ionic liquid. Mater. Sci. Eng. C. 2009;29:1984–1989. [Google Scholar]
- 3.Madhumitha G., Elango G., Roopan S.M. Biotechnological aspects of ZnO nanoparticles: overview on synthesis and its applications. Appl. Microbiol. Biotechnol. 2016;100:571–581. doi: 10.1007/s00253-015-7108-x. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard J., Kesavan L., Piccinini M., He Q., Tiruvalam R., Dimitratos&G N., Hutchings J. Direct synthesis of hydrogen peroxide and benzyl alcohol oxidation using Au− Pd catalysts prepared by sol immobilization. Langmuir. 2010;26:16568–16577. doi: 10.1021/la101597q. [DOI] [PubMed] [Google Scholar]
- 5.Yang F., Cheng K., Wu T., Zhang Y., Yin J., Wang G., Cao D. Au–Pd nanoparticles supported on carbon fiber cloth as the electrocatalyst for H2O2 electroreduction in acid medium. J. Power Sources. 2013;233:252–258. [Google Scholar]
- 6.Nemamcha A., Rehspringer J.L., Khatmi D. Synthesis of Palladium nanoparticles by sonochemical reduction of palladium (II) nitrate in aqueous solution. J. Phys. Chem. B. 2006;110:383–387. doi: 10.1021/jp0535801. [DOI] [PubMed] [Google Scholar]
- 7.Mizukoshi Y., Sato K., Konno T.J., Masahashi N. Dependence of photocatalytic activities upon the structures of Au/Pd bimetallic nanoparticles immobilized on TiO2 surface. Appl. Catal. B Environ. 2010;94:248–253. [Google Scholar]
- 8.Xiong Y., Chen J., Wiley B., Xia Y., Aloni S., Yin Y. Understanding the role of oxidative etching in the polyol synthesis of Pd nanoparticles with uniform shape and size. J. Am. Chem. Soc. 2005;127:7332–7333. doi: 10.1021/ja0513741. [DOI] [PubMed] [Google Scholar]
- 9.Gangula A., Podila R., Karanam L., Janardhana C., Rao A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir. 2011;27:15268–15274. doi: 10.1021/la2034559. [DOI] [PubMed] [Google Scholar]
- 10.Prema P., Iniya P.A., Immanuel G. Microbial mediated synthesis, characterization, antibacterial and synergistic effect of gold nanoparticles using Klebsiella pneumoniae (MTCC-4030) RSC Adv. 2016;6:4601–4607. [Google Scholar]
- 11.Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Sastry M. Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001;40:3585–3588. doi: 10.1002/1521-3773(20011001)40:19<3585::aid-anie3585>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad A., Senapati S., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir. 2003;19:3550–3553. [Google Scholar]
- 13.Kowshik M., Ashtaputre S., Kharrazi S., Vogel W., Urban J., Kulkarni S.K., Paknikar K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2002;14:95. [Google Scholar]
- 14.Wen A.M., Steinmetz N.F. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev. 2016;45:4074–4126. doi: 10.1039/c5cs00287g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam M.N., Das S., Batuta S., Roy N., Chatterjee A., Mandal D., Begum N.A., Murraya koenegii Spreng Leaf extract: an efficient green multifunctional agent for the controlled synthesis of Au nanoparticles. ACS Sustain. Chem. Eng. 2014;2:652–664. [Google Scholar]
- 16.Shabestarian H., Homayouni-Tabrizi M., Soltani M., Namvar F., Azizi S., Mohamad&H R., Shabestarian Green synthesis of gold nanoparticles using Sumac aqueous extract and their antioxidant activity. Mater. Res. 2017;20:264–270. [Google Scholar]
- 17.Bankar A., Joshi B., Kumar A.R., Zinjarde S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surfaces B Biointerfaces. 2010;80:45–50. doi: 10.1016/j.colsurfb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S., Patil S., Ahire M., Kitture R., Gurav D.D., Jabgunde&D A.M., Dhavale D. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 2012;10:17. doi: 10.1186/1477-3155-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sathishkumar G., Jha P.K., Vignesh V., Rajkuberan C., Jeyaraj M., Selvakumar M., Sivaramakrishnan S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016;215:229–236. [Google Scholar]
- 20.Leonard K., Ahmmad B., Okamura &J H. Kurawaki. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surfaces B Biointerfaces. 2011;82:391–396. doi: 10.1016/j.colsurfb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Thirumalai T., Therasa V., Elumalai E.K., David E. Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume. Asian Pac.J.Trop. Biomed. 2011;1:381–385. doi: 10.1016/S2221-1691(11)60084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy S.P., Niranjan C.M., Jyothi T.M., Shankrayya M.M., Vishawanath K.M., Setty K. Prabhu &R.S. Antiulcer and anti-inflammatory activity of aerial parts Enicostemma littorale Blume. J. Young Pharm. 2010;2:369–373. doi: 10.4103/0975-1483.71629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince P.S.M., Srinivasan M. Enicostemma littorale Blume aqueous extract improves the antioxidant status in alloxan induced diabetic rat tissues. Acta Pol. Pharm. 2005;62:363–367. [PubMed] [Google Scholar]
- 24.N Gite V., Pokharkar R.D., Chopade V.V., Takate S.B. Hepato-protective activity of Enicostemma axillare in paracetamol induced hepato-toxicity in albino rats. Int. J. Pharm.Life.Sci.(IJPLS) 2010;1:50–53. [Google Scholar]
- 25.Tanna S., Shukla V.J., Patgiri B.J., Prajapati P.K. Physico-phytochemical evaluation of aqueous extract of mamajjaka [Enicostemma littorale auct. Non bl] Int. J. Pharm.Biol.Arch. 2010;1:309–331. [Google Scholar]
- 26.Desai P.D. Chemical investigation of some Indian medicinal plants: Part II. Indian J. Chem. 1966;4:457–459. [Google Scholar]
- 27.Sathishkumar R., Lakshmi P.T.V., Annamalai A., Antioxidants of Enicostemma littorale BLUME Int. J. Pharma Bio Sci. 2010;1:2. [Google Scholar]
- 28.Gnanavel V., Palanichamy V., Roopan S.M. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116) J. Photochem. Photobiol. B Biol. 2017;171:133–138. doi: 10.1016/j.jphotobiol.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Jamdade D.A., Rajpali D., Joshi K.A., Kitture R., Kulkarni A.S., Shinde V.S., Ghosh S. Gnidia glauca-and plumbago zeylanica-mediated synthesis of novel CuONPs as promising antidiabetic agents. Adv.Pharmacol. Sci. 2019 doi: 10.1155/2019/9080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singare D.S., Marella S., Gowthamrajan K., Kulkarni G.T., Vooturi R., Rao P.S. Optimization of formulation and process variable of nanosuspension: an industrial perspective. Int. J. Pharm. 2010;402:213–220. doi: 10.1016/j.ijpharm.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 31.Sasmal A.K., Dutta S., Pal T. A ternary Cu2O–Cu–CuO nanocomposite: a catalyst with intriguing activity. Dalton Trans. 2016;45:3139–3150. doi: 10.1039/c5dt03859f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.