Abstract
The most commonly used tools for tuberculosis testing in cattle, the tuberculin skin test and the interferon-γ release assay, detect immune reactivity to various antigens of Mycobacterium bovis, including ESAT-6 and CFP-10. However, some non-tuberculous mycobacteria (NTM) can also harbor the cfp-10 and/or esat-6 genes, which can lead to false-positive results. We tested 77 NTM isolates belonging to 22 different species from lymph nodes of healthy slaughtered cattle for the occurrence of cfp-10 and esat-6. Most isolates did not harbor cfp-10 and esat-6. However, M. gordonae, ‘M. lymphaticum’, M. kansasii, and M. persicum were cfp-10 positive. The esat-6 gene was found in M. kansasii and M. persicum. Protein expression of cfp-10 and esat-6 could be detected for M. kansasii and M. persicum. An effective tuberculosis control program based on interferon-γ release assays and tuberculin skin testing is dependent on further monitoring and characterization of NTM in a cattle population.
Keywords: Bovine tuberculosis diagnosis interference, cfp-10, esat-6, lymph nodes, non-tuberculous mycobacteria
Introduction
Non-tuberculous mycobacteria (NTM) are ubiquitous in the environment, mainly in water and soil.8 More than 190 mycobacteria species are known (www.bacterio.net/mycobacterium.html). Given the global importance of tuberculosis (TB) as a public health problem, NTM were neglected for a long time. Studies have revealed various NTM playing an important role as opportunistic and obligate pathogens.6 However, not only are NTM important pathogens, but certain species can further interfere with TB diagnosis in cattle. The most commonly used tools for TB testing, tuberculin skin test and interferon-γ (IFN-γ) release assays, detect immune reactivity to various antigens of Mycobacterium bovis, including a 6-kDa early secretory antigenic target (ESAT-6) and a 10-kDa culture filtrate protein (CFP-10) whose genes are located within the region of difference 1 (RD1).10 Both esat-6 and cfp-10 are essential for virulence in M. tuberculosis.19 The genes code for ESAT-6 and CFP-10, both potent T-cell antigens.3 Deletions of RD1 in M. tuberculosis or esat-6 in M. bovis were shown to result in decreased virulence in animal models.20 On the other hand, reinsertion of RD1 in Bacillus Calmette–Guérin (BCG) increased virulence.12 Even though RD1 was initially identified as a virulence factor of M. tuberculosis, there are some reports showing that RD1 can also be found in certain NTM.2,7,19 We identified NTM isolates originating from cultures of 642 lymph nodes from slaughtered cattle, examined in 2013–2015 in Switzerland, and tested them for the occurrence and expression of cfp-10 and esat-6.
Material and methods
Ethics statement
Our study was carried out in accordance with the recommendations of Swiss federal regulations (TSV 916.401 and VSFK 817.190). Analysis of animal samples was carried out within an official context of monitoring bovine tuberculosis and NTM infections, meaning that no animals were killed for the purposes of this research project and ethical approval was not necessary.
Collection of isolates
From March 2013 until November 2014, ~13,700 animals were tested for M. tuberculosis complex by either using intradermal tuberculin test alone or in combination with IFN-γ testing. In total, as described previously,14 lymph nodes from 534 cattle from 203 dairy herds were further cultured for mycobacteria; 57 NTM isolates were obtained and included in our study. An additional 20 NTM isolates originating from cultures of 108 lymph nodes from 108 slaughtered cattle collected in a prior study5 were also included. This total of 77 mycobacterial isolates were identified and further characterized in our study.
Mycobacterial culture and DNA extraction
Mycobacterial culture was performed as described previously.4 Genomic DNA was extracted from mycobacteria that had been grown in 1.5 mL of mycobacteria growth indicator tubes (MGIT; Becton Dickinson, Franklin Lakes, NJ) and then centrifuged (10 min, 13,000 × g). In brief, the sediment was suspended in 180 μL of ATL buffer (Qiagen, Hilden, Germany), transferred onto a bead-beating matrix in a 2-mL microtube (Omni International, Kennesaw, GA), heat inactivated, and subjected to mechanical cell lysis (TissueLyser II, Qiagen) and enzymatic digestion with proteinase K (Qiagen). Automated DNA preparation was performed (QIAcube, QIAamp cador pathogen mini kit, Qiagen). DNA concentration and purity were determined (NanoDrop 2000c spectrophotometer, Thermo Fisher Scientific, Reinach, Switzerland) and samples stored at −20°C until subsequent experiments.
Identification and characterization of mycobacteria
For identification of mycobacteria, 16S ribosomal RNA,9 rpoB,1 and hsp6517 were amplified and then sequenced using the Sanger sequencing method. Nucleotide sequences were compared with available sequences by BLAST analysis (https://www.ncbi.nlm.nih.gov/). Alignment of sequences was performed (CLC main workbench v.7.6.1, CLC Bio, Qiagen). In addition, to identify the subspecies of M. avium complex, sequence analysis of the 3’-fragment of the hsp65 gene was performed by amplifying the near-complete hsp65 gene using primers MAChsp65F and MAChsp65R.18 Isolates belonging to the M. kansasii complex were assigned to corresponding subtypes by sequencing cfp-10 and esat-619 as detailed below.
cfp-10 and esat-6 analysis
PCR was performed using primer sets Esa-12 and Esa-303 for esat-6, and opBR78 and opBR103 for cfp-10, as described previously.19 Each reaction mixture contained 1× HotStart Taq master mix kit (Qiagen), 0.5 μM of each primer pair, and 10 ng of purified mycobacterial DNA. PCR was performed with an initial 15 min activation step at 95°C followed by 40 cycles of 95°C for 30 s, 60°C for 60 s, 72°C for 30 s, and a final extension step of 72°C for 10 min. For subspecies identification of M. kansasii isolates, sequencing results of the obtained cfp-10 PCR products were compared to M. tuberculosis (GenBank FJ014498.1), M. kansasii subtype I (FJ014492.1), M. kansasii subtype II (FJ014493.1), and M. kansasii subtype V (FJ014497.1). In the case of esat-6, obtained PCR products were compared to M. tuberculosis (FJ014499.1), M. kansasii subtype I (EU888292.1), M. kansasii subtype II (EU888293.1), and M. kansasii subtype V (EU888297.1). M. kansasii subtype II corresponds to the newly described species M. persicum.15
Western blot analysis
Expression of CFP-10 and ESAT-6 proteins by M. kansasii (NMT13 and ZH29), M. persicum (NTM5), M. lymphaticum (ZH21), M. gordonae (NTM38), and M. tuberculosis (H37Rv) were assessed by western blot analysis of cell lysates. Bacteria were harvested by centrifugation (17,000 × g, 15 min, 4°C) after growth in BBL MGIT liquid medium (Becton, Dickinson) at 37°C with added supplements as described previously.4 After washing the bacterial pellet once with cold phosphate-buffered saline (pH 7.4), the pellet was resuspended in 200 μL of RIPA buffer (Santa Cruz, Dallas, TX), according to the manufacturer’s instructions, and transferred into a bead-beating matrix in a 2-mL microtube (Omni International). Afterwards, the tubes were subjected to mechanical lysis at 6,500 rpm for 90 s using a TissueLyser II. Protein concentrations were determined (Bradford reagent, Bio-Rad, München, Germany). An aliquot containing 20 μg of protein per well was loaded and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using a 15% gel. Semi-wet transfer was performed to nitrocellulose membranes (GE Healthcare Life Sciences, Glattbrugg, Switzerland), and immunodetection was carried out using rabbit polyclonal anti–CFP-10 (1:200; Thermo Fisher Scientific ref. PA1-19445) and mouse monoclonal antibody anti–ESAT-6 (1:500; clone 11G4; Abcam ref. ab26246, Cambridge, MA). Membranes were incubated with the corresponding horseradish peroxidase–conjugated secondary antibodies (1:1000; Sigma, Buchs, Switzerland), and signals were developed with an enhanced chemiluminescence system.11
Results
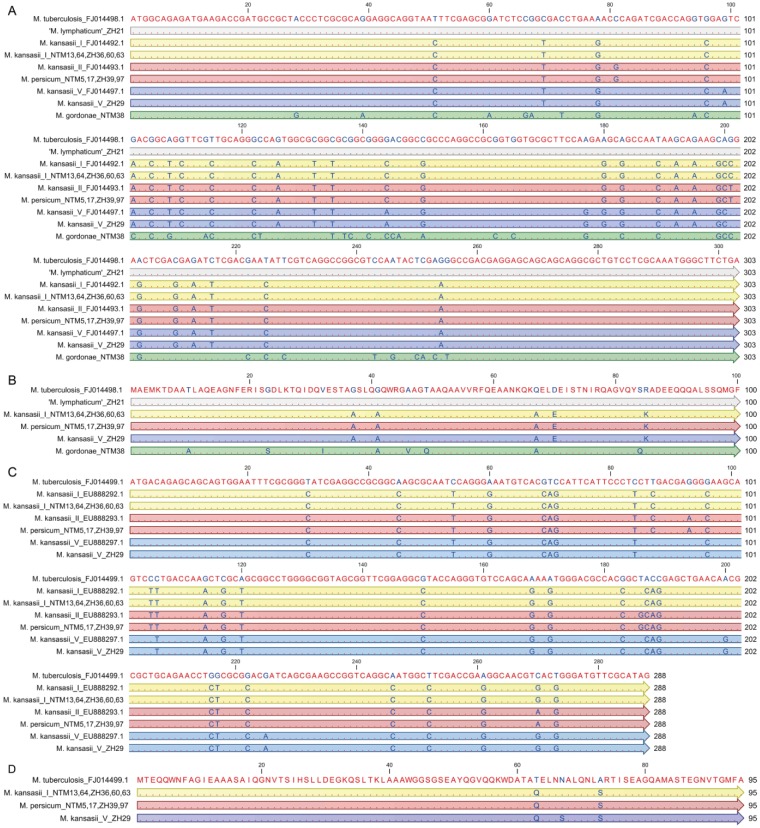
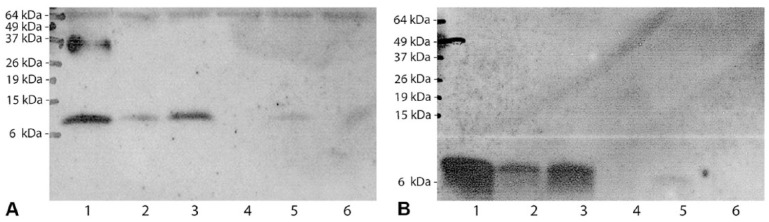
Although most of the 77 NTM isolates were cfp-10 and esat-6 negative, cfp-10 was found in 12 (16%) of the analyzed NTM (M. kansasii subtype I, M. kansasii subtype V, M. persicum, ‘M. lymphaticum’, M. gordonae) and esat-6 in 10 (13%) of the analyzed NTM (M. kansasii subtype I, M. kansasii subtype V, M. persicum; Table 1). Within each subtype of the M. kansasii complex, the sequences were 100% identical (Fig. 1A, C). In comparison to the DNA sequence of M. tuberculosis cfp-10 (303 bp), the similarity of ‘M. lymphaticum’ was 100%, 90.4% for M. kansasii subtype I, and 90.1% for M. kansasii subtype V and M. persicum, respectively (Fig. 1A). The similarity of the “cfp-10–like” gene of M. gordonae was only 85.5% (Fig. 1A). With respect to M. tuberculosis esat-6 (288 bp), the DNA sequences had a similarity of 89.6% for M. kansasii subtype I and M. kansasii subtype V, respectively, and 88.9% for M. persicum (Fig. 1C). In comparison to esat-6 of M. kansasii subtype I, M. persicum and M. kansasii subtype V were closely related with a similarity of 99% and 98.6%, respectively. The deduced amino acid sequences were aligned with M. tuberculosis as a reference (Fig. 1B, D). The putative CFP-10 and ESAT-6 proteins of M. kansasii subtype I, M. persicum, and M. kansasii subtype V differed by only 5 and 2 or 3 amino acids (95% similarity for CFP-10 and 96.8–97.8% similarity for ESAT-6), respectively, from the amino acid sequences of the corresponding M. tuberculosis protein. Interestingly, the putative CFP-10 amino acid sequences were identical for ‘M. lymphaticum’ and M. tuberculosis. The CFP-10 protein sequence of M. gordonae differed the most, with 8 amino acids (92% similarity). Western blot analysis revealed protein expression of cfp-10 (Fig. 2A) and esat-6 (Fig. 2B) for M. kansasii subtype I, M. persicum, and M. kansasii subtype V. For ‘M. lymphaticum’ and M. gordonae, neither CFP-10 nor ESAT-6 could be detected.
Table 1.
Results of examination of non-tuberculous mycobacteria obtained from cattle lymph nodes. The presence (+) and absence (−) of cfp-10 and esat-6 is indicated.
| Mycobacterium species | No. of isolates | cfp-10 | esat-6 |
|---|---|---|---|
| M. avium subsp. hominissuis | 26 (33.8) | − | − |
| M. kansasii subtype I | 5 (6.5) | + | + |
| M. kansasii subtype V | 1 (1.3) | + | + |
| M. persicum | 4 (5.2) | + | + |
| M. nonchromogenicum | 7 (9.1) | − | − |
| M. neoaurum | 6 (7.8) | − | − |
| M. monacense | 3 (3.9) | − | − |
| M. xenopi | 3 (3.9) | − | − |
| M. elephantis | 2 (2.6) | − | − |
| M. vaccae | 2 (2.6) | − | − |
| M. abscessus sp. | 1 (1.3) | − | − |
| M. bourgelatii | 1 (1.3) | − | − |
| M. chitae | 1 (1.3) | − | − |
| M. diernhoferi | 1 (1.3) | − | − |
| M. engbaekii | 1 (1.3) | − | − |
| M. europaeum | 1 (1.3) | − | − |
| M. gordonae | 1 (1.3) | + | − |
| M. intermedium | 1 (1.3) | − | − |
| M. intracellulare | 1 (1.3) | − | − |
| ‘M. lymphaticum’ | 1 (1.3) | + | − |
| M. palustre | 1 (1.3) | − | − |
| M. parafortuitum | 1 (1.3) | − | − |
| M. phlei | 1 (1.3) | − | − |
| Not identifiable | 5 (6.5) | − | − |
| Total | 77 (100) |
Numbers in parentheses indicate frequency, in percentages.
Figure 1.
Alignment of the nucleotide sequence of cfp-10 (A) and esat-6 (C) of Mycobacterium tuberculosis (used as a reference) with the nucleotide sequences of homologues analyzed in our study. In order to illustrate the corresponding subtypes of M. kansasii, they are each compared to the reference strains (FJ014492.1 for M. kansasii subtype I, FJ014493.1 for M. kansasii subtype II, and FJ014497.1 for M. kansasii subtype V). Alignment of the amino acid sequence of CFP-10 (B) and ESAT-6 (D) of M. tuberculosis with the deduced amino acid sequences of the homologues analyzed in our study. Matching residues are indicated with red letters; blue letters represent variable nucleotides. Accessions in GenBank are indicated in the case of reference strains. Yellow indicates M. kansasii subtype I, M. persicum (corresponding to M. kansasii subtype II former labeling) is in red, M. kansasii subtype V in blue, and M. gordonae in green.
Figure 2.
Western blot analysis of cfp-10 and esat-6 expression. Protein lysates were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with polyclonal anti–CFP-10 antibody (A) and monoclonal ESAT-6 antibody (B). Lane 1: Mycobacterium tuberculosis (H37Rv); lane 2: M. persicum (NTM5); lane 3: M. kansasii subtype I (NMT13); lane 4: ‘M. lymphaticum’ (ZH21); lane 5: M. kansasii subtype V (ZH29); lane 6: M. gordonae (NTM38). Molecular masses are indicated in kilodaltons.
Discussion
We identified cfp-10 in M. persicum, M. gordonae, and ‘M. lymphaticum’. Moreover, we identified M. persicum to harbor esat-6. In previous studies, authors have reported the occurrence of cfp-10 and esat-6 in M. kansasii, M. szulgai, M. marinum, M. riyadhense, and M. smegmatis.19 Furthermore, esat-6 was found in M. bovis, M. africanum, M. flavescens, M. gastri, and in 1 of 2 tested M. gordonae isolates.2,7 The presence of identical (‘M. lymphaticum’) or similar (M. gordonae, M. persicum, M. kansasii subtypes I and V) CFP-10 and ESAT-6 amino acid sequences compared to M. tuberculosis might result in immunologic cross-reactivity of existing proteins. However, the antigenicity of the resulting antigens depends to a large extent on their secondary and tertiary structure; therefore, we studied protein expression. Interestingly, protein expression was only detected for M. kansasii subtype I and V as well as for M. persicum, both harboring cfp-10 and esat-6 at the same time. In contrast, no expression of cfp-10 could be shown for ‘M. lymphaticum’ and M. gordonae, even though both isolates carried cfp-10. Because ‘M. lymphaticum’ showed an identical cfp-10 sequence in comparison to M. tuberculosis, it is possible that there are transcriptional regulators that have silenced the gene, or that mutations of the promoter region or other DNA regions are required for expression. Another possible explanation for the missing CFP-10 antigen in both ‘M. lymphaticum’ and M. gordonae could be that CFP-10 and ESAT-6 form a closely interacting heterodimer, implying that the precise level of production of ESAT-6 compared to that of CFP-10 may be relevant for the formation of an active complex.13 The expression level of cfp-10 and esat-6 was found to be highest for M. kansasii subtype I, consistent with the higher pathogenicity of this subtype for humans compared to other subtypes.16 M. persicum showed an intermediate expression level of cfp-10 and esat-6 in comparison to the lowest expressed cfp-10 and esat-6 by M. kansasii subtype V, with the latter considered to belong to a subgroup of environmental isolates, which generally belong to less pathogenic M. kansasii subtypes.
When an immune reaction in cattle occurs by natural exposure to environmental NTM harboring cfp-10 and esat-6, the IFN-γ release assay or skin test could lead to false-positive results as a result of the reduced specificity of the tests. Depending on the local epidemiologic situation in regions with a high or low occurrence of TB, our results suggest that it may be necessary to modify parameters such as antigen (type, composition, and concentration) or cutoff values of any applied screening tests. As well, algorithms used to interpret test results may need to be modified.
If tests comprising antigens such as CFP-10 and ESAT-6 are used for surveillance of animal disease or confirmation of TB-suspicious animals in regions with a low prevalence of TB, such as Switzerland, it is especially important to be aware of potential false-positive results. Given that no alternative TB confirmatory test system in living animals exists, it is advantageous to know the occurrence of NTM in a cattle population. The fact that we isolated several cfp-10 and esat-6 harboring NTM from lymph nodes of slaughtered cattle highlights the specificity problem that might occur by applying the tuberculin skin test or the IFN-γ release assay in a TB-free cattle population if TB-suspicious animals must be tested.
Acknowledgments
We thank Brigitte Sigrist for excellent technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Simone Scherrer  https://orcid.org/0000-0001-9548-8798
https://orcid.org/0000-0001-9548-8798
References
- 1. Adekambi T, et al. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol 2003;41:5699–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arend SM, et al. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J Infect Dis 2005;191:1301–1310. [DOI] [PubMed] [Google Scholar]
- 3. Berthet FX, et al. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 1998;144:3195–3203. [DOI] [PubMed] [Google Scholar]
- 4. Ghielmetti G, et al. Epidemiological tracing of bovine tuberculosis in Switzerland, multilocus variable number of tandem repeat analysis of Mycobacterium bovis and Mycobacterium caprae. PLoS One 2017;12:e0172474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghielmetti G, et al. Non-tuberculous mycobacteria isolated from lymph nodes and faecal samples of healthy slaughtered cattle and the abattoir environment. Transbound Emerg Dis 2018;65:711–718. [DOI] [PubMed] [Google Scholar]
- 6. Good RC. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol 1985;39:347–369. [DOI] [PubMed] [Google Scholar]
- 7. Harboe M, et al. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun 1996;64:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson MM, et al. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 2014;6:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirschner P, et al. Species identification of mycobacteria using rDNA sequencing. Methods Mol Biol 1998;101:349–361. [DOI] [PubMed] [Google Scholar]
- 10. Millington KA, et al. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 2011;108:5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mruk DD, et al. Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis 2011;1:121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pym AS, et al. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 2002;46:709–717. [DOI] [PubMed] [Google Scholar]
- 13. Renshaw PS, et al. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J Biol Chem 2002;277:21598–21603. [DOI] [PubMed] [Google Scholar]
- 14. Scherrer S, et al. Molecular characterization of Mycobacterium avium subsp. hominissuis of two groups of lymph nodes, being intradermal tuberculin or interferon-gamma test positive and negative, isolated from Swiss cattle at slaughter. Front Vet Sci 2018;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahraki AH, et al. Mycobacterium persicum sp. nov., a novel species closely related to Mycobacterium kansasii and Mycobacterium gastri. Int J Syst Evol Microbiol 2017;67:1766–1770. [DOI] [PubMed] [Google Scholar]
- 16. Taillard C, et al. Clinical implications of Mycobacterium kansasii species heterogeneity: Swiss National Survey. J Clin Microbiol 2003;41:1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Telenti A, et al. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 1993;31:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turenne CY, et al. Sequencing of hsp65 distinguishes among subsets of the Mycobacterium avium complex. J Clin Microbiol 2006;44:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Ingen J, et al. Region of difference 1 in nontuberculous Mycobacterium species adds a phylogenetic and taxonomical character. J Bacteriol 2009;191:5865–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wards BJ, et al. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber Lung Dis 2000;80:185–189. [DOI] [PubMed] [Google Scholar]