Abstract
To achieve a contemporary understanding of the common and rare lesions that affect wild, urban Norway rats (Rattus norvegicus), we conducted a detailed pathology analysis of 672 rats from Vancouver, British Columbia, Canada. Grossly evident lesions, such as wounds, abscesses, and neoplasms, were present in 71 of 672 rats (11%) and tended to be severe. The most common and significant lesions were infectious and inflammatory, most often affecting the respiratory tract and associated with bite wounds. We assessed a subset of rats (up to n = 406 per tissue) for the presence of microscopic lesions in a variety of organ systems. The most frequent lesions that could impact individual rat health included cardiomyopathy (128 of 406; 32%), chronic respiratory tract infections as indicated by pulmonary inducible bronchus-associated lymphoid tissue (270 of 403; 67%), tracheitis (192 of 372; 52%), and thyroid follicular hyperplasia (142 of 279; 51%). We isolated 21 bacterial species from purulent lesions in rats with bacterial infections, the most frequent of which were Escherichia coli, Enterococcus sp., and Staphylococcus aureus. Parasitic diseases in rats resulted from infection with several invasive nematodes: Capillaria hepatica in the liver (242 of 672; 36%), Eucoleus sp. in the upper gastrointestinal tract (164 of 399; 41%), and Trichosomoides crassicauda in the urinary bladder (59 of 194; 30%). Neoplastic, congenital, and degenerative lesions were rare, which likely reflects their adverse effect on survival in the urban environment. Our results establish a baseline of expected lesions in wild urban rats, which may have implications for urban rat and zoonotic pathogen ecology, as well as rat control in cities worldwide.
Keywords: Bacterial infections, parasitic diseases, pathology, Rattus, Rodentia, trauma
Introduction
“Very little work has been conducted on the subject of normative biology or natural disease of wild rats. It is presumed that much of the data derived from laboratory rats can be extrapolated to wild rats.”52
Laboratory rats are among the most ubiquitous animals used in biomedical research, and their wild conspecifics are equally pervasive. Norway rats (Rattus norvegicus, hereafter, “rats”) inhabit every continent on Earth with the exception of Antarctica, leading to their designation as one of the most successful free-ranging mammalian species. As 1 of 6 designated globally invasive “urban exploiter species,” rats thrive in human-modified environments including cities, where they capitalize on the constructed environmental habitats with abundant year-round food sources.71 Rats are associated with substantial infrastructure damage and social stigma.30,104 They destroy crops, food, and agricultural infrastructure, leading to food insecurity and annual losses sufficient to feed millions of people.73,88,104 Rats also threaten many populations of endangered wildlife species, and carry numerous zoonotic pathogens, some of which cause serious illness in people.48,95 Such pathogens include Yersinia pestis, which causes plague; Seoul hantavirus (species Seoul orthohantavirus), which causes hemorrhagic fever with renal syndrome; and Leptospira spp., which causes Weil’s disease.
Despite their impact and interwoven association with human societies, the biology, ecology, and diseases of wild rats are understudied. As the opening quote emphasizes, equivalence between rats in the wild and in laboratories is assumed, but there are few data to support this assumption. Previous studies of disease in wild rats have been limited by small sample sizes and/or a narrow focus on specific pathogens or disease processes.10,28,38,41,54,64,67,90,99 Studies by our group describe respiratory and cardiovascular pathology, as well as lesions associated with Capillaria hepatica, Eucoleus sp., and Notoedres muris infections.1,83-86 Many historical studies of wild rat disease describe obvious and common gross anatomic lesions (e.g., chronic lung disease and C. hepatica infection of the liver) rather than undertaking a systematic histologic evaluation of major organ systems.23,87 Investigators during historical plague-eradication efforts examined thousands of rats, but non–plague-associated lesions were of low priority and thus were not documented in detail.104 Further, lesion descriptions and ancillary testing lacked the rigor of modern standards, and pathogen nomenclature and knowledge of disease pathogenesis have since advanced considerably. A modern, holistic disease assessment of a large sample is lacking. Given the wide distribution and thriving numbers of wild rats worldwide and their close association with humans, there is an urgent need for more information about wild rat diseases.
Understanding of the breadth and characteristics of diseases in wild rats is important for several reasons. Generally, a nuanced understanding of any wild animal species’ life history should include natural disease.55,101 The ecology and biology of urban rats must be understood to enable integrated population control and management strategies.30,88 Understanding spontaneous disease in urban rats may also be crucial in the event of a bio- or agroterrorism event, given that rats have the capacity to carry weaponized pathogens.66 Observing the presence or absence of certain diseases in wild rats may provide insight into diseases of laboratory rats used in biomedical research (e.g., cardiomyopathy).19,86 Finally, naturally occurring pathogens and diseases in rats may influence zoonotic pathogen ecology and public health.48,97 The aim of our study was to broaden the understanding of health and disease in wild Norway rats by examining macroscopic and microscopic lesions in a large sample of urban rats.
Materials and methods
Rat collection
As part of a study of zoonoses in urban rats (http://www.vancouverratproject.com), we collected 685 Norway rats from Vancouver, British Columbia, Canada from September 2011 to August 2012, as described previously.46 Briefly, we randomly assigned city blocks to a single 2-wk trapping period and actively trapped rats in back alleys (n = 674). A professional pest control company also collected rats for our study within an international shipping port adjacent to the study area using snap-type lethal traps (n = 11). None of the rats was collected by deliberate use of anticoagulant rodenticides. Following general anesthesia with isoflurane, we euthanized the rats with intracardiac sodium pentobarbital. Our study was carried out in accordance with the Canadian Council on Animal Care national guidelines (http://www.ccac.ca) and with approval by the University of British Columbia’s Animal Care Committee (A11-0087).
Autopsy and sample collection
Following storage at −30°C, we thawed carcasses for a systematic autopsy and tissue collection protocol conducted at the Animal Health Centre (AHC), British Columbia Ministry of Agriculture, Abbotsford, British Columbia. We recorded sex, sexual maturity, body mass, and body condition score either at the time of capture or during autopsy (Supplementary Wild Rat Autopsy Form). It was not possible to autopsy fresh specimens given the large sample size and geographic distance between the study site and laboratory.
Pathology analyses
For each rat, we recorded macroscopic lesions and trimmed tissues for histopathology from most rats with grossly evident lesions (6 rats were erroneously excluded, and rats with liver lesions typical of C. hepatica as the sole macroscopic lesion were not trimmed). For grossly evident purulent lesions, we collected sterile swabs or tissue samples for routine aerobic and anaerobic bacterial isolation and identification at the AHC. We also randomly selected 341 rats from the 601 rats with no macroscopic lesions for histopathology (Fig. 1). The following tissues were included in histologic examinations: adrenal gland, esophagus, heart, intestines, liver, lungs, lymph node, kidney, pharynx, skeletal muscle, spleen, stomach, tongue, thyroid gland, trachea, and urinary bladder, as well as any additional tissues in which gross anatomic lesions were observed. Not all tissues were available from each rat because of collection errors or autolysis (Supplementary Table 1). For 25 randomly selected rats, we examined a transverse section through the demineralized head, including both eyes. We fixed all tissues in 10% neutral-buffered formalin for at least 48 h, then processed tissues by routine methods. Using light microscopy, we examined 4 μm-thick sections of paraffin-embedded tissues stained with hematoxylin and eosin. As required, additional sections were stained with Gram, Steiner silver, and Masson trichrome stains to enhance diagnostic interpretation. Immunohistochemistry on thyroid tissues from 6 rats was performed using monoclonal antibodies against thyroglobulin (EPR9730/ab-156008, monoclonal rabbit anti-mouse, -rat, -human, 1:15,000; Abcam, Cambridge, MA) and calcitonin (A0576, polyclonal rabbit anti-human, 1:30,000; Dako, Santa Clara, CA).
Figure 1.
Sample selection protocol for studying the pathology of urban Norway rats (Rattus norvegicus) from Vancouver, British Columbia, Canada.
We performed transmission electron microscopy on skin tissue for ultrastructural analysis of pox-like lesions. The skin was post-fixed in 2% aqueous OsO4/0.2 M cacodylate buffer (Electron Microscopy Sciences, Hatfield, PA), dehydrated, and embedded (Epon 812; Polysciences, Warrington, PA). Ultrathin sections were stained with uranyl acetate (Electron Microscopy Sciences), followed by lead citrate (Leica Microsystems, Bannockburn, IL). One observer (PMT) visualized sections with a JEOL 1230 transmission electron microscope equipped with an 11-megapixel SC1000 ORIUS side-mount Gatan CCD camera (JEOL, Tokyo, Japan).
Lesion categorization
A veterinary pathologist (JLR) certified by the American College of Veterinary Pathologists (ACVP) screened all tissues. From these data and after review of lesions with at least one other ACVP-certified pathologist (CGH, FAL, NMN, PMT, and/or KMDL), we developed a binary classification scheme to conservatively categorize tissues for the presence or absence of the most frequently observed microscopic lesions (Supplementary Table 1). Tissue artifacts from freeze–thaw, autolysis, and intracardiac barbiturate euthanasia may have obscured subtle lesions. The scheme was based on observed lesions and laboratory rat pathology references.7,9,27,70,82,92 The primary observer (JLR) then re-examined each specimen to confirm the presence or absence of the lesions in the defined categories, and noted infrequent lesions that were not captured by the categories and also microscopic lesions that corresponded to the macroscopic lesions noted at autopsy.
Statistical analyses
For the lesions deemed important to individual rat health that occurred at >10% prevalence and that were not analyzed in previous studies in this sample, we investigated associations with rat demographic characteristics. We fitted univariable logistic regression models with lesion status (positive or negative) as the outcome, and sex, sexual maturity (mature vs. immature), body mass, body condition score (subjective score of 0–2 based on volume of internal fat), and season (spring = March–June, summer = June–August, fall = September–November, and winter = December–February) as explanatory variables. Using Lowess curves (i.e., locally weighted scatterplot smoothing), we assessed linearity between the log odds of each lesion against body mass (continuous variable). If the relationship was nonlinear, we determined the significance of a quadratic term and its main effect in a multi-level logistic regression model (with a random effect for city block) and assessed the shape of the Lowess curve. If the quadratic term was nonsignificant and/or the curve was not quadratic, we categorized weight by quartiles or dichotomized by median weight, depending on the distribution of the data. All models included a random effect for city block to control for clustering among rats. We considered variables for a multivariable model based on their significance in univariable analyses (α = 0.1). Using manual backwards selection, the goal of the model-building process was to identify the most parsimonious set of explanatory variables that predicted the outcome. We used R (R Development Core Team, Vienna, Austria) for all statistical analyses, except for Lowess curves, which were generated in Stata (v.14; StataCorp, College Station, TX).
Results
Of the 685 Norway rats trapped, we excluded 13 from analysis because of advanced autolysis and/or incomplete data that resulted from data collection or entry errors (Fig. 1). Among the remaining 672 rats, 374 (56%) were male, 290 (43%) were female, and sex could not be determined for 8 rats because of recording errors. Most rats were sexually mature (n = 390; 58%; open vaginal orifices in females and scrotal testes in males); maturity could not be determined for 64 rats because of recording errors. The median weight was 145 g (range: 20–466 g). There were macroscopic lesions in 71 rats (11%), of which 6 rats were erroneously eliminated from histologic examination.
The most frequent and major histopathologic findings in the 406 rats examined for microscopic lesions are tabulated (Table 1). The full lesion classification scheme, including minor and/or less frequent lesions, is provided in Supplementary Table 1. Summaries and descriptions of neoplastic and proliferative lesions, rare lesions, and all parasites are provided in Supplementary Tables 2–4, respectively. The most frequent parasitic infections associated with lesions and bacteria isolated from lesions are summarized in Table 2 and Supplementary Table 5, respectively. Patterns of parasitic coinfections are provided in Supplementary Table 6.
Table 1.
Major histologic lesions that may impact individual rat health observed at ≥10% prevalence, and demographic characteristics associated with increased odds of having the lesion, among a sample of wild urban Norway rats (Rattus norvegicus) trapped in Vancouver, British Columbia, Canada.
| Category | Histologic description | n/N* | Prevalence | 95% CI | Demographic characteristics† |
|---|---|---|---|---|---|
| Cardiomyopathy | Lymphoplasmacytic myocarditis, fibrosis, and/or myocardial degeneration | 128/406 | 32 | 27–36 | Male sex & heavy body mass |
| Stomach lesions‡ | Hyperkeratosis, mucosal hyperplasia, granulocytic inflammation | 231/388 | 60 | 54–65 | Heavy body mass & sexual maturity |
| Thyroid follicular hyperplasia | Hypertrophic follicular cells surround small or absent follicular lumens lacking colloid§ | 142/279 | 51 | 45–57 | Male sex & heavy body mass |
| Inducible BALT | Peribronchiolar and/or perivascular lymphoplasmacytic cuffs¦ | 270/403 | 67 | 62–72 | Heavy body mass |
| Tracheitis | Lymphoplasmacytic and/or granulocytic submucosal and/or periglandular inflammation | 192/372 | 52 | 47–59 | Heavy body mass |
| Interstitial nephritis | Interstitial lymphoplasmacytic inflammation# | 121/405 | 30 | 26–35 | Heavy body mass |
| Pyelitis | Lymphoplasmacytic inflammation in interstitium immediately adjacent to the renal pelvis | 85/255 | 33 | 28–40 | Heavy body mass, body condition score, & Leptospira interrogans infection |
BALT = bronchus-associated lymphoid tissue; CI = confidence interval.
The number of rats examined for each lesion varies because not all tissues were available for each individual rat because of sampling error, tissue artifacts, and/or autolysis.
Demographic characteristic(s) associated with increased odds of having the lesion as determined by multivariable logistic regression modeling with a random effect for city block to control for clustering.
Lesions were associated with Eucoleus sp. infection including hyperkeratosis (keratin layer >45 μm thick in areas without artificial separation and not adjacent to the limiting ridge [junction with glandular stomach]), mucosal hyperplasia (mucosa is >4–6 cells thick, affecting mainly the basal layers and >25% of the section), and inflammation (keratin pustules and submucosal inflammation with granulocytes infiltrating >2 foci not adjacent to the limiting ridge). Details of statistical associations are available elsewhere.85
Follicular cells were tall cuboidal-to-columnar and had to affect >50% of the thyroid gland and with ≤10 normal follicles.
Lymphocytes and plasma cells surrounding ≥3 secondary or tertiary bronchioles or 1 primary bronchiole and/or ≥3 blood vessels.
Present in ≥2 locations within the renal interstitium and excluding areas immediately adjacent to the renal pelvis.
Table 2.
Major parasitic infections, associated lesions, and demographic characteristics associated with increased odds of having the parasite among a sample of wild urban Norway rats (Rattus norvegicus) trapped in Vancouver, British Columbia, Canada.
| System | Location | Organism | Associated lesion | n/N* | Demographic characteristics† | Ref. |
|---|---|---|---|---|---|---|
| Digestive | Upper GI tract‡ | Eucoleus spp. | Mucosal hyperkeratosis, hyperplasia, inflammation | 164/399 (41) | Heavy body mass & sexual maturity | 85 |
| Hepatic | Liver | Capillaria hepatica | Multifocal granulomatous hepatitis and fibrosis | 242/672 (36) | Sexual maturity | 83 |
| Integumentary | Ears, nose, distal limbs, tail | Notoedres muris | Proliferative and crusting dermatitis | 2/672 (0.3) | NA | 1 |
| Urinary | Urinary bladder§ | Trichosomoides crassicauda | Variable, mild submucosal lymphoplasmacytic inflammation | 59/194 (30) | Female sex & heavy body mass | —¦ |
NA = not available; Ref. = reference to previous studies of a subsample of rats in the current study. We found no evidence of Angiostrongylus cantonensis, Echinococcus multilocularis, Sarcocystis spp., Toxoplasma gondii, or Trichinella spiralis infection in any of the rats.
Rats were positive if there was at least one characteristic egg and/or adult cross-section in the sections examined. The number of rats examined for each lesion varies because not all tissues were available for each individual rat because of sampling error, tissue artifacts, and/or autolysis. Numbers in parentheses are prevalences.
Demographic characteristic(s) associated with increased odds of having the parasite as determined by multivariable logistic regression modeling with a random effect for city block to control for clustering.
Adults and/or eggs were present in upper gastrointestinal tract including tongue, oropharynx, esophagus, and/or non-glandular stomach.
Adults and/or eggs were also rarely present within the renal pelvis.
There are no previous studies of these parasites in this population of rats.
Cardiovascular lesions
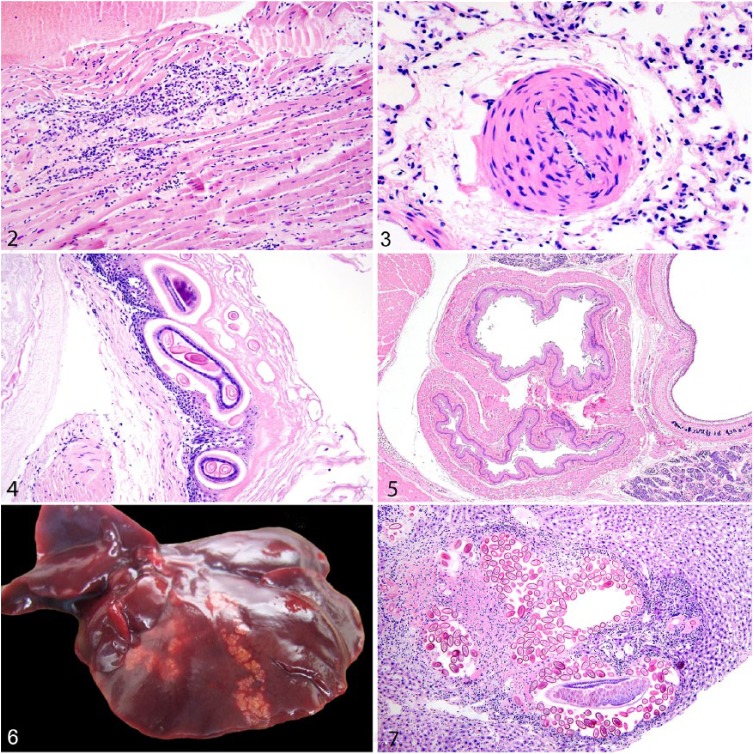
The most common cardiovascular lesion was cardiomyopathy (32%), consisting of mononuclear cell myocarditis, fibrosis, and/or myocardial degeneration as described previously (Fig. 2).86 Myocardial mineralization, which was not typically associated with cardiomyopathy, was evident in 8.6% of rats, and 4.5% of rats had right ventricular hypertrophy. Vascular lesions were most prominent in the lungs and included pulmonary arteriolar medial hypertrophy in 22% of rats (Fig. 3) and intimal mineralization of pulmonary blood vessels in 15% of rats.
Figures 2–7.
Microscopic lesions in urban Norway rats. Figure 2. Lymphocytic myocarditis, myocyte necrosis, and fibrosis indicative of cardiomyopathy. H&E. Figure 3. Medial hypertrophy of pulmonary blood vessels featuring thickening of the tunica media by disorganized smooth muscle cells. H&E. Figure 4. Eucoleus sp. adults and eggs in the non-glandular gastric mucosa in association with hyperkeratosis and mucosal hyperplasia. H&E. Figure 5. Two distinct esophageal lumens (double esophagus) are adjacent to the trachea. H&E. Figure 6. Multiple tortuous tan parasite tracts caused by Capillaria hepatica on the liver surface. Figure 7. An adult C. hepatica in oblique section and eggs efface hepatocytes and are surrounded by granulomatous inflammation and fibrosis in the liver parenchyma. H&E.
Digestive tract lesions
A capillarid nematode, Eucoleus sp., was present in the upper gastrointestinal tract of many rats (41%) and was associated with hyperkeratosis, mucosal hyperplasia, and submucosal inflammation in the forestomach, as described previously in a subset of rats included in our study (Fig. 4).85 The previous study also observed that infection and/or associated stomach lesions were associated with heavier and sexually mature rats.85 Rarely, rats were coinfected with Eucoleus sp. and the nematode Gongylonema neoplasticum.12,85 Nematodes and cestodes (species unidentified) were within small intestinal lumina in 42% and 3.0% of rats, respectively, and a few rats (6.6%) were infected with coccidian protozoa, also in the small intestines. Intestinal contents of at least 2 rats contained bright turquoise material consistent with commercial bait products containing anticoagulant rodenticide (Supplementary Fig. 1; see section on hemorrhage in “Other Lesions and General Conditions” below).
Dental disease was infrequent and most often involved mild subjective overgrowth of lower incisors or other malocclusion (Supplementary Fig. 2). In the most severe case of malocclusion, there were multiple missing teeth and suppurative osteomyelitis and fracture of alveolar bones. Several rats had variably severe stomatitis, glossitis, pharyngitis, and/or esophagitis. Rare lesions included squamous papillomas of the tongue and forestomach85 (Supplementary Table 2); Meckel diverticulum, double esophagus (Fig. 5), and gastric ulceration, each in 1 rat; and fungal gastritis and sialadenitis in 3 and 5 rats, respectively (Supplementary Table 3).
The most common hepatic lesions were associated with the presence of C. hepatica (syn. Calodium hepaticum) in 36% of rats, as described previously (Table 2; Figs. 6, 7).83 The previous study recorded that rats with bite wounds and those that were sexually mature had increased odds of being infected.83 One rat each had multifocal hepatic necrosis, periportal hepatitis, and hepatic lipidosis (Supplementary Table 3). Rarely, peripancreatic fat was saponified and/or infiltrated by granulocytes and macrophages.
Endocrine lesions
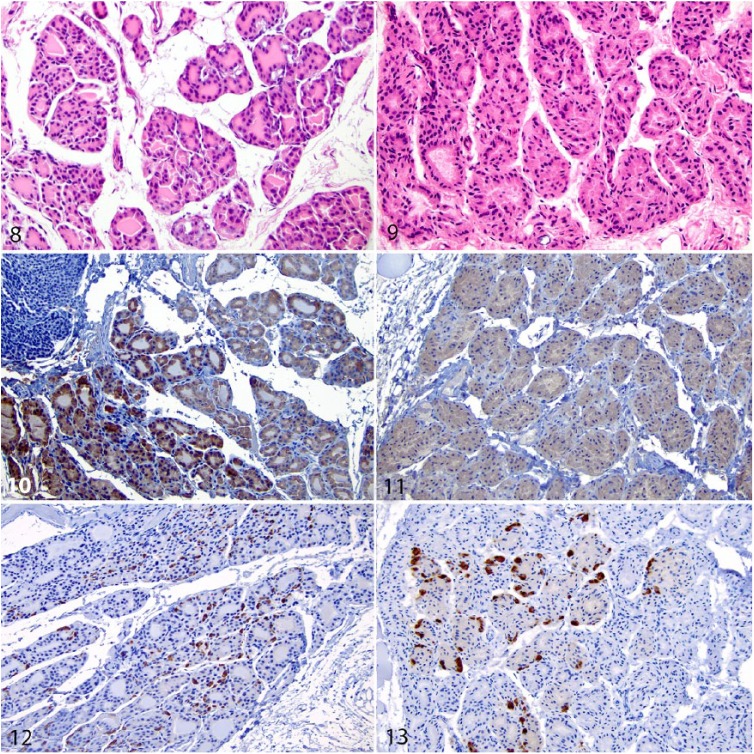
Thyroid glands of 51% of rats had diffuse follicular hyperplasia (i.e., hyperplastic goiter) consistent with dietary iodine deficiency (Figs. 8–13). Follicles were lined by tall cuboidal-to-columnar epithelial cells. Follicular lumens were not evident or barely appreciable and lacked colloid. This lesion was differentiated from C-cell hyperplasia using immunohistochemistry for calcitonin and thyroglobulin in 6 exemplar rats. Immunostaining for thyroglobulin was weak, diffuse, and intracytoplasmic in the majority of hyperplastic cells (consistent with follicular cells). Calcitonin immunostaining was variably strong, punctate-to-weak, and diffuse within the cytoplasm of interstitial cells located centrally within the thyroid gland (consistent with C cells). Multivariable logistic regression modeling revealed that the odds of a rat having thyroid follicular hyperplasia were increased in males versus females (odds ratio [OR] = 1.97; 95% confidence interval [CI] = 1.14–3.45; p = 0.016) and heavy versus light rats (>256 g; OR = 3.69; 95% CI = 1.30–11.1; p = 0.016; Supplementary Table 7).
Figures 8–13.
Normal and diffuse thyroid follicular hyperplasia in the thyroid gland of urban Norway rats. Figure 8. Normal thyroid follicular cells are low cuboidal and surround follicles that contain abundant, homogeneous, eosinophilic colloid. H&E. Figure 9. Affected thyroid follicular cells are cuboidal to low columnar and surround follicles that lack colloid. H&E. Figure 10. Normal immunostaining that is intense and intracytoplasmic within follicular cells. Immunohistochemistry (IHC) for thyroglobulin. Figure 11. Within affected hyperplastic cells, immunostaining is weak, diffuse, and intracytoplasmic, consistent with follicular cells. IHC for thyroglobulin. Figures 12, 13. Scattered normal interstitial C cells have intense cytoplasmic immunostaining, whereas hyperplastic follicular cells are negative. IHC for calcitonin.
Residual colloid was mineralized in 47% of rats. Additional, albeit rare, lesions included adrenal cortical hyperplastic nodules (Supplementary Table 2), adrenal gland mineralization, adrenalitis, and thyroiditis (Supplementary Table 3).
Integumentary lesions
Skin wounds were the most frequent integumentary lesion (33%) and likely resulted from conspecific bites. The mean number of wounds was 0.6 per rat (range: 0–15). Wounds were most often over the caudal back and base of the tail (Supplementary Fig. 3); facial and tail wounds were less frequent. Wounds ranged from focal puncture wounds (1 mm) to large (≤2 cm) and sharply demarcated lacerations. Chronicity ranged from acute to completely healed with scar tissue. In some cases, bite wounds were associated with severe myositis, cellulitis, and/or osteomyelitis, particularly those occurring on the dorsal lumbar area and hindlimbs. Some rats with and without bite wounds had subcutaneous abscesses along the ventral head, neck, and lateral thorax that were often associated with local or generalized lymph node enlargement (Supplementary Fig. 4). A previous study in these rats describes the bacterial culture results from infected bite wounds—most infections were polymicrobial and the most common isolate was Staphylococcus aureus; male and heavier rats had increased odds of having bite wounds.50
Many rats had bilaterally symmetrical alopecia over the dorsal lumbar region (exact number not recorded; Supplementary Figs. 3, 5). In severe cases, alopecia was accompanied by yellow-tan epidermal crusting and extended down the thighs and cranially to the neck. Histologically, these areas featured decreased numbers of hair follicles, mild mononuclear cell infiltration, sebaceous gland atrophy, epidermal hyperplasia, and orthokeratotic hyperkeratosis. In severe cases, inflammation extended to the deep dermis and into the panniculus muscle.
Many rats were infested by ectoparasites—fleas (Nosopsyllus fasciatus), mites (Ornithonyssus bacoti), and lice (Polyplax spinulosa).7 Two rats were affected by proliferative dermatitis associated with the ear mite, N. muris (Table 2; Supplementary Fig. 6).1 Rare lesions included proliferative skin lesions associated with poxvirus in 2 rats (confirmed by electron microscopy; Supplementary Figs. 7–10, Supplementary Table 2) and pinnal dermatitis, hyperkeratosis, and chemical or thermal dermatosis in 1 rat each (Supplementary Table 3).
Lymphatic, musculoskeletal, adipose, and connective tissue lesions
Generalized or locally enlarged lymph nodes were common (exact number not recorded) and were typically associated with skin wounds or abscesses. Granulocytic lymphadenitis was observed in 3.2% of rats. Two rats had splenic fibrosis (Supplementary Table 3).
Occasionally, rats were affected by granulocytic-to-lymphoplasmacytic myositis in one or more of the following skeletal muscle groups: tongue, larynx, diaphragm, and triceps or quadriceps. Some of these rats had simultaneous infections or traumatic injuries. For example, one rat with pyelonephritis and another with chronic traumatic lesions on the distal tail had multifocal lymphoplasmacytic myositis of the tongue, diaphragm, triceps, and quadriceps. Glossal myositis was often perineural. Severe and deep skin wounds were often associated with suppurative myositis and/or abscesses in the subjacent musculature. One rat each had an ascending limb infection, generalized muscle atrophy, and coxofemoral arthritis (Supplementary Table 3, Supplementary Fig. 23). One rat had a hibernoma in the perirenal adipose tissue (Supplementary Table 2).
Respiratory lesions
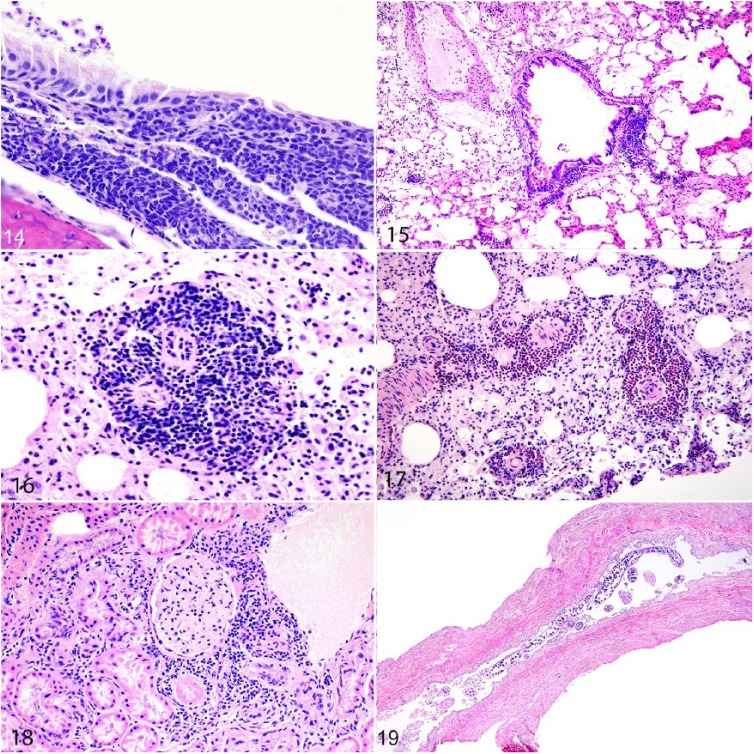
The most common histologic lesion in the upper respiratory tract was lymphoplasmacytic or granulocytic tracheitis (52%; Fig. 14), followed by lymphoplasmacytic rhinitis (40%), and lymphoplasmacytic or granulocytic laryngitis and/or epiglossitis (39%). Cilia-associated respiratory bacillus (CAR bacillus), which was evident histologically as mats of filamentous bacteria among respiratory epithelium lining the nasal cavity or trachea, was present in 24% of rats. One rat had laryngeal hyperkeratosis (Supplementary Table 3).
Figures 14–19.
Microscopic lesions in urban Norway rats. Figure 14. Lymphoplasmacytic tracheitis, featuring severe infiltration by lymphocytes and plasma cells within the submucosa; a few granulocytes are adhered to the mucosal surface. Figure 15. The bronchiole is cuffed by asymmetrical clusters of lymphocytes indicative of inducible bronchus-associated lymphoid tissue (iBALT). Figure 16. Blood vessels are cuffed by thick clusters of lymphocytes indicative of iBALT in the lungs. Figure 17. Arterioles are cuffed by a mixed population of granulocytes and lymphocytes indicating perivascular mixed inflammation. Figure 18. The interstitium adjacent to glomeruli and tubules is infiltrated by lymphocytes and plasma cells indicative of interstitial nephritis. Figure 19. Infection by the nematode parasite, Trichosomoides crassicauda, in the urinary bladder. Multiple adults in cross- and longitudinal sections are within the urinary bladder lumen.
The most frequent histologic lesions in the lower respiratory tract were cuffs of lymphocytes and plasma cells that surrounded bronchioles and/or pulmonary blood vessels (consistent with inducible bronchus-associated lymphoid tissue [iBALT]) in 67% of rats (Figs. 15, 16). Less frequently, a mixed inflammatory cell population that included granulocytes, lymphocytes, and plasma cells surrounded pulmonary blood vessels (20%; Fig. 17).
Grossly evident respiratory pathology for all rats in our current study, including abscesses/bronchiectasis and pulmonary neoplasms (Supplementary Table 2), and rare microscopic lesions, were described previously in a subsample of rats in our current study.84 The previous study identified increased odds of having upper respiratory tract inflammatory lesions and iBALT in heavier rats.84
Reproductive lesions
Three male rats had orchitis (Supplementary Table 3). Lesions of the female reproductive tract were infrequent (exact number not recorded unless explicitly stated). Mucohemorrhagic vaginal discharge was present in a few sexually mature rats that were pregnant or lactating, or had lesions consistent with anticoagulant rodenticide toxicity or mild granulocytic endometritis. A few had clear-to-hemorrhagic fluid in the uterine lumen. Endometritis of variable severity was present in at least 7 rats, and at least 2 rats had clitoral adenitis (Supplementary Table 3). Placental scars from previous pregnancies were observed frequently (Supplementary Fig. 11). Two female rats with no reproductive tract lesions were pregnant in only one uterine horn.
Urinary lesions
The most common urinary tract lesion was intratubular renal crystals present in 34% of rats. Lymphoplasmacytic interstitial nephritis occurred in 30% of rats and was typically mild and perivascular in distribution (Fig. 18). Multivariable logistic regression modeling revealed that heavier rats had significantly increased odds of having interstitial nephritis (145–256 g: OR = 21.0, 95% CI = 4.0–390, p = 0.004; >256 g: OR = 84.7, 95% CI = 16.2–1,580, p < 0.001; Supplementary Table 8). There was no significant association with Leptospira interrogans (pathogen test and prevalence was described previously).46 Interstitial lymphoplasmacytic pyelitis immediately adjacent to the renal pelvis occurred in 33% of rats. Multivariable logistic regression modeling revealed significantly increased odds of a rat having pyelitis in heavier rats (>145 g vs. <144 g: OR = 9.69, 95% CI = 3.20–37.7, p < 0.001), those with higher body condition score (BCS = 1: OR = 4.92, 95% CI = 1.40–23.1, p < 0.021; BCS = 2: OR = 5.24, 95% CI = 1.41–25.4, p < 0.020), and those infected with L. interrogans (OR = 3.29, 95% CI = 1.42–8.62, p < 0.010; Supplementary Table 9).
The nematode parasite, Trichosomoides crassicauda, was present in the lumen or superficial mucosa of the urinary bladder or, less frequently, of the renal pelvis, in 30% of rats (Fig. 19).6 In a few infected rats, there was mild lymphoplasmacytic inflammation of the submucosa and/or muscularis. Based on multivariable logistic regression modeling, there were significantly increased odds of T. crassicauda infection in female rats (OR = 2.11; 95% CI = 1.04–4.30; p = 0.040) and those that weighed >145 g (OR = 24.4, 95% CI = 6.96–155, p < 0.001; Supplementary Table 10). Urinary bladders of 9 of 119 (7.6%) male rats contained agonal proteinaceous plugs (Supplementary Fig. 12). Rare lesions included pyelonephritis (Supplementary Fig. 13) and perirenal abscesses in 2 rats each, and a renal cyst and lesions consistent with bilateral chronic progressive nephropathy in 1 rat each (Supplementary Table 3).
Other lesions and general conditions
Hemorrhages consistent with anticoagulant rodenticide exposure were present in 8.8% (59 of 672) of rats (Supplementary Figs. 1, 14–17). Most often, these hemorrhages were within the subcutaneous tissues. Periarticular, parenchymal (lung, intestine, testis or scrotum), and intracranial hemorrhages, as well as hemothorax and hemoperitoneum, were less frequent. Hemorrhagic discharge from the penis or vagina, epistaxis, and retroperitoneal hemorrhages were rare. Lungs of 2 rats with pulmonary hemorrhages contained hemosiderin-laden macrophages, suggesting that the hemorrhagic events were chronic but ongoing.84 In at least 2 rats, stomach and intestines contained bright turquoise material morphologically similar to commercial anticoagulant rodenticides (Supplementary Fig. 1).
Eight rats were affected by severe, chronic traumatic injuries, including one rat with an intrathoracic air gun pellet as described previously.84 The skin of one rat contained a sharply demarcated, deep, linear wound with associated crusting and alopecia that surrounded the neck and continued caudally to the right front leg (Supplementary Fig. 18). One rat had proliferation of the dorsal spinous processes of the cranial thorax and thickening of the spine at the base of the tail. This rat also had debris and alopecia on the caudal portion of the body, collectively suggestive of paralysis. Five rats had femoral fractures or amputations affecting the right hindlimb. These lesions were frequently associated with wounds, suppurative cellulitis, and osteomyelitis. Tail amputations were frequent (exact number not recorded; Supplementary Fig. 19).
Acute traumatic injuries in at least 36 rats were likely related to the live trapping activities we undertook to collect rats: hyperemia of the distal extremities (Supplementary Fig. 20), and less often, nail devolving, tail tip amputations, and nostril lacerations. The whiskers, facial fur, and ears of 2 rats were singed. Additional rare conditions including bilateral panophthalmitis are described in Supplementary Table 3 and depicted in Supplementary Figs. 21–23.
Discussion
Overall, the most severe diseases of wild rats in Vancouver appear to be infectious and inflammatory conditions in a variety of systems. Lesions in the cardiovascular, respiratory, endocrine, integumentary, and digestive systems from a variety of causes were common and apparently important based on their severity. Grossly evident lesions were relatively rare yet severe. Although individual microscopic lesions may have limited significance, the cumulative effects of multi-systemic lesion patterns (i.e., total disease burden) may have negative impacts on individual rat health and longevity.89 Given the breadth and severity of lesions we observed, it is possible that many individuals in this sample were in poor overall health. However, we were unable to directly link lesions with evidence of ill health, such as emaciation and decreased fecundity. Among the most important lesions, there was often a statistical association with heavy body mass. If mass is considered a proxy for age, these results suggest that rats accumulate lesions as part of the aging process.
Lesions associated with bacterial infections
There was a variety of bacterial pathogens and associated diseases in our sample of wild rats. The most common bacterium isolated from purulent lesions was Escherichia coli, followed by Enterococcus spp., and Staphylococcus spp. The latter was frequently isolated from abscesses, a result that is consistent with rats examined in San Francisco, CA, during a plague control effort, and also a survey of infections in New Zealand rats.17,104 Other studies have also identified a diverse array of bacterial infections in wild rats, often in abscesses, including Bordetella bronchiseptica and Corynebacterium sp.17 Conspecific bite wounds were among the lesions most frequently associated with bacterial infections in our study (see “Traumatic and Anthropogenic Lesions” below). In total, 28 of 672 (4.2%) rats had abscesses in a variety of systems, which may have resulted from bacteria inoculated into bite wounds.
In our study, rats frequently had respiratory tract lesions (perivascular and/or peribronchiolar lymphoplasmacytic cuffs and tracheitis) that a previous study associated with Mycoplasma pulmonis and/or CAR bacillus infection.84 The infections were not microscopically distinguishable, and many rats were coinfected in the previous study.84 Similar respiratory coinfections in wild rats have also been described.28 In laboratory and pet rats, respiratory infections with these pathogens can progress to severe bronchiectasis, bronchopneumonia, and pulmonary abscesses.24,34,37,59,100 We observed these types of severe lesions in a small number of rats,84 which suggests that rats experience similar disease progression in the wild. Other studies of wild rats have also documented severe respiratory disease in the form of bronchiectasis or pulmonary abscesses.10,43,58,62,67,81,87 Among all of the lesions that we observed, those associated with the respiratory system likely had the most impact on rat health.
Bacteria also infected the reproductive and urinary tracts. Purulent lesions in the seminal vesicles and uterus were rare occurrences in other studies of wild rats.5,104 We observed an unusual Salmonella enterica ser. Enteritidis–associated metritis, perhaps from an ascending infection, although fecal Salmonella spp. were rare in this population (3 of 633; 0.5%; this individual was not tested for fecal carriage of this bacterium).49 Within the urinary system were rare cases of pyelonephritis and perirenal abscesses that were also rare in other wild rat studies.40,96
No rats had lesions consistent with plague (i.e., subcutaneous hyperemia, multifocal hepatic necrosis, splenomegaly, and bilateral pleural effusions).104 We did observe abscessed lymph nodes, but these were purulent rather than caseous as is typical of plague in rats. Albeit rarely, rats may be infected with Yersinia pestis but lack lesions; however, bacteriologic results yielded no evidence of Y. pestis infection, indicating that rats in Vancouver were likely free of plague during the time frame when rats were collected. However, as a major port city, there is the ongoing potential for plague introductions.33
Parasitic lesions
Parasites and/or associated lesions were common and included nematode infections in the liver (C. hepatica), upper gastrointestinal system (Eucoleus sp.), and intestines, as well as the urinary bladder and kidney (T. crassicauda); ectoparasites on the skin (N. muris, N. fasciatus, O. bacoti, and P. spinulosa); and unidentified cestodes and suspected coccidian protozoa in the intestines. Of the most common parasites, Eucoleus sp. and C. hepatica were consistently associated with host responses to infection: Eucoleus sp. with hyperkeratosis and mucosal hyperplasia in the non-glandular stomach, and C. hepatica with fibrosis and hepatic parenchymal destruction.83,85 In general, even severe C. hepatica infections are not associated with clinical signs of hepatic dysfunction or mortality in rats.29 And although the biology of Eucoleus sp. is less understood, it is similarly unlikely to directly cause clinical disease or mortality. But in addition to these lesions, nematodes may also negatively affect rat health through their systemic influence on the immune system and response to microparasite infections (i.e., viruses, bacteria, and protozoa).36 Other studies have identified a similarly diverse array of parasites in wild rats.69,74 However, histopathology is required to detect the full array and distribution of parasites along with associated lesions (e.g., Eucoleus sp. in the oral cavity and esophagus; T. crassicauda in the kidney).
We identified T. crassicauda in 30% of rats, which is in agreement with previous studies in North America in which 25–56% of rats were infected.32,45 We most often observed adults and/or eggs in the urinary bladder—they were only rarely in the renal pelvis. The rarity of renal infection differs from a previous study that documented either adults or eggs in ~25% of rat kidneys examined.40 The prevalence of urinary and enteric parasites may be underestimated in our study as a result of freeze–thaw of carcasses and the use of histopathology without direct examination—both factors may decrease parasite detection.87 T. crassicauda was associated with heavier (presumably older) rats and females, which suggests the possibility of age and sex-based differences in how rats acquire the parasite.
Four zoonotic parasites were notably absent in our study: Angiostrongylus cantonensis, Echinococcus multilocularis, Trichinella spiralis, and Toxoplasma gondii. The range of the globally emerging parasite, A. cantonensis, is expanding in southern North America but our results indicate that it had not yet established in Vancouver rats at the time our samples were collected.21 The absence of E. multilocularis is expected because rats are only rarely intermediate hosts and likely have no-to-minimal roles in the epidemiology of this pathogen.80 The absence of T. spiralis is also not surprising given that the parasite, as of 2019, is rare in Canada and virtually absent from Canadian domestic pigs. A study of rats collected in the 1940s from farms in British Columbia reported T. spiralis in 25% of wild rats (n = 260).77 Given that there are no pig farms near the study site, our results support the theory that the domestic transmission cycle in rats requires infected pigs.2 We did not observe tissues cysts consistent with T. gondii in any of the rats examined even though it is a common infection in rats elsewhere.98 The apparent absence may be a reflection of our laboratory technique, given that histology is less sensitive than other methods.26
Larvae of Taenia taeniaeformis were also absent, despite being present in relatively high numbers in rats elsewhere (e.g., up to 45% prevalence in Montreal).32,63 This result may be related to deworming of domestic cats and limiting their outdoor access.
Idiopathic inflammatory lesions
Idiopathic inflammatory lesions were present in nearly every body system. With the exception of cardiomyopathy, most of these lesions were likely of no clinical significance. The lesions included interstitial nephritis, sialoadenitis, peripancreatic fat saponification and inflammation, adrenalitis, orchitis, cerebral gliosis, and pustular stomatitis and/or esophagitis that was distinct from lesions typically associated with Eucoleus sp. infection (i.e., hyperkeratosis, mucosal hyperplasia, and submucosal inflammation).85
Cardiomyopathy, consisting of myocarditis, fibrosis, and/or myocardial degeneration, was present in approximately one-third of the rats in our study. In a subsample of these rats, lesions of cardiomyopathy were associated with heavier (presumably older) male rats, which mirrors the epidemiology of this condition in laboratory rat populations.86 Cardiomyopathy is a frequent, spontaneous lesion in laboratory rats, in which it most often affects aged males fed standard commercial diets ad libitum.19,57 The disease can be fatal in laboratory rats, and it may be similarly important to wild rat health.57
We identified interstitial nephritis in approximately one-third of the rats examined, which is consistent with the results from previous studies in wild rats.18,40,58,62,103,104 This lesion may be incidental, an early manifestation of chronic progressive nephropathy or the result of chronic Leptospira spp. infection.44,90 Although ~11% of the rats in our sample tested positive for L. interrogans,46 there was no statistical association between interstitial nephritis and detection of this pathogen. Results from other studies that assessed an association between interstitial nephritis and Leptospira in rats are inconsistent.3,14,76,90 For instance, one study observed no difference in the prevalence of interstitial nephritis based on Leptospira sp. culture status in wild rats.96 In experimental studies in laboratory rats, acute infections were not associated with lesions, but chronic infections led to interstitial nephritis.76 These studies also differed in pathogen detection methods and statistical analyses. Pyelitis, a specific type of interstitial nephritis in which lymphocytes and plasma cells aggregate immediately adjacent to the renal pelvis, was frequently present in our study and was statistically associated with L. interrogans infection (as identified by PCR), as well as heavy body mass and body condition score. This distinctive lesion has also been identified previously in wild rats.40 Interstitial nephritis and pyelitis tended to surround renal blood vessels and could result from migration of T. crassicauda larvae44; however, in our study, there was no statistical association between the lesion and the parasite infection.
Traumatic and anthropogenic lesions
Conspecific bite wounds were common and seemingly important lesions. Wounds ranged in severity, were frequently associated with lymphadenopathy, and were often infected. Infected wounds harbored 1 or more of 22 species of aerobic and anaerobic bacteria.50 The bacterial species isolated from wounds were often also present in internal infections, suggesting that the bite wounds were the portals of entry. Wounds and associated infections likely had an important impact on individual rat health. Fighting-induced wounds, and especially those that become infected, can be fatal.13 Most bite wounds occurred in the lumbosacral region near the base of the tail, as in previous observations of wild rats.8,13 Dominant male rats tend to preferentially bite the back of subordinate conspecifics, whereas females bite the head,94 which suggests that most bites were inflicted by males. Disruption of a colony’s established dominance hierarchy may increase fighting,8,30 which may occur following anthropogenic control efforts. Fighting and the resulting bite wounds may also be an important mechanism for transmitting zoonotic and other pathogens among rats.39,46
Although conspecific bite wounds were the most prevalent traumatic lesions, human activities likely resulted in other traumatic injuries. One rat was shot with a metal pellet in the thorax.84 We suspected that 7 of 672 rats (1.0%) with chronic wounds, limb fractures, and/or amputations survived snap-type rat traps based on wound appearance and location. One study attributed amputations in wild rats to frostbite,87 but temperatures in Vancouver are rarely below freezing. Most chronic limb injuries occurred in the right hindlimb, without apparent reason. It is remarkable that these individuals survived, in spite of the apparent severity of these lesions given that animals that survived severe limb injuries have been reported only rarely in wildlife.102 These injuries likely exerted a substantial impact on individual health. Collectively, these results suggest that rats in urban environments frequently experience non-lethal trauma and raise animal welfare concerns about trapping methodologies for rat control. Although snap-type traps are generally considered to be humane, there are serious welfare implications if traps are improperly set and fail to kill.68
There were also incidents of acute trauma that were attributable to our live-trapping techniques. The cage-type traps likely caused injury to the nose and toes of a small number of rats. There were 2 instances in which we suspected that members of the public intentionally burnt rats in traps. Given the overall objectives of the project, live trapping was necessary to obtain the required biological samples (serum, fresh tissues); therefore, alternative methods were not considered. Future studies could potentially avoid these types of injuries by lining cage traps with a solid bottom to prevent pinched toes and by not startling the trapped rats to prevent nose injuries that may result when they rush into the wire mesh. Reducing time in traps may also improve welfare and deter malicious activities, although the standard approach for nocturnal mammals such as rats, is to leave traps set overnight, as we did in our study.35,68
Sublethal exposure to anticoagulant rodenticide was suspected in many of the rats based on the presence of characteristic turquoise bait in the stomach and/or intestinal contents, as well as subcutaneous, periarticular, and internal hemorrhages.68 We did not use rodenticide as a means to collect rats for our study, therefore rats were exposed through other sources (e.g., property owners, pest control companies). Information about rodenticide use in the study location was not available, and toxicology testing was not included in our study. Poisoned rats can live for at least several days, depending on the dosage and type of anticoagulant rodenticide.68 We live-trapped the poisoned rats detected in our study—these individuals were actively foraging at the time of capture, which contradicts the assumption that poisoned rats die in burrows and are not readily recovered.20 An alternative explanation for the presence of live-trapped rats with evidence of rodenticide exposure is that these rats may have genetic resistance to this class of poisons. Whatever the underlying mechanism, there may be a substantial risk of secondary poisonings to cats, dogs, predatory and scavenging birds, and mammals if they capture and consume poisoned rats.68,91
Degenerative conditions
Degenerative conditions were infrequent in wild rats in our study compared to laboratory rats, and included chronic progressive nephropathy (CPN), alopecia, mineralization of various soft tissues, dental disease, right ventricular cardiac hypertrophy, and coxofemoral arthritis. Degenerative conditions have also been infrequently observed in other free-ranging wild animals.31,79
The presence of CPN in only one rat was unexpected given that it is a frequent background lesion of laboratory rats and has been identified in other studies of wild rats.44,96,104 It may be that the early lesions of CPN, which are subtle (i.e., tubular basophilia and basement membrane thickening, hyaline casts, and glomerulosclerosis)44 were obscured by tissue artifacts from autolysis and freezing. The low prevalence of CPN may also be a function of environmental and dietary differences between wild and laboratory populations. Diets of free-ranging rats may consist of less protein and fewer calories compared to commercial rations. Low protein and calorie-restricted diets reduce the prevalence of CPN in laboratory rats.44,56 Rat burrows may be more humid than typical laboratory animal facilities and thus may support maintenance of hydration and kidney function. Alternatively, it is possible that wild rats die at younger ages from other causes before CPN can progress to the severity seen in laboratory rats, or that affected individuals are selectively removed from the population early in the course of disease.
Many rats had bilaterally symmetrical alopecia. Previous trauma is a frequent cause of alopecia in laboratory rats,72 and although alopecia was often concurrent with bite wounds in our study, many alopecic rats had no evidence of previous skin injury. In the absence of traumatic injury, other potential causes include age-related senescence, endocrine diseases, and ectoparasites. A seminal observational study of wild rats maintained in an outdoor enclosure associated alopecia with normal senescence in rats of both sexes, as well as social rank among males.13 The study also noted that hair thinned over the lumbosacral area before progressing to generalized alopecia in most rats. Given that many rats in our study were affected by hyperplastic goiter, endocrine-associated alopecia is a possibility. Ectoparasitism was also common in this population and may have contributed to hair loss.
A range of tissues had mild mineralization, which probably had no adverse health effects. Affected tissues included pulmonary blood vessels, heart, adrenal glands, kidney, and thyroid glands. Other studies of wild rats describe mineralization in the kidneys and mammary glands.25,96 Although vitamin D toxicity may cause tissue mineralization, vitamin D analogue rodenticides are not licensed for pest control in Canada, making exposure to these substances unlikely (Heath Canada: Pesticides and Pest Management, Government of Canada, http://www.hc-sc.gc.ca/cps-spc/pest/index-eng.php). Similarly, multi-tissue metastatic mineralization is often associated with CPN in laboratory rats,44 but CPN was rare in our study. Given the likely exposure of many rats to anticoagulant rodenticides, it is possible that this contributed to tissue mineralization, as occurs in experimental settings.51
Several rare degenerative lesions likely had important impacts on the health of individual rats. Although rare in our study, dental disease is frequent in laboratory rat populations.65 Right ventricular hypertrophy was rarely identified in our study and was not associated with heart failure.86 We observed arthritis in the coxofemoral joint of one rat that may have resulted from a traumatic injury.
Proliferative, neoplastic, and congenital lesions
The most frequent proliferative lesion was diffuse thyroid follicular hyperplasia, present in 51% of rats. Male rats and those with body mass >256 g had increased odds of having this lesion; there was no significant association with season. Given that thyroid function has a major influence on a variety of physiological processes, including the immune system, the overall poor health of many rats in this population may be attributed in part to the high prevalence of this lesion. Although the cause is unknown, iodine deficiency is likely. The association with body mass suggests that older rats may be predisposed to developing this lesion. It is also possible that rats were exposed to a goitrogenic substance in the urban environment. Although it is widely believed that urban rats subsist on human refuse (i.e., food waste),11 this diet should contain iodine. The possibility of iodine deficiency in urban rats may call into question the true source of urban rat diets. Further studies are necessary to determine if the follicular hyperplasia is associated with iodine deficiency and hypothyroidism.
There were several rare tumors and proliferative lesions in our sample of rats; however, only a round cell lung tumor was deemed malignant and was associated with severe cachexia.84 The rarity of neoplasia is consistent with previous studies, and the absence of other malignant neoplasms may have been a function of reduced survival. For instance, among 23,000 wild rats examined during the 1907–1908 plague eradication effort in San Francisco, CA, only 14 epithelial and 8 mesenchymal tumors were observed, and half of these were deemed malignant.103 The estimated prevalence of tumors among these Californian rats was 0.1%, much lower than the 1.5% prevalence (10 of 672) in our study.104 Half of the tumors in our study were only identified via histopathology; the prevalence of grossly identifiable tumors was 0.7%, which is closer to the historical estimate. Interestingly, most neoplastic lesions affected the mammary tissues in this previous study, whereas we did not observe any mammary tumors.104 Another historical study did not describe any neoplasms in 444 wild Norway rats, suggesting that large sample sizes are necessary to detect these rare lesions.5 Other neoplasms described in wild rats include uterine fibroma, leiomyosarcoma, lipoma, renal carcinoma, and testicular angiosarcoma.53,58,104 In our study, benign neoplasia, proliferative lesions (e.g., pulmonary mucous metaplasia,84 hibernoma, adrenal cortical hyperplastic nodules), and the 2 cutaneous pox lesions were likely of minimal health significance.
We observed 2 congenital anomalies: a double esophagus and a Meckel diverticulum. Both are similarly rare in laboratory rats.15,42 The low prevalence of congenital anomalies in wild rats may be the result of high genetic diversity and poor survival of rats with severe anomalies.
Implications
There are some similarities between the lesions in our sample of wild rats and those in laboratory settings. Historically, infectious diseases were a major cause of morbidity and mortality in laboratory rats prior to their elimination through cesarean derivation and enhanced biosecurity measures,4,100 so it is not surprising to find similar infections in wild rats. Yet several lesions were notably rare or absent in our study compared to their captive conspecifics: chronic progressive nephropathy, gastric ulceration, and malignant neoplasia. Similarly, we did not identify histologic evidence of viral diseases, with the exception of cutaneous pox, although we did not pursue viral molecular testing. It is probable that severely diseased individuals are rapidly removed from the population through conspecific aggression, starvation, and predation. If this is true, then the prevalence of all lesions in our study is likely an underestimate. However, our selection of rats for histologic examination (most rats with grossly evident lesions and roughly half of rats with none) and lack of accounting for geographical clustering may have created artificially high prevalence estimates. Nevertheless, our results suggest that the type and prevalence of lesions in wild rats are remarkably different than laboratory populations. Thus, it cannot be assumed that results in laboratory rats are directly applicable to wild rats.52
Considering that we assessed euthanized, live-trapped rats rather than collected carcasses, the frequency of lesions and disease afflicting our study population was striking. It is unclear whether live trapping biased the sample toward either diseased or healthy individuals.75 Diseased rats may have been more willing to enter traps to consume food bait. Conversely, healthy rats may have foraged more widely and thus encountered traps. Regardless, our live trapping efforts seem to have sampled diverse individuals along the spectrum of health and disease. This range is consistent with the mass rat trapping efforts in San Francisco, CA, in which apparently sick rats were frequently captured.104 It would be advantageous to study disease in rats that die naturally; however, carcass recovery is impeded by their small body size, rapid decomposition, and subterranean habitat preference (i.e., burrows and infrastructure such as sewers).13,30,104
Despite the potential impacts of disease on individual rats, there was evidence of robust reproduction,47 suggesting that population-level effects were unlikely.5,22,55 Specifically, among sexually mature female rats in our study, 21% were visibly pregnant with a median of 9 embryos per pregnancy, 50% were lactating, and over 60% were parous.47 Infectious diseases such as Leptospira sp., Salmonella sp., and C. hepatica have no apparent effect on rat populations.23 Even plague outbreaks—the most virulent infectious disease to afflict rats—fail to suppress populations.104
Our large, live-trapped sample and in-depth analysis of lesions and causative agents differs from typical wildlife pathology studies. Most are retrospective surveys that use cause-of-death analyses of sick or dead specimens collected through passive surveillance.31,78 The scale and systematic approach of our study is analogous to disease surveys undertaken in fish, which are often actively caught rather than collected through passive surveillance.61 The lack of analogous wild mammal studies is likely the result of logistical and ethical limitations given that histology requires invasive and/or lethal sampling that is expensive, requires skilled personnel, and may be only ethically feasible for certain species such as pests (e.g., rats and mice) and those sustainably harvested by subsistence hunting.16,60,93 One of the most comprehensive health studies in any terrestrial mammal included active sample collection from 835 reindeer and caribou (Rangifer ssp.) and the primary focus was on body condition indices, pathogen detection, heavy metals, and contaminants rather than a systematic pathology assessment. Yet, the use of general diagnostic techniques including histopathology for wild animals is an invaluable tool to detect novel and emerging diseases in this era of molecular testing and provides important baseline disease data.16,55
Our cross-sectional study captured the occurrence of lesions in an urban population of wild rats live-trapped during a 1-y period. Infectious and inflammatory lesions were present in a variety of organ systems, and their severity had the most potential to impact rat health, as did the high prevalence of thyroid follicular hyperplasia. It is unknown if this amount and type of disease is reflective of rats in other cities or environments (e.g., farms and natural areas) so future studies should compare our results to other wild rat populations. Given that rats carry many zoonotic pathogens, understanding the influence of comorbidities and coinfections on zoonotic pathogen status in individual rats is an intriguing area of future research. Studies that document and assess the impact of disease on rat survival would also increase our general knowledge of these large and persistent populations. Ultimately, accounting for disease will allow for a more nuanced understanding of urban rat ecology.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638719833436 for Pathology of wild Norway rats in Vancouver, Canada by Jamie L. Rothenburger, Chelsea G. Himsworth, Krista M. D. La Perle, Frederick A. Leighton, Nicole M. Nemeth, Piper M. Treuting and Claire M. Jardine in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Kirbee Parsons and Alice Feng for their assistance with rat collection; Victoria Chang, Heather Anholt, and Debra Rempel for their assistance with sample collection; and Erin Zabek (bacterial culture), and Sandra Etheridge and Joanne Taylor (histology slide preparation) at the British Columbia Ministry of Agriculture Animal Health Centre. The fieldwork was made possible by the assistance of the City of Vancouver (Murray Wightman, Stuart McMillan), the Urban Health Research Initiative, the Vancouver Injection Drug Users Study, and the Vancouver Area Network of Drug Users.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The Canadian Institutes of Health Research funded this study (MOP-119530). J Rothenburger’s research is supported by the Natural Sciences and Engineering Research Council Alexander Graham Bell Canada Graduate Scholarship–Doctoral, Canadian Federation of University Women Dr. Margaret McWilliams Pre-Doctoral Fellowship, Imperial Order Daughters of the Empire War Memorial Scholarship, Ontario Veterinary College Graduate Student Fellowship, and the University of Guelph Dean’s Tri-Council Scholarship.
Supplementary material: Supplementary material for this article is available online.
ORCID iDs: Jamie L. Rothenburger  https://orcid.org/0000-0003-1634-9154
https://orcid.org/0000-0003-1634-9154
Piper M. Treuting  https://orcid.org/0000-0002-1812-1519
https://orcid.org/0000-0002-1812-1519
References
- 1. Anholt H, et al. Ear mange mites (Notoedres muris) in black and Norway rats (Rattus rattus and Rattus norvegicus) from inner-city Vancouver, Canada. J Wildl Dis 2014;50:104–108. [DOI] [PubMed] [Google Scholar]
- 2. Appleyard GD, Gajadhar AA. A review of trichinellosis in people and wildlife in Canada. Can J Public Health 2000;91:293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Athanazio DA, et al. Rattus norvegicus as a model for persistent renal colonization by pathogenic Leptospira interrogans. Acta Trop 2008;105:176–180. [DOI] [PubMed] [Google Scholar]
- 4. Baker DG. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clinical Microbiol Rev 1998;11:231–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balfour A. Observations on wild rats in England, with an account of their ecto- and endoparasites. Parasitology 1922;14:282–298. [Google Scholar]
- 6. Barthold SW. Trichosomoides crassicauda infection, urinary bladder, rat. In: Jones TC, et al., eds. Urinary System. Monographs on Pathology of Laboratory Animals. Berlin, DE: Springer, 1998:463–465. [Google Scholar]
- 7. Barthold SW, et al. Rat. In: Barthold SW, et al., eds. Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IA: Blackwell, 2016:119–171. [Google Scholar]
- 8. Blanchard RJ, et al. Conspecific wounding in free-ranging R. norvegicus from stable and unstable populations. Psychol Rec 1985;35:329–335. [Google Scholar]
- 9. Boorman GA. Lung. In: Boorman GA, ed. Pathology of the Fischer Rat. San Diego, CA: Elsevier Academic Press, 1990:339–367. [Google Scholar]
- 10. Brogden KA, et al. Cilia-associated respiratory bacillus in wild rats in central Iowa. J Wildl Dis 1993;29:123–126. [DOI] [PubMed] [Google Scholar]
- 11. Brooks JE, Jackson WB. A review of commensal rodents and their control. CRC Crit Rev Environ Cont 1973;3:405–453. [Google Scholar]
- 12. Brown HR, Hardisty JF. Oral cavity, esophagus, and stomach. In: Boorman GA, ed. Pathology of the Fischer Rat. San Diego, CA: Elsevier Academic Press, 1990:15–30. [Google Scholar]
- 13. Calhoun JB. The Ecology and Sociology of the Norway Rat. Bethesda, MD: U.S. Department of Health, Education, and Welfare, 1963:237–239. Public Health Service Publication 1008. [Google Scholar]
- 14. Cameron GC, Irwin DA. Leptospira icterohæmorrhagiæ occurrence in wild rats at Toronto. Can Public Health J 1929;20:386–392. [Google Scholar]
- 15. Canpolat L, et al. Case report: a rare congenital esophageal malformation on double esophagus in the rat. Kaibogaku Zasshi 1998;73:13–17. [PubMed] [Google Scholar]
- 16. Carnegie RB, et al. Managing marine mollusc diseases in the context of regional and international commerce: policy issues and emerging concerns. Philos Trans R Soc Lond B Biol 2016;371(1689):20150215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter ME, Cordes DO. Leptospirosis and other infections of Rattus rattus and Rattus norvegicus. N Z Vet J 1980;28:45–50. [DOI] [PubMed] [Google Scholar]
- 18. Ceruti R, et al. Wild rats as monitors of environmental lead contamination in the urban area of Milan, Italy. Environ Pollut 2002;117:255–259. [DOI] [PubMed] [Google Scholar]
- 19. Chanut F, et al. Spontaneous cardiomyopathy in young Sprague-Dawley rats: evaluation of biological and environmental variability. Toxicol Pathol 2013;41:1126–1136. [DOI] [PubMed] [Google Scholar]
- 20. Conlogue G, et al. Capillaria hepatica (Bancroft) in select rat populations of Hartford, Connecticut, with possible public health implications. J Parasitol 1979;65:105–108. [PubMed] [Google Scholar]
- 21. Cowie RH. Angiostrongylus cantonensis: agent of a sometimes fatal globally emerging infectious disease (rat lungworm disease). ACS Chem Neurosci 2017; 2102–2104. [DOI] [PubMed] [Google Scholar]
- 22. Davis DE. The characteristics of rat populations. Q Rev Biol 1953;28:373–401. [DOI] [PubMed] [Google Scholar]
- 23. Davis DE. The relation between the level of population and the prevalence of Leptospira, Salmonella, and Capillaria in Norway rats. Ecology 1951;32:465–468. [Google Scholar]
- 24. Davis JK, Cassell GH. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol 1982;19:280–293. [DOI] [PubMed] [Google Scholar]
- 25. De Oliveira D, et al. Leptospira in breast tissue and milk of urban Norway rats (Rattus norvegicus). Epidemiol Infect 2016;144:2420–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubey JP, Frenkel JK. Toxoplasmosis of rats: a review, with considerations of their value as an animal model and their possible role in epidemiology. Vet Parasitol 1998;77:1–32. [DOI] [PubMed] [Google Scholar]
- 27. Dungworth DL, et al. Nonneoplastic lesions in the lungs. In: Mohr U, et al., eds. Pathobiology of the Aging Rat. Vol 1 Washington, DC: ILSI Press, 1992:143–160. [Google Scholar]
- 28. Easterbrook JD, et al. A survey of rodent-borne pathogens carried by wild-caught Norway rats: a potential threat to laboratory rodent colonies. Lab Anim 2008;42:92–98. [DOI] [PubMed] [Google Scholar]
- 29. Farhang-Azad A. Ecology of Capillaria hepatica (Bancroft 1893) (Nematoda). 1: Dynamics of infection among Norway rat populations of the Baltimore Zoo, Baltimore, Maryland. J Parasitol 1977;63:117–122. [PubMed] [Google Scholar]
- 30. Feng AYT, Himsworth CG. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst 2014;17:149–162. [Google Scholar]
- 31. Fenton H, et al. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis Aquat Organ 2017;122:171–183. [DOI] [PubMed] [Google Scholar]
- 32. Firlotte WR. Parasites of the brown Norway rat. Can J Comp Med Vet Sci 1948;12:187. [PubMed] [Google Scholar]
- 33. Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol 2005;50:505–528. [DOI] [PubMed] [Google Scholar]
- 34. Ganaway JR, et al. Isolation, propagation, and characterization of a newly recognized pathogen, cilia-associated respiratory bacillus of rats, an etiological agent of chronic respiratory disease. Infect Immun 1985;47:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gannon WL, Sikes RS. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 2007;88:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garza-Cuartero L, et al. The worm turns: trematodes steering the course of co-infections. Vet Pathol 2014;51:385–392. [DOI] [PubMed] [Google Scholar]
- 37. Giddens WE, et al. Morphologic and microbiologic features of trachea and lungs in germfree, defined-flora, conventional, and chronic respiratory disease-affected rats. Am J Vet Res 1971;32:115–129. [PubMed] [Google Scholar]
- 38. Giusti AM, et al. Gastric spiral bacteria in wild rats from Italy. J Wildl Dis 1998;34:168–172. [DOI] [PubMed] [Google Scholar]
- 39. Glass GE, et al. Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus). Epidemiol Infect 1988;101:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gray JE, et al. Observations on the kidneys and urine of the wild Norway rat, Rattus norvegicus. Vet Pathol 1974;11:144–152. [DOI] [PubMed] [Google Scholar]
- 41. Gregson RL, et al. Bronchus-associated lymphoid tissue (BALT) in the laboratory-bred and wild rat, Rattus norvegicus. Lab Anim 1979;13:239–243. [DOI] [PubMed] [Google Scholar]
- 42. Gupta BN. Meckel’s diverticulum in a rat. Lab Anim Sci 1973;23:426–427. [PubMed] [Google Scholar]
- 43. Habermann RT, et al. Common infections and disease conditions observed in wild Norway rats kept under simulated natural conditions. Am J Vet Res 1954;15:152–156. [PubMed] [Google Scholar]
- 44. Hard GC, et al. Non-proliferative lesions of the kidney and lower urinary tract in rats. In: Guides for Toxicologic Pathology. Washington, DC: STP/ARP/AFIP, 1999. [Google Scholar]
- 45. Harkema R. The parasites of some North Carolina rodents. Ecol Monogr 1936;7:151–232. [Google Scholar]
- 46. Himsworth CG, et al. Ecology of Leptospira interrogans in Norway rats (Rattus norvegicus) in an inner-city neighborhood of Vancouver, Canada. PLoS Negl Trop Dis 2013;7:e2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Himsworth CG, et al. The characteristics of wild rat (Rattus spp.) populations from an inner-city neighborhood with a focus on factors critical to the understanding of rat-associated zoonoses. PLoS One 2014;9:e91654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Himsworth CG, et al. Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis 2013;6:349–359. [DOI] [PubMed] [Google Scholar]
- 49. Himsworth CG, et al. Prevalence and characteristics of Escherichia coli and Salmonella spp. in the feces of wild urban Norway and black rats (Rattus norvegicus and Rattus rattus) from an inner-city neighborhood of Vancouver, Canada. J Wildl Dis 2015;51:589–600. [DOI] [PubMed] [Google Scholar]
- 50. Himsworth CG, et al. Bacteria isolated from conspecific bite wounds in Norway and black rats: implications for rat bite-associated infections in people. Vector Borne Zoonotic Dis 2014;14:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Howe AM, Webster WS. Warfarin exposure and calcification of the arterial system in the rat. Int J Exp Pathol 2000;81:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hulin MS, Quinn R. Wild and black rats. In: Suckow MA, et al., eds. The Laboratory Rat. Burlington, MA: Elsevier, 2006:865–882. [Google Scholar]
- 53. Jeon B-S, et al. Uterine leiomyosarcoma in a wild rat (Rattus norvegicus): usefulness of Ki-67 labeling index for diagnosis. Lab Anim Res 2013;29:127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kakrada MK, et al. Cilia-associated respiratory bacillus infection in rats in New Zealand. N Z Vet J 2002;50:81–82. [DOI] [PubMed] [Google Scholar]
- 55. Karesh WB, Cook RA. Applications of veterinary medicine to in situ conservation efforts. Oryx 1995;29:244–252. [Google Scholar]
- 56. Keenan KP, et al. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: II. Effects on age-related proliferative and degenerative lesions. Toxicol Pathol 1995;23:287–302. [DOI] [PubMed] [Google Scholar]
- 57. Keenan KP, et al. Diet, overfeeding, and moderate dietary restriction in control Sprague-Dawley rats: I. Effects on spontaneous neoplasms. Toxicol Pathol 1995;23:269–286. [DOI] [PubMed] [Google Scholar]
- 58. Kilham L, et al. Intranuclear inclusions and neoplasms in the kidneys of wild rats. J Natl Cancer Inst 1962;29:863–885. [PubMed] [Google Scholar]
- 59. Kling MA. A review of respiratory system anatomy, physiology, and disease in the mouse, rat, hamster, and gerbil. Vet Clin North Am Exot Anim Pract 2011;14:287–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kutz S, et al. Standardized monitoring of Rangifer health during International Polar Year. Rangifer 2013;33:91–114. [Google Scholar]
- 61. Lang T, et al. Diseases of dab (Limanda limanda): analysis and assessment of data on externally visible diseases, macroscopic liver neoplasms and liver histopathology in the North Sea, Baltic Sea and off Iceland. Mar Environ Res 2017;124:61–69. [DOI] [PubMed] [Google Scholar]
- 62. Laurain AR. Lesions of skeletal muscle in leptospirosis: review of reports and an experimental study. Am J Pathol 1955;31:501–519. [PMC free article] [PubMed] [Google Scholar]
- 63. Lee B-W, et al. Cysticercus fasciolaris infection in wild rats (Rattus norvegicus) in Korea and formation of cysts by remodeling of collagen fibers. J Vet Diagn Invest 2016;28:263–270. [DOI] [PubMed] [Google Scholar]
- 64. Lewis DJ. A comparison of the pathology of the larynx from SPF, germ-free, conventional, feral and mycoplasma-infected rats. J Comp Pathol 1982;92:149–160. [DOI] [PubMed] [Google Scholar]
- 65. Losco PE. Dental dysplasia in rats and mice. Toxicol Pathol 1995;23:677–688. [DOI] [PubMed] [Google Scholar]
- 66. Lõhmus M, et al. Rodents as potential couriers for bioterrorism agents. Biosecur Bioterror 2013;11(Suppl 1):247–257. [DOI] [PubMed] [Google Scholar]
- 67. MacKenzie WF, et al. A filamentous bacterium associated with respiratory disease in wild rats. Vet Pathol 1981;18:836–839. [DOI] [PubMed] [Google Scholar]
- 68. Mason GJ, Littin KE. The humaneness of rodent pest control. Anim Welf 2003;12:1–37. [Google Scholar]
- 69. McGarry JW, et al. Zoonotic helminths of urban brown rats (Rattus norvegicus) in the UK: neglected public health considerations? Zoonoses Public Health 2015;62:44–52. [DOI] [PubMed] [Google Scholar]
- 70. McInnes EF. Wistar and Sprague-Dawley rats. In: Background Lesions in Laboratory Animals: A Color Atlas. China: Saunders Elsevier, 2012:17–36. [Google Scholar]
- 71. McKinney ML. Urbanization as a major cause of biotic homogenization. Biol Conserv 2006;127:247–260. [Google Scholar]
- 72. Mecklenburg L, et al. Proliferative and non-proliferative lesions of the rat and mouse integument. J Toxicol Pathol 2013;26(Suppl 3):S27–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meerburg BG, et al. The year of the rat ends—time to fight hunger! Pest Manag Sci 2009;65:351–352. [DOI] [PubMed] [Google Scholar]
- 74. Milazzo C, et al. Helminths of the brown rat (Rattus norvegicus) (Berkenhout, 1769) in the city of Palermo, Italy. Helminthologia 2010;47:238–240. [Google Scholar]
- 75. Mitchell D. Experiments on neophobia in wild and laboratory rats: a reevaluation. J Comp Physiol Psychol 1976;90:190–197. [DOI] [PubMed] [Google Scholar]
- 76. Monahan AM, et al. Host-pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol 2009;46:792–799. [DOI] [PubMed] [Google Scholar]
- 77. Moynihan IW, Musfeldt IW. A study of the incidence of trichinosis in rats in British Columbia. Can J Comp Med Vet Sci 1949;13:152–155. [PMC free article] [PubMed] [Google Scholar]
- 78. Mörner T, et al. Surveillance and monitoring of wildlife diseases. Rev Sci Tech 2002;21:67–76. [DOI] [PubMed] [Google Scholar]
- 79. Munson L, et al. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J Wildl Dis 2005;41:542–548. [DOI] [PubMed] [Google Scholar]
- 80. Okamoto M, et al. Natural Echinococcus multilocularis infection in a Norway rat, Rattus norvegicus, in southern Hokkaido, Japan. Int J Parasitol 1992;22:681–684. [DOI] [PubMed] [Google Scholar]
- 81. Owen D. Some parasites and other organisms of wild rodents in the vicinity of an SPF unit. Lab Anim 1976;10:271–278. [DOI] [PubMed] [Google Scholar]
- 82. Renne R, et al. Proliferative and non-proliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol 2009;37(Suppl 7):S5–S73. [DOI] [PubMed] [Google Scholar]
- 83. Rothenburger JL, et al. Capillaria hepatica in wild Norway rats (Rattus norvegicus) from Vancouver, Canada. J Wildl Dis 2014;50:628–633. [DOI] [PubMed] [Google Scholar]
- 84. Rothenburger JL, et al. Respiratory pathology and pathogens in wild urban rats (Rattus norvegicus and Rattus rattus). Vet Pathol 2015;52:1210–1219. [DOI] [PubMed] [Google Scholar]
- 85. Rothenburger JL, et al. Lesions associated with Eucoleus sp. in the non-glandular stomach of wild urban rats (Rattus norvegicus). Int J Parasitol Parasites Wildl 2014;3:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rothenburger JL, et al. Survey of cardiovascular pathology in wild urban Rattus norvegicus and Rattus rattus. Vet Pathol 2015;52:201–208. [DOI] [PubMed] [Google Scholar]
- 87. Schiller EL. Ecology and health of Rattus at Nome, Alaska. J Mammal 1956;37:181–188. [Google Scholar]
- 88. Singleton GR, et al. Unwanted and unintended effects of culling: a case for ecologically-based rodent management. Integr Zool 2007;2:247–259. [DOI] [PubMed] [Google Scholar]
- 89. Snyder JM, et al. Cause-of-death analysis in rodent aging studies. Vet Pathol 2016;53:233–243. [DOI] [PubMed] [Google Scholar]
- 90. Sterling C, Thiermann A. Urban rats as chronic carriers of leptospirosis: an ultrastructural investigation. Vet Pathol 1981;18:628–637. [DOI] [PubMed] [Google Scholar]
- 91. Stone WB, Okoniewski JC. Poisoning of wildlife with anticoagulant rodenticides in New York. J Wildl Dis 1999;35:187–193. [DOI] [PubMed] [Google Scholar]
- 92. Suckow MA, et al., eds. The Laboratory Rat. 2nd ed. Burlington, MA: Elsevier Academic Press, 2006. [Google Scholar]
- 93. Sullivan TP, et al. Impact of removal-trapping on abundance and diversity attributes in small-mammal communities. Wildl Soc Bull 2003;31:464–474. [Google Scholar]
- 94. Takahashi LK, Blanchard RJ. Attack and defense in laboratory and wild Norway and black rats. Behav Processes 1982;7:49–62. [DOI] [PubMed] [Google Scholar]
- 95. Towns DR, et al. Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions 2006;8:863–891. [Google Scholar]
- 96. Tucunduva de Faria M, et al. Morphological alterations in the kidney of rats with natural and experimental Leptospira infection. J Comp Pathol 2007;137:231–238. [DOI] [PubMed] [Google Scholar]
- 97. Vaumourin E, et al. The importance of multiparasitism: examining the consequences of co-infections for human and animal health. Parasit Vectors 2015;8:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Webster JP. Prevalence and transmission of Toxoplasma gondii in wild brown rats, Rattus norvegicus. Parasitology 1994;108:407–411. [DOI] [PubMed] [Google Scholar]
- 99. Webster JP, Macdonald DW. Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology 1995;111:247–255. [DOI] [PubMed] [Google Scholar]
- 100. Weisbroth SH. Post-indigenous disease: changing concepts of disease in laboratory rodents. Lab Anim 1996;25:25–33. [Google Scholar]
- 101. Wobeser GA. Essentials of Disease in Wild Animals. Ames, IA: Blackwell, 2006. [Google Scholar]
- 102. Woodman N. Survival of the less-fit: a least shrew (Mammalia, Soricidae, Cryptotis parvus) survives a separated leg fracture in the wild. J Wildl Dis 2013;49:735–737. [DOI] [PubMed] [Google Scholar]
- 103. Woolley PG, Wherry WB. Notes on twenty-two spontaneous tumors in wild rats (M. norvegicus). J Med Res 1911;25:205–216. [PMC free article] [PubMed] [Google Scholar]
- 104. Wyman W, ed. The Rat and Its Relation to the Public Health. Washington, DC: Treasury Department, Public Health and Marine-Hospital Service of the United States, 1910. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638719833436 for Pathology of wild Norway rats in Vancouver, Canada by Jamie L. Rothenburger, Chelsea G. Himsworth, Krista M. D. La Perle, Frederick A. Leighton, Nicole M. Nemeth, Piper M. Treuting and Claire M. Jardine in Journal of Veterinary Diagnostic Investigation