Abstract
Polycystic liver is usually considered an incidental finding in human and veterinary medicine. Two unrelated adult llamas (Lama glama) with a history of marked anorexia and weight loss were received for autopsy and diagnostic workup. The main gross change in the liver of both animals was multiple variably sized cysts randomly distributed throughout the parenchyma. Histologically, the cysts compressed the adjacent parenchyma and were lined by a single layer of cuboidal-to-columnar epithelium, surrounded by a fibrous collagen capsule. The lumen of the cysts contained finely granular-to-homogeneous basophilic material. The lining epithelium displayed strong immunoreactivity for pancytokeratin AE1/AE3 and cytokeratins 7, 8, 8/18, and 19, and was negative for vimentin, confirming the biliary epithelial origin of the cysts. No parasitic or infectious agents, or neoplastic changes, were detected. All other laboratory tests performed in both llamas were negative or non-diagnostic, suggesting that the congenital hepatic cysts described may have been at least partly responsible for clinical disease in both animals.
Keywords: Congenital hepatic cysts, llamas, liver disease
Polycystic liver (often referred to as polycystic liver disease) has been described in several species of domestic, wild, and laboratory animals,5,11,14,20,22 and in humans.4 In both humans and animals, this condition is congenital and has usually been considered to be an incidental finding. A literature search revealed that polycystic liver has not previously been reported in llamas. We describe and characterize herein polycystic livers in 2 adult llamas and possible association of the lesions with clinical disease in both animals.
Two llamas from different herds were presented to the California Animal Health and Food Safety (CAHFS), Tulare and San Bernardino branches, respectively, for autopsy and diagnostic workup. Case 1 was a 22-y-old intact male, and case 2 was a 14-y-old intact female. The clinical history of both animals included multiple episodes of anorexia with progressive weight loss for ~3 wk. Given the poor prognosis, the owners opted for euthanasia in both cases. No blood work or other antemortem test results were available from either case. Euthanasia was performed with an overdose of barbiturate, and a postmortem examination was performed on both carcasses a few hours after death.
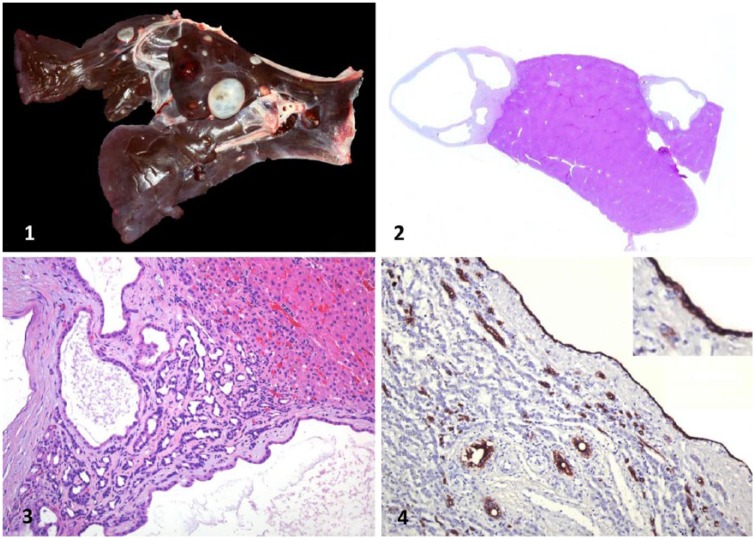
Both carcasses were in poor-to-fair nutritional condition, with scant fat reserves and mild but generalized muscle atrophy. The livers of both animals were smaller than normal, had softer than normal consistency, and had many 0.2–2.5 cm diameter cysts. The cysts were roughly spherical, contained clear-to-yellow–tinged translucent fluid, and had white, semi-transparent capsules. Cysts were randomly distributed and replaced ~30% of the hepatic parenchyma (Fig. 1). A few small (0.5 cm diameter), firm, mineralized nodules were observed in the lungs and liver of cases 1 and 2, respectively. No other significant gross abnormalities were observed in either carcass. In particular, no icterus was observed.
Figures 1–4.
Polycystic liver in an adult llama (Lama glama). Figure 1. Liver with multiple round-to-oval biliary cysts of various sizes. The cysts are surrounded by a capsule of fibrous collagenous tissue. Figure 2. Sub-gross photograph of liver with multiple cysts. H&E. Figure 3. Hepatic cysts composed of a single cuboidal-to-columnar epithelium surrounded by a thin fibrous capsule. H&E. Figure 4. Intense intracytoplasmic immunohistochemical reactivity of cystic and bile duct epithelium for cytokeratin 19. Inset: higher magnification of cyst epithelium.
Samples of liver, spleen, lung, heart, kidney, skeletal muscle, adrenal gland, the 3 gastric compartments, and small and large intestine were collected and fixed in 10% neutral-buffered formalin for 24 h, processed routinely for the production of 4-μm thick sections, and stained with hematoxylin and eosin. Selected sections were also stained with periodic acid–Schiff (PAS), Perl Prussian blue, Gram, and Hall bilirubin stains.
Microscopic examination of the liver in both animals showed the cysts to be lined by a single layer of cuboidal-to-columnar epithelium, surrounded by a thick, well-defined capsule consisting of organized, concentric layers of mostly acellular mature collagen (Figs. 2, 3), which was negative on PAS staining. Finely granular-to-homogeneous and basophilic material was seen in the lumen of most cysts (Fig. 3). The adjacent hepatic parenchyma was markedly compressed by the cystic structures (Fig. 3). Elsewhere in the liver, the hepatocytes were ~50% smaller than normal, contained many sharply demarcated cytoplasmic vacuoles, and there was moderate bile duct proliferation in the portal areas. Mild-to-moderate cholestasis was also observed. Occasionally, Kupffer cells were laden with Perl Prussian blue–positive brown granules (iron), and a mild inflammatory infiltrate composed of lymphocytes and plasma cells was present in the surrounding hepatic parenchyma in portal and periportal areas. A few small foci of mineralization without associated inflammatory cells were also observed in the liver of case 2 and in the lungs of case 1. No parasitic or infectious organisms were identified in the liver of either animal. No other significant microscopic abnormalities were observed in any of the organs of either animal examined.
Immunohistochemistry (IHC) for pancytokeratin (CK AE1/AE3), cytokeratin 7 (CK 7), CK 8/18, CK 19, and vimentin was performed on liver sections following standard operating procedures (SOPs) of the Veterinary Teaching Hospital of the School of Veterinary Medicine, University of California–Davis. Briefly, antigen retrieval was performed (Target retrieval solution, pH 6, Dako North America, Carpinteria, CA) for CK AE1/AE3 and vimentin in a steamer for 30 min, and with proteinase K (Dako) for 7, 8, and 10 min at room temperature for CK 8 and CK 8/18, CK 19, and CK 7, respectively. The following primary antibodies were used: anti-CK AE1/AE3 (dilution 1:300; Product MU0110-UC, BioGenex, Fremont, CA), anti-CK 7 (dilution 1:100; Product MU255-UC, BioGenex), anti-CK 8 (dilution 1:5; Product 349205, Becton Dickinson, San Jose, CA), anti-CK 8/18 (dilution 1:250; Product 18-0213, Life Technologies, Carlsbad, CA), anti-CK 19 (dilution 1:100; Product VP-C415, Vector Laboratories, Burlingame, CA), and anti-vimentin (dilution 1:300; Product M7020, Dako). Peroxidase anti-mouse polymer (Product MC541, Biocare Medical, Concord, CA) was used as a secondary reagent. All reactions were visualized with NovaRed for peroxidase (SK-4800, Vector Laboratories). Liver sections from a normal llama were used as positive controls. Sections were counterstained with Mayer hematoxylin and coverslipped. Buffer was used instead of the primary antibody in negative control sections.
The epithelium lining the hepatic cysts showed strong staining for all studied cytokeratins (AE1/AE3, CK 7, CK8, CK 8/18, and CK 19; Fig. 4) and negative staining for vimentin.
The following ancillary tests were performed following CAHFS SOPs: bacterial aerobic culture of lung and liver (cases 1 and 2), PCR for Salmonella sp. on liver (case 2), reverse-transcription quantitative PCR (RT-qPCR) on spleen for bovine viral diarrhea virus (BVDV, cases 1 and 2), and fecal float (cases 1 and 2). Liver samples from both cases were also screened for heavy metals, including lead, manganese, iron, mercury, arsenic, molybdenum, zinc, cooper, cadmium, and selenium.
Bacterial aerobic culture, PCR for Salmonella sp., and RT-qPCR for BVDV were negative. No parasites or parasite eggs were detected in the feces of either animal. The liver of case 2 had slightly increased molybdenum (1.7 ppm; reference range: <1.0 ppm) and selenium (1.7 ppm; reference interval [RI]: 0.25–1.0 ppm), and moderately reduced levels of cooper (7.5 pm; RI: 25–100 ppm). Other heavy metals in this animal and all heavy metals in the liver of case 1 were within RIs.
We based the diagnosis of polycystic liver in these 2 adult llamas on gross and microscopic features, including IHC. The presence of one layer of cuboidal-to-columnar epithelial cells lining the cysts suggested biliary system epithelial origin, which was confirmed by positive immunoreactivity of these cells for CK 7, CK8, CK 8/18, and CK 19, all markers of biliary epithelium.23
The main differential diagnoses considered in these cases were parasitic cysts and neoplasia. Hepatic cysts caused by parasitic infections in camelids are most commonly produced by Echinococcus spp. and Cysticercus spp. Echinococcosis is considered to be one the most important zoonotic diseases worldwide with significant public health and socioeconomic concerns.2,3,6,18 Neoplasms producing hepatic cysts include biliary cystadenomas and cystic adenocarcinomas.5 Microscopic examination of both livers showed no evidence of parasitic infestation or neoplasia.
Congenital polycystic liver disease is caused by defects in the ductal plates of the intrahepatic biliary tree.4 These structures have an endodermal origin and are considered the precursors of bile ducts.5,17 The cysts may not develop until late in life, or they may develop early and undergo gradual enlargement with age.4,13 In humans, it has been reported that estrogen can promote the growth of congenital hepatic cysts; this phenomenon is thought to be related to the expression of α- and β-estrogen receptors by the epithelium of intrahepatic ducts.1,8,19 Hepatic cysts in humans therefore tend to increase in size with puberty.
Polycystic livers have been reported previously in several animal species, including 2 alpacas9 and 17 camels.10 The hepatic cysts previously described in alpacas and camels were morphologically similar to those described in our report, but the cells of origin were not determined in either case. We demonstrated that the cysts in our 2 llamas were lined by biliary epithelium.
Polycystic liver has been also described in humans and cats as part of so-called autosomal dominant polycystic kidney disease. That is, however, a well-defined syndrome that is characterized by cysts of various sizes in the renal cortex and medulla and occasionally in other abdominal organs, and which almost always leads to renal failure.7
Most cases of hepatic congenital cysts in many animal species and humans are asymptomatic, the only concern in animals being offal value depreciation during slaughter because of condemnation of the affected organs in deer. However, compression of adjacent tissues has been occasionally associated with liver failure in deer,16 chamois,11 dogs,22 hamsters,20 and 2 alpacas.9
Both llamas in our study had anorexia and weight loss. Although we cannot rule out that this was a consequence of hepatic insufficiency associated with the replacement of a significant amount of normal tissue by multiple cysts,5 this is unlikely given that >50% of the parenchyma remained. It is well-known that even if up to two-thirds of the liver is removed, an animal can survive without showing clinical signs of hepatic insufficiency.5
Perhaps more plausible is the possibility that the effect of the compression of the cysts on the surrounding hepatic parenchyma, which showed gross and microscopic signs of atrophy, had clinical significance, as this would have affected much more hepatic tissue than that replaced by the cysts. The finding of bile stasis in the liver of both animals supports this hypothesis, although the absence of icterus does not allow confirmation of this possibility. Unfortunately, no blood tests for liver function were carried out in either animal before death and, therefore, we cannot confirm or rule out that these animals suffered liver failure. Although we cannot confirm a relationship of the findings in these 2 llamas with clinical disease, it is important that pathologists and diagnosticians are aware that these lesions (incidental or not) may be seen in llamas.
In case 2, there was also copper deficiency, which might have been primarily or secondarily responsible for the loss of condition of this animal.5,12,15,21,24 Although the selenium and molybdenum concentrations in the liver of this llama were slightly elevated, they were not considered to be at toxic levels. Such elevated mineral levels may have been the result of recent supplementation, although no information was available in this regard.
Other possible causes of loss of condition considered in our 2 cases were intestinal parasites and dentition problems. Intestinal parasitism was ruled out by fecal float and the absence of adult parasites in the gastrointestinal content; no significant gross abnormalities were observed in the dentition of either animal. Our study suggests that non-infectious, non-parasitic, and non-neoplastic intrahepatic cysts (most likely congenital) might be associated with health impairment in adult llamas, although this could not be confirmed.
Acknowledgments
We thank Juliann Beingesser, EJ Hurley, Janelle Dominguez, and Mike Manzer for their help with histology and immunohistochemistry preparations.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our study was supported by California Animal Health and Food Safety Laboratories. Benjamín Doncel-Díaz received an Externship Award from the Latin Comparative Pathology Group.
References
- 1. Alvaro D, et al. Alfa and beta estrogen receptors and the biliary tree. Mol Cell Endocrin 2002;193:105–108. [DOI] [PubMed] [Google Scholar]
- 2. Andresiuk MV, et al. Echinococcus granulosus genotype G1 dominated in cattle and sheep during 2003–2006 in Buenos Aires province, an endemic area for cystic echinococcosis in Argentina. Acta Tropica 2013;127:136–142. [DOI] [PubMed] [Google Scholar]
- 3. Cardona GA, Carmena D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet Parasitol 2013;192:10–32. [DOI] [PubMed] [Google Scholar]
- 4. Cnossen WR, Drenth JP. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis 2014;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cullen JM, Brown DL. Hepatobiliary system and exocrine pancreas. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. Maryland Heights, MO: Elsevier Mosby, 2012:405–422. [Google Scholar]
- 6. Dakkak A. Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Vet Parasitol 2010;174:2–11. [DOI] [PubMed] [Google Scholar]
- 7. Domanjko-Petric A, et al. Polycystic kidney disease: a review and occurrence in Slovenia with comparison between ultrasound and genetic testing. J Feline Med Surg 2008:10:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Everson GT, et al. Advances in management of polycystic liver disease. Expert Rev Gastroenterol Hepatol 2008;2:563–576. [DOI] [PubMed] [Google Scholar]
- 9. Foster A, et al. Adult polycystic liver disease in alpacas. Vet Rec 2013;172:666–667. [DOI] [PubMed] [Google Scholar]
- 10. Gameel AA, Bakhsh AA. Non-parasitic hepatic cysts in camels (Camelus dromedarius). J Camel Pract Res 2002;9:79–82. [Google Scholar]
- 11. Glawischnig W, et al. Polycystic liver disease in senile chamois (Rupicaprae rupicaprae). J Wildl Dis 2010;46:669–672. [DOI] [PubMed] [Google Scholar]
- 12. Grace ND, et al. Impact of molybdenum on the copper status of red deer (Cervus elaphus). NZ Vet J 2005;53:137–141. [DOI] [PubMed] [Google Scholar]
- 13. Melnick PJ. Polycystic liver; analysis of seventy cases. AMA Arch Pathol 1955;59:162–172. [PubMed] [Google Scholar]
- 14. Munro R. Intrahepatic biliary cysts in deer. J Comp Pathol 1991;105:05–107. [DOI] [PubMed] [Google Scholar]
- 15. Naylor JM, et al. Diagnosis of copper deficiency and effects of supplementation in beef cows. Can J Vet Res 1989;53:343–348. [PMC free article] [PubMed] [Google Scholar]
- 16. Picone J, et al. Polycystic liver in four white-tailed deer. J Wild Dis 1981;17:395–400. [DOI] [PubMed] [Google Scholar]
- 17. Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anatom Rec 2008;291:628–635. [DOI] [PubMed] [Google Scholar]
- 18. Sánchez E, et al. Echinococcus granulosus genotypes circulating in alpacas (Lama pacos) and pigs (Sus scrofa) from an endemic region in Peru. Mem Inst Oswaldo Cruz 2012;107:275–278. [DOI] [PubMed] [Google Scholar]
- 19. Sherstha R, et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 1997;26:1282–1286. [DOI] [PubMed] [Google Scholar]
- 20. Somvanshi R, et al. Polycystic liver disease in golden hamsters. J Comp Pathol 1987;97:615–618. [DOI] [PubMed] [Google Scholar]
- 21. Suttle NF. The interactions between copper, molybdenum, and sulphur in ruminant nutrition. Ann Rev Nutr 1991;11:121–140. [DOI] [PubMed] [Google Scholar]
- 22. Van Den Ingh TS, Rothuizen J. Congenital cystic disease of the liver in seven dogs. J Comp Pathol 1985;95:405–414. [DOI] [PubMed] [Google Scholar]
- 23. Van Eyken P, et al. The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology 1988;8:1586–1595. [DOI] [PubMed] [Google Scholar]
- 24. Xiao-Yun S, et al. Studies of a naturally occurring molybdenum-induced copper deficiency in the yak. Vet J 2006;171:352–357. [DOI] [PubMed] [Google Scholar]