Abstract
Common variable immunodeficiency (CVID) is a rare condition in adult horses characterized by hypogammaglobulinemia and increased susceptibility to parasitic and bacterial infections, including recurrent respiratory diseases, septicemia, and meningitis. Lyme disease is often included as a differential diagnosis in CVID horses with signs of meningitis; however, the Borrelia burgdorferi organism has not been demonstrated previously within central nervous system tissues of CVID horses with neurologic disease, to our knowledge. We report herein a case of neuroborreliosis in a CVID horse, confirmed by combined immunologic testing, histopathology, real-time PCR assay, fluorescent in situ hybridization, and immunohistochemical staining. Implications of these findings include heightened monitoring of CVID horses for Lyme disease in endemic areas and appropriate therapy in the case of neurologic disease.
Keywords: Borrelia burgdorferi, common variable immunodeficiency, horses, Lyme disease, neuroborreliosis, neurologic signs
A 7-y-old American Quarter Horse mare from the northeastern United States was presented to a referring veterinarian in mid-June 2015 with bilateral mucopurulent ocular discharge and was treated for conjunctivitis. Two days later, the horse was lame on the right forefoot with moderate distal limb edema and a rectal temperature of 38.1°C (normal 37.5–38.6°C). Hoof abscess was suspected, and she was treated appropriately. Although the lameness and ocular discharge resolved within 1 wk of presentation, enlarged submandibular lymph nodes and moderate mucopurulent discharge in the left nostril were noted. Temperature was still within normal range at 37.9°C. Oral examination revealed no significant findings. Bloodwork at this time revealed anemia, lymphopenia, hypoproteinemia, and hypoglobulinemia (Table 1). Fibrinogen was also elevated (7.8 g/L; reference interval: 1.5–4.0 g/L) and would remain persistently elevated for the next 6 mo. A Lyme multiplex assay19 performed at the New York State Animal Health Diagnostic Center (AHDC, Ithaca, NY) to measure serum antibodies to the outer surface proteins (Osps) of Borrelia burgdorferi revealed negative OspA (18), OspC (15), and OspF (9) titers. Given a suspected viral cause and resolution of the hoof abscess, antibiotic therapy was not administered at the time, and the horse was placed in isolation.
Table 1.
Hematology values in a CVID horse with neuroborreliosis from the time of presentation (June 2015) and the time of euthanasia (December 2015).
| Analyte | Unit | June 2015 | December 2015 | Reference interval |
|---|---|---|---|---|
| Hematocrit | L/L | 0.25 | 0.35 | 0.32–0.53 |
| Red blood cells | ×1012/L | 5.7 | 7.4 | 6.8–12.9 |
| Platelets | ×109/L | 177 | 209 | 100–400 |
| White blood cells | ×109/L | 5.0 | 6.3 | 5.4–14.3 |
| Lymphocytes | ×109/L | 1.4 | 1.9 | 1.5–7.7 |
| Monocytes | ×109/L | 0.4 | 0.4 | 0.0–1.5 |
| Neutrophils | ×109/L | 3.1 | 4.6 | 2.3–9.5 |
| Eosinophils | ×109/L | 0.1 | 0.1 | 0.0–1.0 |
| Basophils | ×109/L | 0.0 | 0.0 | 0.0–0.1 |
| Gamma-glutamyl transferase | µkat/L | 0.37 | >0.97 | 0.08–0.40 |
| Total protein | g/L | 55 | 56 | 57–80 |
| Albumin | g/L | 29 | 33 | 22–37 |
| Gamma globulins | g/L | 26 | 23 | 27–50 |
| Fibrinogen | g/L | 7.8 | 10.0 | 1.5–4.0 |
By early July 2015, acute uveitis with an anterior chamber fibrin clot was noted in the left eye. There was still left nasal discharge, although the horse remained afebrile (38.0°C). Blood was submitted for Anaplasma spp. and Leptospira spp. titers, which were negative. A respiratory PCR panel was negative for equine influenza virus, equid herpesvirus 1 (EHV-1), equid herpesvirus 4, and Streptococcus equi subsp. equi. Treatment was started with intravenous oxytetracycline (Boehringer Ingelheim Vetmedica, St. Joseph, MO), and the horse was referred to an equine ophthalmologist for fibrin treatment and resolution. For continued eye care, the horse was sent to a rehabilitation facility that specializes in ophthalmology. While at the facility, the horse developed urticaria.
Upon return from the rehabilitation facility in late July 2015, the horse became febrile (39.0°C), had continued bilateral ocular discharge, and began shifting weight in the forelimbs. Thoracic ultrasound suggested pneumonia. Injections of ceftiofur crystalline-free acid (Zoetis, Kalamazoo, MO) antibiotic and flunixin meglumine (Aspen Veterinary Resources, Liberty, MO) were begun as needed for control of fever. Repeat PCR for S. equi subsp. equi remained negative. In early August 2015, after a week of antibiotics, the horse developed bilateral mucopurulent nasal discharge and acute non–weight bearing cellulitis in the left hindlimb. Skull radiographs were within normal limits. Antibiotic therapy was changed to ceftiofur sodium (Zoetis) and gentamicin (Vedco, St. Joseph, MO). Intravenous Propionibacterium acnes (Neogen, Lansing, MI) was added to the treatment regimen. One week later, while still on ceftiofur, the horse developed right forefoot lameness. All clinical signs resolved after 2 wk of ceftiofur sodium and gentamicin treatment. However, the referring veterinarian suspected an underlying immune system abnormality given the persistent elevated fibrinogen levels, despite antibiotic and anti-inflammatory therapies.
Initial lymphocyte immunophenotyping was performed at Cornell University Equine Immunology Laboratory (Ithaca, NY) in mid-August, and results were consistent with common variable immunodeficiency (CVID). The final peripheral blood lymphocyte phenotyping (Table 2) showed a markedly decreased B-cell concentration measured by flow cytometry with a 1.0% distribution of CD19+ B cells in the lymphocyte-gated area, 0.2% CD21+ B cells, and 0.9% IgM+ B cells. The CD4+ and CD8+ T-cell distributions were normal to slightly increased, and the CD4-to-CD8 ratio was within the low normal reference interval. Serum IgG concentrations measured by immunoturbidimetric assay in August 2015 and December 2015 were markedly decreased; serum IgM concentrations measured by radial immunodiffusion at the same times were within the normal reference interval (Table 3).
Table 2.
Phenotyping of peripheral blood lymphocytes using flow cytometry, December 2015.
| Lymphocyte distribution | Unit | Lymphocyte | Median | Confidence interval |
|---|---|---|---|---|
| Total CD19+ count | ×109/L | 0.02 | ||
| CD19+ B cells | % | 1.0 | 9.0 | 2.0 |
| CD21+ B cells | % | 0.2 | 10.2 | 4.2 |
| IgM+ B cells | % | 0.9 | 10.2 | 2.1 |
| Total CD3+ T cell count | ×109/L | 1.91 | ||
| Total CD4+ T cell count | ×109/L | 1.42 | ||
| Total CD8+ T cell count | ×109/L | 0.41 | ||
| CD3+ T cells | % | 98.6 | 92.0 | 2.1 |
| CD4+ T cells | % | 73.2 | 64.4 | 4.7 |
| CD8+ T cells | % | 21.1 | 18.3 | 2.6 |
| CD4+/CD8+ ratio | 2.5 | 3.5 | 0.8 |
Table 3.
Serum immunoglobulin concentrations at initial immunophenotyping (August 2015) and near the time of euthanasia (December 2015).
| Unit | August 2015 | December 2015 | Reference interval | |
|---|---|---|---|---|
| IgG | g/L | 4.67 | 4.23 | 11.57–23.63 |
| IgM | mg/L | 630 | 630 | 500–1,500 |
In late August 2015, the horse exhibited narcolepsy signs such as slow head movements and abrupt lying down on the knees and sides. No seizures were noted. On neurologic examination, there was weak bilateral tail pull; the left pupil was mildly dilated as a result of atropine treatments while at the referral hospital. There was also continued nasal discharge, but no lameness or fever. Oral doxycycline (Wedgewood Pharmacy, Swedesboro, NJ) and tapering doses of injectable flunixin meglumine were started. The horse remained relatively stable over the next 3 mo with monthly examinations and monthly 7-d courses of oral sulfamethoxazole and trimethoprim (Vista Pharmaceuticals, West Orange, NJ). Bloodwork in December showed hypoproteinemia and hypoglobulinemia, as well as markedly elevated gamma-glutamyl transferase activity (Table 1). By January 2016, bilateral ocular discharge was still noted, and the horse was reported to be falling down. Given the potential danger to caretakers and the poor prognosis, the horse was submitted for euthanasia under the owner’s consent. An autopsy was performed on the same day at the anatomic pathology service at the AHDC for complete tissue collection.
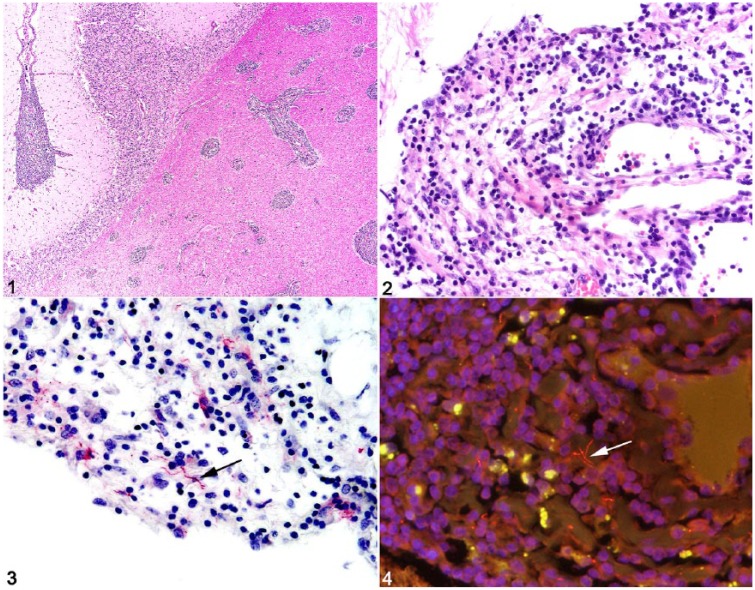
On autopsy, gross anatomic findings included moderate serous abdominal effusion, extensive fibrosis of the hepatic capsule and parenchyma, numerous coalescing white hepatic nodules, variably sized pulmonary and thymic white nodules, 2 chronic gastric ulcers, mild bilateral forelimb laminitis, and a chronic sole abscess of the left forelimb. Microscopically, meninges of the spinal cord, cerebellum, and cerebrum were diffusely expanded by marked infiltrates of lymphocytes, histiocytes, fewer neutrophils, and rare plasma cells (Figs. 1, 2). Within the parenchyma of the white and gray matter, the Virchow–Robin spaces of blood vessels had cuffs of similar inflammatory cells, and blood vessels were prominent with endothelial hypertrophy and variable branching. Other significant histologic findings included moderate, chronic lymphocytic hypophysitis and lymphohistiocytic eosinophilic choroid plexitis; thymic lymphoid depletion; absent splenic lymphoid follicles and small periarteriolar lymphoid sheaths; loss of normal architecture and abundant homogeneous acellular eosinophilic material within a mesenteric lymph node interpreted as amyloid-like material; severe myeloid and erythroid hypoplasia of the bone marrow; chronic lymphoplasmacytic portal hepatitis with portal-to-portal and portal-to-central bridging fibrosis and acute lobular hepatocellular necrosis; and chronic mineralized granulomas in the liver, lung, and thymus. Although no metazoan parasites were noted in any of the tissue sections examined, the mineralized granulomas were presumed to be caused by chronic parasitic migration, given that excessive gastrointestinal tract parasitism has been noted in another CVID horse.3
Figures 1–4.
Neuroborreliosis in a horse with common variable immunodeficiency. Figure 1. Cerebral Virchow–Robin spaces are expanded by large numbers of lymphocytes and histiocytes. Figure 2. Lymphohistiocytic and neutrophilic cerebral meningitis. Figure 3. Rare immunoreactive Borrelia burgdorferi spirochetes within areas of meningitis (arrow). Figure 4. Probe-positive spirochetes within the cerebrum (arrow).
The constellation of gross and histologic findings, namely chronic parasitic migration and depletion of lymphoid tissues in the spleen, thymus, and mesenteric lymph node, was consistent with a severely immunocompromised animal with meningoencephalomyelitis. Given the histologic findings and clinical history of uveitis, lymphadenopathy, and, finally, narcolepsy, B. burgdorferi was considered to be a likely etiologic agent. However, other causes of equine meningoencephalomyelitis, such as eastern equine encephalitis virus (EEEV), EHV-1, West Nile virus (WNV), equine arteritis virus (EAV), and rabies virus, were also considered. Real-time PCR (rtPCR) assay for detection of the Borrelia spp. flagellin gene in fresh frozen cerebrum was positive with a cycle threshold value of 32.3 (positive is considered <33.4, equivalent to ~100 copies in reaction) using forward primer sequence TCTTTTCTCTGGTGAGGGAGCT, reverse primer sequence TCCTTCCTGTTGAACACCCTCT, and probe sequence FAM-AAACTGCTCAGGCTGCACCGGTTC-BHQ1 as described previously.11 Sections of cerebrum, deparaffinized and processed for antigen retrieval and immunohistochemistry (IHC) with affinity-purified rabbit polyclonal IgG anti–B. burgdorferi (Biodesign, Kennebunk, ME) prepared from whole cell B. burgdorferi,10 revealed strong immunoreactivity of rare spiral organisms within areas of inflammation (Fig. 3). IHC for EEEV, EHV-1, WNV, and rabies virus antigens yielded no immunoreactivity. Using the Borrelia genus–specific Borr4 probe4 and a range of bacteria, including Leptospira kirschneri serovar Grippotyphosa, Streptococcus spp., Escherichia coli, and B. burgdorferi to control for probe specificity as described previously,4,13 fluorescent in situ hybridization (FISH) assay for B. burgdorferi messenger RNA (mRNA) revealed ~15-μm spirochetes within areas of cerebral inflammation consistent with B. burgdorferi (Fig. 4).8 Additionally, immunofluorescent antibody staining of sections of cerebrum showed no immunoreactivity for EHV-1 or EAV antigens. Gram stain of cerebrum sections for bacteria revealed no organisms, and aerobic bacteriologic culture was negative. Further IHC staining of cerebrum sections to characterize the leukocyte component of meningoencephalitis detected widespread CD3 membranous and Iba1 cytoplasmic immunoreactivity for T cells and macrophages, respectively (data not shown). Markers for B cells (Pax5 and CD20) revealed only rare immunoreactive cells within areas of meningeal and cerebral inflammation (data not shown).
CVID is a primary immunodeficiency disease of humans and horses that encompasses a group of heterogeneous disorders characterized by hypogammaglobulinemia and increased susceptibility to bacterial and parasitic infections. Generally, at least 2 isotypes of antibodies are affected, although IgG deficiency alone is also recognized.9 Human CVID patients have a high frequency of autoimmune and lymphoproliferative disease, and such patients often are seen clinically with recurrent respiratory disease18 and persistent herpesviral infection.14 B cells may be present or absent in human patients with CVID; however, more commonly, differentiation into plasma cells is disrupted by an unknown mechanism that results in reduced humoral immunity to common pathogens.9
In horses, CVID is a rare condition. Relatively few cases have been reported,2,3,6,12,17 though the Equine Immunology Laboratory at Cornell College of Veterinary Medicine (Ithaca, NY) has identified this condition in >50 horses since 2002 and has been actively investigating potential genetic and epigenetic mechanisms of the disease.16 Age of onset is 2–23 y, with an average of 11.3 y. Like human patients, horses with CVID most often have recurrent infections of the respiratory tract. In addition, persistent bacterial meningitis caused by common skin contaminants such as Staphylococcus spp. has been described, although B. burgdorferi has been highly suspected in some cases.3,12 One report of central and peripheral nervous system inflammation in a CVID horse described a positive western blot analysis result with low-to-moderate B. burgdorferi antibody response in serum and a positive PCR assay result from cerebrospinal fluid (CSF) using primers for the ospA DNA.6 Although western blot analysis and PCR assay confirmed exposure to B. burgdorferi and the presence of bacteria within CSF, respectively, neither test demonstrated active B. burgdorferi infection within areas of encephalitis.
Clinical syndromes of B. burgdorferi infection include neuroborreliosis, uveitis, and cutaneous pseudolymphoma.1 Equine neuroborreliosis, as with most species, is characterized by neutrophilic or lymphoplasmacytic, histiocytic, perivascular-to-diffuse inflammation, most severely affecting the central nervous system (CNS), including the meninges, ganglia, and cranial and spinal nerve roots, with various degrees of necrosis, fibrosis, and neuroparenchymal invasion.5,7 In our clinical case, the inflammation was predominantly lymphocytic and histiocytic, and the distribution included the spinal cord and ganglia, meninges, choroid plexus, pituitary gland, and neuroparenchyma. Using IHC, rare spiral organisms were present within areas of perivascular inflammation; rtPCR and FISH confirmed the presence of B. burgdorferi nucleic acid and mRNA, respectively, within regions of severe cerebral inflammation. In addition to the CNS lesions, this horse had chronic hepatitis with marked capsular and bridging fibrosis. Therefore, hepatic encephalopathy cannot be completely ruled out as contributing to the neurologic signs. Interestingly, B. burgdorferi has been speculated to be the cause of hepatitis in other cases of equine neuroborreliosis.7 The hepatic fibrosis could be attributed in part to chronic hepatitis caused by B. burgdorferi; however, fibrosis could also be the result of the presumed parasitic granulomas, as well as another bacterial, viral, or toxic cause. No matter the cause of the liver lesions, the history of uveitis and narcolepsy, clinical data, microscopic meningoencephalomyelitis, choroid plexitis, hypophysitis, and ancillary testing are consistent with Lyme neuroborreliosis in a CVID horse.5-7,12,13
Another histologic feature that supports the diagnosis of CVID, in addition to lymphoid depletion in the mesenteric lymph node, spleen, and thymus, was rare B-cell immunoreactivity within sections of cerebrum with florid inflammation. This finding mirrors the antemortem peripheral blood immunophenotyping that reported <1% B cells of total lymphocytes. Indeed, to date, research on equine CVID focuses on the disruption of B-cell development in bone marrow and has identified decreased mRNA expression and incomplete demethylation of the PAX5 gene required for commitment and differentiation of B cells.15,16 In addition, massive parasitic migration is consistent with lack of antibody response to parasitic antigens.3
A low Lyme antibody concentration should be expected in humoral immunodeficiency, and a negative result should not rule out CVID. Nevertheless, the lack of humoral immunity, the primary host defense mechanism against B. burgdorferi, likely contributed to chronic Lyme disease in this horse with CVID. Implications of these findings include heightened monitoring of CVID horses for Lyme disease in endemic areas and appropriate therapy when there is clinical evidence of neurologic disease.
Acknowledgments
We thank the staff of the New York State Animal Health Diagnostic Center histology, bacteriology, virology, and molecular diagnostic laboratories at Cornell University for technical assistance, including Drs. Amy Glaser and Laura Goodman for interpretation of and information on the Borrelia burgdorferi rtPCR assay. Special thanks to Dr. Sean McDonough for consultation on the liver lesions.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Heidi L. Pecoraro  https://orcid.org/0000-0001-5475-5482
https://orcid.org/0000-0001-5475-5482
References
- 1. Divers TM, et al. Borrelia burgdorferi infection and Lyme disease in North American horses: a consensus statement. J Vet Intern Med 2018;32:617–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flaminio MJBF, et al. Common variable immunodeficiency in a horse. J Am Vet Med Assoc 2002;221:1296–1302. [DOI] [PubMed] [Google Scholar]
- 3. Flaminio MJBF, et al. Common variable immunodeficiency in horses is characterized by B cell depletion in primary and secondary lymphoid tissues. J Clin Immunol 2009;29:107–116. [DOI] [PubMed] [Google Scholar]
- 4. Hammer B, et al. Visualization of Borrelia burgdorferi sensu lato by fluorescence in situ hybridization (FISH) on whole-body sections of Ixodes ricinus ticks and gerbil skin biopsies. Microbiology 2001;147:1425–1436. [DOI] [PubMed] [Google Scholar]
- 5. Imai DM, et al. Lyme neuroborreliosis in 2 horses. Vet Pathol 2011;48:1151–1157. [DOI] [PubMed] [Google Scholar]
- 6. James FM, et al. Meningitis, cranial neuritis, and radiculoneuritis associated with Borrelia burgdorferi infection in a horse. J Am Vet Med Assoc 2010;237:1180–1185. [DOI] [PubMed] [Google Scholar]
- 7. Johnstone LK, et al. Retrospective evaluation of horses diagnosed with neuroborreliosis on postmortem examination: 16 cases (2004–2015). J Vet Intern Med 2016;30:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudryashev M, et al. Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Mol Microbiol 2009;71:1415–1434. [DOI] [PubMed] [Google Scholar]
- 9. Kumar V, et al. Diseases of the immune system. In: Kumar V, et al. eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Saunders/Elsevier, 2015:185–264. [Google Scholar]
- 10. Marques AR, et al. Detection of immune complexes is not independent of detection of antibodies in Lyme disease patients and does not confirm active infection with Borrelia burgdorferi. Clin Diagn Lab Immunol 2005;12:1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pahl A, et al. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol 1999;37:1958–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pellegrini-Masini A, et al. Common variable immunodeficiency in three horses with presumptive bacterial meningitis. J Am Vet Med Assoc 2005;227:114–122. [DOI] [PubMed] [Google Scholar]
- 13. Priest HL, et al. Diagnosis of Borrelia-associated uveitis in two horses. Vet Ophthalmol 2012;15:398–405. [DOI] [PubMed] [Google Scholar]
- 14. Raeiszadeh M, et al. The T cell response to persistent herpes virus infections in common variable immunodeficiency. Clin Exp Immunol 2006;146:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tallmadge RL, et al. Expression of essential B cell development genes in horses with common variable immunodeficiency. Mol Immunol 2012;51:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tallmadge RL, et al. Bone marrow transcriptome and epigenome profiles of equine common variable immunodeficiency patients unveil block of B lymphocyte differentiation. Clin Immunol 2015;160:261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tennet-Brown BS, et al. Common variable immunodeficiency in a horse with chronic peritonitis. Equine Vet Educ 2010;22:393–399. [Google Scholar]
- 18. Verma N, et al. Lung disease in primary antibody deficiency. Lancet Respir Med 2015;3:651–660. [DOI] [PubMed] [Google Scholar]
- 19. Wagner B, et al. Development of a multiplex assay for the detection of antibodies to Borrelia burgdorferi in horses and its validation using Bayesian and conventional statistical methods. Vet Immunol Immunopathol 2011;144:374–381. [DOI] [PubMed] [Google Scholar]