Abstract
Reports of raw meat pet food containing zoonotic foodborne bacteria, including Salmonella, Escherichia coli, and Listeria monocytogenes, are increasing. Contaminated raw pet food and biological waste from pets consuming those diets may pose a public health risk. The U.S. Food and Drug Administration Veterinary Laboratory Investigation and Response Network conducted 2 case investigations, involving 3 households with animal illnesses, which included medical record review, dietary and environmental exposure interviews, animal sample testing, and whole genome sequencing (WGS) of bacteria isolated from the pets and the raw pet food. For each case investigation, WGS with core genome multi-locus sequence typing analysis showed that the animal clinical isolates were closely related to one or more raw pet food bacterial isolates. WGS and genomic analysis of paired animal clinical and animal food isolates can confirm suspected outbreaks of animal foodborne illness.
Keywords: Bacteriology, cats, dogs, foodborne illness, raw pet food, whole genome sequencing
Pets may contract foodborne pathogens, including Salmonella, Listeria monocytogenes, and Shiga toxin–producing Escherichia coli (STEC), from their diet, environment, and infected humans and animals.17,18,20 A 2014 study reported Salmonella, L. monocytogenes, and STEC in 7.7%, 16.3%, and 4.1%, respectively, of 196 samples of commercial raw pet foods tested.18 In addition, a 2017 multi-laboratory study of Salmonella prevalence in pets demonstrated that dogs testing positive for Salmonella were more likely to have consumed raw food than cooked food.20 The U.S. Food and Drug Administration (FDA; https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm403350.htm) and the Centers for Disease Control and Prevention (https://www.cdc.gov/salmonella/schwarzengrund_faq.html) have issued statements to consumers that feeding raw meat diets to pets may pose a public health risk. Despite the public health risk, previous surveys on pet feeding practices in the United States, Canada, and Australia indicate that 16–30% of dogs and 9.6% of cats may receive raw diets or bones.5,14 Infected animals, however, may not show clinical signs, complicating the diagnosis of pet foodborne illness.20 Because of the potential for human exposure, identifying and diagnosing pet foodborne illness is crucial to protecting animal and human health.18 We report the results of 2 case investigations involving 3 breeders, each with pet illness associated with consumption of raw pet food.
For each case investigation, FDA received consumer complaints either through the FDA Safety Reporting Portal (https://www.safetyreporting.hhs.gov/) or through a FDA District Office. The FDA Center for Veterinary Medicine evaluated the complaints (https://www.fda.gov/Safety/ReportaProblem/QuestionsandAnswersProblemReporting/ucm056069.htm), and the FDA Veterinary Laboratory Investigation and Response Network (Vet-LIRN; https://www.fda.gov/AnimalVeterinary/ScienceResearch/ucm247334.htm) requested medical records and conducted dietary and environmental exposure interviews to obtain signalment and significant clinical histories from both case investigations (Table 1).
Table 1.
Signalment and significant clinical history from 2 case investigations.
| Case investigation | Animal | Signalment | Medical history | Diet and environment |
|---|---|---|---|---|
| 1 | Cat 1 | 1-y-old, female intact Scottish Fold | Asymptomatic | Owner is a breeder with 10 cats and boarding an 11th (cat 2); recently started food 1 but previously fed an unreported raw food (food 2) |
| Cat 2 | 7-mo-old, male castrated Scottish Straight | Vomiting, watery diarrhea, lethargy; febrile | ||
| Cats 3–11 | Unknown | Asymptomatic | ||
| 2 | Household 1: dog 1 | 6-wk-old, female intact Saint Bernard | Autopsy showed septic bacterial enterocolitis: Salmonella enterica (large intestine), E. coli (liver, kidney) | Owner is a breeder with an 11-dog kennel and a new litter of puppies; the sire and 1 other puppy were ill and recovered; recently fed food 7 |
| Household 2: kittens | Various, Persian kittens | Ataxia, tetraplegia, and anuria; some kittens were asymptomatic; 2 deaths, autopsy inconclusive | Owner is a breeder; an unknown number of cats in the household had minor diarrhea; recently fed food 9 |
Clinical samples, leftover open products, and closed unopened products were tested for foodborne pathogens, primarily Salmonella and Listeria in case 1, with the addition of E. coli in case 2. In both cases, product pathogen testing was informed by the results of the animal pathogen testing and prior reports of Salmonella, E. coli, and Listeria in raw pet foods.18 The matrices tested, requested pathogens, and test methods used from the 2 case investigations are summarized in Supplementary Table 1, and brief details are provided below.
Culturing of fecal and large intestinal contents for Salmonella was performed by 2 laboratories. The Animal Disease Diagnostic Laboratory of the Ohio Department of Agriculture (OH-ADDL) performed enriched culture of the fecal samples. Fecal samples (~1 g) were added to tetrathionate broth (TTB) and buffered peptone water (BPW; Hardy Diagnostics, Santa Maria, CA) and incubated at 37°C for 24 h. The incubated TTB was plated onto brilliant green with novobiocin agar (BGN; Hardy Diagnostics) and xylose–lysine–tergitol 4 agar (XLT-4, Hardy Diagnostics) and incubated at 37°C for 24 h. The incubated BPW was inoculated into Rappaport–Vassiliadis broth (RV; Becton, Dickinson, Franklin Lakes, NJ) and TTB, and the broths were incubated at 42°C and 37°C, respectively, for 24 h. The RV and TTB broths were plated onto BGN and XLT-4 and incubated at 37°C for 24 h. All agar plates were observed for 48 h, and suspect Salmonella colonies were subcultured onto MacConkey agar and confirmed as Salmonella sp. by matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Billerica, MA).
The Athens Veterinary Diagnostic Laboratory at the University of Georgia College of Veterinary Medicine (GA-AVDL) examined the large intestinal contents for Salmonella. Intestinal content (~1 g) was added to selenite broth (SE; Remel, Lenexa, KS) and incubated at 40 ± 2°C for 18 h. The intestinal content was also plated directly onto MacConkey agar (Remel) and incubated at 35 ± 2°C for 18 h. The incubated SE was plated onto brilliant green agar (Remel) and xylose–lysine–deoxycholate agar (Remel) and incubated at 35 ± 2°C for 18 h. All agar plates were observed for 48 h; suspect Salmonella colonies were confirmed as Salmonella sp. by MALDI-TOF MS (Vitek MS, bioMérieux, St. Louis, MO), and were serogrouped (Poly A-I antiserum, BD Diagnostics, Sparks, MD). Salmonella sp. isolates were sent to National Veterinary Services Laboratories for serotyping.
Salmonella cultures of food were performed by 2 laboratories. The Consumer Protection Laboratory of the Ohio Department of Agriculture (OH-CPL) used a VIDAS Salmonella Screen (bioMérieux, Durham, NC), described by the Association of Analytical Communities (AOAC) International Official Method of Analysis (OMA) 2004.03,3 and any positive samples were confirmed using a method for Salmonella described in the FDA Bacteriological Analytical Manual (BAM).1 The FDA Office of Regulatory Affairs (ORA) Laboratory used a culture method described in the FDA BAM for Salmonella.1
The OH-ADDL cultured feces for Listeria sp. by inoculating BPW and UVM modified Listeria enrichment broth (Becton Dickinson) with ~1 g of feces. After 24 h of incubation at 37°C, both the BPW and UVM broth were inoculated into fresh UVM broth and incubated for an additional 24 h at 37°C. The UVM broths were plated onto modified Oxford medium (Hardy Diagnostics) and incubated for 72 h at 37°C, checking for growth every 24 h. The OH-CPL screened the food using the VIDAS Listeria sp. Screen (bioMérieux), described in the AOAC OMA 999.06,2 and positive samples were culture confirmed using the method described in the FDA BAM for Listeria.9 The FDA ORA Laboratory also cultured the food for Listeria sp. using the method described in the FDA BAM for Listeria.9
The GA-AVDL cultured for E. coli from liver and kidney tissue using an aerobic culture method. Each tissue was macerated and plated directly onto 5% sheep blood agar and MacConkey agar (Remel) and incubated at 35 ± 2°C for 18 h. All agar plates were observed for 48 h, and suspect colonies were confirmed as E. coli by MALDI-TOF MS (Vitek MS). The FDA ORA Laboratory isolated and identified E. coli from the food using the method described in the FDA BAM for E. coli.7
The bacteria isolated from the animal clinical samples and raw pet foods are summarized in Table 2. OH-ADDL performed antimicrobial susceptibility testing by a minimum inhibitory concentration (MIC) method for the cat Salmonella isolate (Sensititre companion animal MIC plate, Thermo Fisher Scientific, Waltham, MA). OH-ADDL and OH-CPL serotyped the animal and case 1 food isolates using SISTR (https://lfz.corefacility.ca/sistr-app/) based on the WGS data of each isolate. The FDA ORA laboratory serotyped the isolates using the methods described in the FDA BAM for Salmonella.1 The serovars were identified based on the antigenic formulas as described previously.8
Table 2.
Summary of animal clinical and raw pet food bacterial isolates from 2 case investigations.
| Case investigation | Source | Matrix | No. of isolates | Pathogens identified | Strain name |
|---|---|---|---|---|---|
| 1 | Cat 1 | Feces | 1‡ | Salmonella Reading | OH-16-3844 |
| Cats 2–4 | Feces | 0 | Negative | NA | |
| Food 1†A | Freeze-dried raw duck/turkey/goose cat food | 0 | Negative | NA | |
| Food 2*B | Raw turkey cat food | 4§ | Listeria monocytogenes | OH-16-8884-5 OH-16-8884-6 |
|
| Salmonella Reading | OH-16-8884-7 OH-16-8884-8 |
||||
| Food 3*B | Raw chicken cat food | 2§ | L. monocytogenes | OH-16-8884-1 OH-16-8884-2 |
|
| Food 4*B | Raw beef cat food | 2§ | L. monocytogenes | OH-16-8884-3 OH-16-8884-4 |
|
| Food 5*B | Raw lamb cat food | 0 | Negative | NA | |
| Food 6*B | Raw venison cat food | 0 | Negative | NA | |
| 2 | Dog 1 | Tissues | 2¦ | Salmonella Anatum | OH-16-25847-A |
| Escherichia coli | OH-16-25847-B | ||||
| Food 7†C | Raw beef dog food | 7# | Salmonella Montevideo | FDA00011060 | |
| Salmonella Newport | FDA00011059 FDA00011061 |
||||
| L. monocytogenes | FDA00011068– FDA00011071 |
||||
| Food 8*C | Raw beef dog food | 6# | Salmonella Anatum | FDA00011063 | |
| Salmonella Newport | FDA00011062 FDA00011064 |
||||
| L. monocytogenes | FDA00011065– FDA00011067 | ||||
| Food 9†C | Raw beef cat food | 8# | Salmonella Anatum | FDA00011009 | |
| E. coli | FDA00011145–FDA00011151 |
A, B, C = manufacturer A, B, and C, respectively; NA = not available.
Purchased food.
Food collected from complainant.
Performed by the OH-ADDL.
Performed by the Consumer Protection Laboratory of the Ohio Department of Agriculture.
Bacteria isolated at GA-AVDL and sequenced at OH-ADDL.
Performed by the FDA ORA Laboratory. Whole genome sequencing results were uploaded to GenBank.
All isolates from the cases were sequenced. The OH-ADDL method involved library preparation (Nextera XT kit, Illumina, San Diego, CA) and sequencing (MiSeq v2 [250×250] reagent kit, Illumina). The FDA ORA Laboratory method for library preparation and DNA sequencing follows the GenomeTrakr standard operating protocol.23 After sequencing, the OH-ADDL and FDA ORA laboratories uploaded the WGS data through the GenomeTrakr network to GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
For all isolates, the OH-ADDL performed core genome multi-locus sequence typing (cgMLST) analysis on the bacterial isolates following a previously described method15 and using SeqSphere v.3.5 (Ridom, Münster, Germany) and online WGS databases.16,24 The animal and pet food isolate sequences were phylogenetically compared for relatedness. OH-ADDL identified genotypic antimicrobial resistance genes and plasmids using PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/).
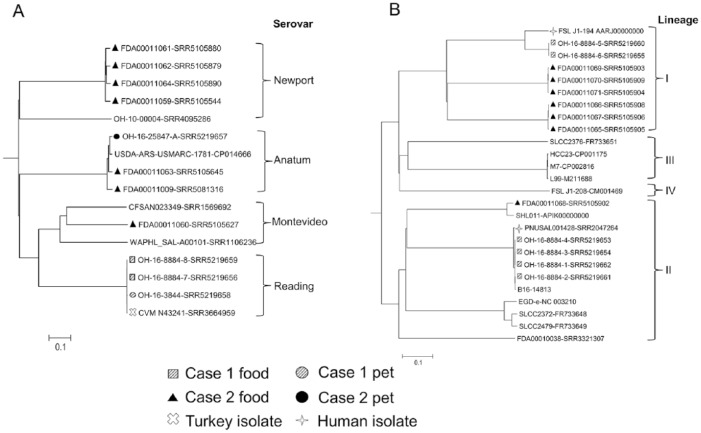
Case investigation 1 involved a breeder housing 11 cats and 2 raw cat foods (Table 1). One of 4 cats tested positive for Salmonella enterica subsp. enterica serovar Reading, but the initially reported product, food 1, tested negative for Salmonella (Table 2). The owner interview identified an unreported product, food 2, which was fed to the cats. No food 2 product was available for testing, but Vet-LIRN tested store-bought samples. One of 5 store-bought samples of food 2 tested positive for Salmonella Reading (Table 2). The Salmonella Reading isolates from the asymptomatic cat 1 fecal and raw turkey food 2 clustered together (Fig. 1A); the isolates also clustered with a 2012 Tennessee ground turkey isolate (CVM N43241). The Salmonella Reading phenotypic and genotypic antimicrobial resistance patterns are provided in Supplementary Table 2.
Figure 1.
Phylogenetic trees were generated for A. Salmonella enterica, B. Listeria monocytogenes, and C. Escherichia coli based on the allelic profiles of 3,288, 1,573, and 3,100 cgMLST target genes defined by SeqSphere v.3.5 (Ridom, Münster, Germany), respectively. The serovars of S. enterica and E. coli isolates were predicted based on the whole genome sequencing data using online databases.14,15,23 Scale bars indicate the distance of 10% dissimilarity.
Case investigation 2 involved complaints from 2 households feeding food made by the same manufacturer. The first household was a breeder housing adult dogs and a new litter of puppies, feeding a raw dog food (Table 1). One puppy that died of septic bacterial enterocolitis cultured positive for Salmonella Anatum. The second household was a breeder housing an unknown number of adult cats and multiple litters of kittens, feeding a raw cat food (Table 1). Two kittens died, but no clinical samples were tested for bacterial pathogens. Three different Salmonella serovars were isolated from the raw pet foods (food 7, 8, 9; Table 2). The cgMLST analysis (Fig. 1A) showed that the Salmonella Anatum isolate from the puppy was closely related to the food 8 isolate and also clustered with the food 9 isolate that was collected from the cat household. The remaining 5 Salmonella isolates (Fig. 1A), serovars Montevideo and Newport, from foods 7 and 8 were distantly correlated to the puppy clinical isolate. In both case investigations, WGS analysis suggests that the pets likely contracted Salmonella after ingesting their respective raw pet foods.
Collectively, 13 L. monocytogenes isolates were recovered from the 2 cases (Table 2). In case 1, 3 of 5 store-bought products tested positive for L. monocytogenes. The cgMLST analysis (Fig. 1B) of the whole genome sequences indicated that 4 L. monocytogenes isolates from food 3 and 4 closely correlated with each other and clustered together with a 2015 human clinical isolate (PNUSAL001428) in lineage II. By contrast, the food 2 isolates clustered together and were relatively related to a 2006 human clinical isolate (FSL J1-194) in lineage I. WGS suggests that the products from case investigation 1 were likely contaminated by a common source of L. monocytogenes. In case investigation 2, 2 samples of raw dog food tested positive for L. monocytogenes. Three (FDA00011069–FDA00011071) of 4 L. monocytogenes isolates (Fig. 1B) from food 7 and all 3 L. monocytogenes isolates from food 8 formed 2 genetic clusters under lineage I. One isolate from food 7 (FDA00011068) belonged to lineage II. It is unclear based on WGS if the 2 products from case investigation 2 were contaminated by the same or different sources of L. monocytogenes.
In case investigation 2, E. coli was recovered from the puppy that died of septic bacterial enterocolitis and from the raw food fed to the cats (food 9). The cgMLST analysis showed that the puppy E. coli isolate was distantly related to only 1 of the 7 E. coli isolates (FDA00011151; Fig. 1C) from food 9. The remaining 6 E. coli isolates (FDA00011145–FDA00011150) from food 9 clustered together and were distinct from the puppy clinical isolate (Fig. 1C). Both E. coli and Salmonella can cause diarrheal illness and sequelae, such as neurologic disease as a result of encephalitis.6 WGS analysis showed a connection between the Salmonella, but not the E. coli, isolates in the raw food and ill cats in case investigation 2. Thus, the cause of the illnesses in case 2 was very likely a consequence of ingesting the raw cat food.
The manufacturers from case investigations 1 and 2 voluntarily recalled the raw pet foods matching the lot and best-by date of the contaminated products.
Case investigations of human foodborne illness are often multidisciplinary, involving epidemiology, consumer exposure interviews, clinical information, product trace-back, and laboratory techniques including WGS.4,10,12,13 Investigations of animal foodborne pathogens including WGS information are not commonly reported in the literature. A multidisciplinary approach using pulsed-field gel electrophoresis (PFGE) and ribotyping for a Salmonella enterica outbreak at a Greyhound breeding facility was reported in 2006,17 and 2 previous articles reported WGS data for Salmonella and Campylobacter spp. isolates from dog feces.19,20 Our study documents a valuable approach that will enable veterinary diagnosticians to identify cases of pet illness caused by contaminated raw pet foods.
Our report reinforces the important role of veterinary diagnosticians in safeguarding public health. If foodborne illness–causing bacteria are isolated from companion animals, diagnosticians should encourage veterinarians to conduct a thorough feeding history review. All 3 consumers in our report were breeders. A prior study indicated that 4% of dog breeders feed a raw diet to puppies, and that most breeders make dietary recommendations.5 Puppies and kittens fed contaminated raw pet food may be a health risk for other animals and people, especially when those animals are sold or commercially transported. It is critical that veterinarians educate pet owners, especially animal breeders, and retail employees who sell pet foods about the risks of feeding and handling raw pet food.
Our case report also highlights the importance of testing fecal samples from multiple animals within a household. The literature indicates that, even though dogs and cats may not be diarrheic, they can still carry Salmonella,20 a situation observed in case investigation 1. Therefore, it may be prudent to test multiple animals, both diarrheic and non-diarrheic, that eat a suspected diet or live within the same household environment (e.g., cats sharing a litter box) to increase the likelihood of isolating a foodborne pathogen. Pet owners, veterinary staff, and veterinary diagnosticians should be cautious when handling clinical samples (e.g., feces) and potentially contaminated raw pet foods that could contain zoonotic bacteria.
WGS has emerged as a powerful tool for comparing foodborne isolates. Several lines of evidence have demonstrated that WGS provides a significantly higher level of discriminatory power than the traditional typing methods, including serotyping, PFGE, MLST, and multiple-locus variable number of tandem repeat analysis.10,12,16,21 Additionally, by using online databases, WGS data can be used to correctly predict serovars of Salmonella and E. coli, help track the occurrence of antimicrobial resistance genes, and potentially identify clusters of human or animal foodborne illness outbreaks.11,15,22,24 Veterinary diagnostic laboratories can play a significant role in public health by conducting WGS and genomic analysis of paired animal clinical and animal food isolates to identify animal foodborne illness outbreaks and thus, in partnership with FDA, safeguard animal food.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638718823046 for Whole genome sequencing confirms source of pathogens associated with bacterial foodborne illness in pets fed raw pet food by Jennifer L. Jones, Leyi Wang, Olgica Ceric, Sarah M. Nemser, David S. Rotstein, Dominika A. Jurkovic, Yamir Rosa, Beverly Byrum, Jing Cui, Yan Zhang, Cathy A. Brown, Anne L. Burnum, Susan Sanchez and Renate Reimschuessel in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Sierra Huff and Paula Bartlett from the Athens Veterinary Diagnostic Laboratory for the microbiology support. We also thank the FDA Office of Regulatory Affairs, including Ruiqing Pamboukian, Terri McConnell, and Ruth Timme, for the microbiology support, sharing the food isolate information, and for providing information about the ORA methods used.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article. Disclaimer: The views expressed in this presentation are those of the authors and may not reflect the official policy of the Department of Health and Human Services, the U.S. Food and Drug Administration, or the U.S. Government, nor do they endorse either the products or companies mentioned.
Funding: Work performed by the OH-ADDL was funded by a Vet-LIRN Infrastructure and Methods Grant (FOA PA-12-194, grant 1U18FD004622-01 and FOA PA-13-244, grant 1U18FD005143-01). Work performed by the GA-AVDL was funded by a Vet-LIRN Infrastructure Grant (FOA PA-12-194, grant 1U18FD004623-01).
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Jennifer L. Jones  https://orcid.org/0000-0003-0472-2994
https://orcid.org/0000-0003-0472-2994
References
- 1. Andrews WH, et al. Chapter 5: Salmonella. In: Bacteriological Analytical Manual. Online edition. Silver Spring, MD: US FDA, 2018. [Google Scholar]
- 2. AOAC International. Official Methods of Analysis. 17th ed. Gaithersburg, MD: AOAC International, 2000. Method 999.06-Listeria in Foods. [Google Scholar]
- 3. AOAC International. Official Methods of Analysis. 17th ed. Gaithersburg, MD: AOAC International, 2000. Method 2004.03-Salmonella in Foods. [Google Scholar]
- 4. Chen Y, et al. Core genome multilocus sequence typing for identification of globally distributed clonal groups and differentiation of outbreak strains of Listeria monocytogenes. J Appl Environ Microbiol 2016;82:6258–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connolly KM, et al. Feeding practices of dog breeders in the United States and Canada. J Am Vet Med Assoc 2014;245:669–676. [DOI] [PubMed] [Google Scholar]
- 6. Ephros M, et al. Encephalopathy associated with enteroinvasive Escherichia coli 0144:NM infection. J Clin Microbiol 1996;34:2432–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng P, et al. Enumeration of Escherichia coli and the coliform bacteria. In: Bacteriological Analytical Manual. Online edition. Silver Spring, MD: US FDA, 2017. [Google Scholar]
- 8. Grimont PAD, Weill F. Antigenic formulae of the Salmonella serovars. 9th ed. Paris: Institut Pasteur, 2007. [Google Scholar]
- 9. Hitchins AD, et al. Detection of Listeria monocytogenes in foods and environmental samples, and enumeration of Listeria monocytogenes in foods. In: Bacteriological Analytical Manual. Online edition. Silver Spring, MD: US FDA, 2017. [Google Scholar]
- 10. Hoffmann M, et al. Tracing origins of the Salmonella Bareilly strain causing a food-borne outbreak in the United States. J Infect Dis 2016;213:502–508. [DOI] [PubMed] [Google Scholar]
- 11. Joensen KG, et al. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol 2015;53:2410–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kozyreva VK, et al. Laboratory investigation of Salmonella enterica serovar Poona outbreak in California: comparison of pulsed-field gel electrophoresis (PFGE) and whole genome sequencing (WGS) results. PLoS Curr 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwong JC, et al. Prospective whole-genome sequencing enhances national surveillance of Listeria monocytogenes. J Clin Microbiol 2016;54:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laflamme DP, et al. Pet feeding practices of dog and cat owners in the United States and Australia. J Am Vet Med Assoc 2008;232:687–694. [DOI] [PubMed] [Google Scholar]
- 15. Larsen MV, et al. Multilocus sequence typing of total genome sequenced bacteria. J Clin Microbiol 2012;50:1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller JM. Whole-genome mapping: a new paradigm in strain-typing technology. J Clin Microbiol 2013;51:1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morley PS, et al. Evaluation of the association between feeding raw meat and Salmonella enterica infections at a greyhound breeding facility. J Am Vet Med Assoc 2006;228:1524–1532. [DOI] [PubMed] [Google Scholar]
- 18. Nemser SM, et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog Dis 2014;11:706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olkkola S, et al. Population genetics and antimicrobial susceptibility of canine Campylobacter isolates collected before and after a raw feeding experiment. PLoS One 2015;10:e0132660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reimschuessel R, et al. Multi-laboratory survey to evaluate Salmonella prevalence in diarrheic and non-diarrheic dogs and cats in the USA between 2012 and 2014. J Clin Microbiol 2017;55:1350–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roetzer A, et al. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med 2013;10:e1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rusconi B, et al. Whole genome sequencing for genomics-guided investigations of Escherichia coli O157:H7 outbreaks. Front Microbiol 2016;7:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Timme RE, et al. GenomeTrakr proficiency testing for foodborne pathogen surveillance: an exercise from 2015. Microb Genom 2018;4:e000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshida CE, et al. The Salmonella in silico typing resource (SISTR): an open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS One 2016;11:e0147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638718823046 for Whole genome sequencing confirms source of pathogens associated with bacterial foodborne illness in pets fed raw pet food by Jennifer L. Jones, Leyi Wang, Olgica Ceric, Sarah M. Nemser, David S. Rotstein, Dominika A. Jurkovic, Yamir Rosa, Beverly Byrum, Jing Cui, Yan Zhang, Cathy A. Brown, Anne L. Burnum, Susan Sanchez and Renate Reimschuessel in Journal of Veterinary Diagnostic Investigation