Abstract
Background
Intracranial aneurysms are increasingly being treated by the placement of flow diverters; however, the factors affecting the outcome of aneurysms treated using flow diverters remain unclarified.
Methods
The present study investigated 94 aneurysms treated with pipeline embolisation device placement, and used a computational fluid dynamics method to explore the factors influencing the outcome of aneurysms.
Results
Seventy-six completely occluded aneurysms and 18 incompletely occluded aneurysms were analysed. Before treatment, inflow jets were found in 13 (72.2%) aneurysms in the incompletely occluded group and 34 (44.7%) in the completely occluded group (P = 0.292). After deployment of the pipeline embolisation device, inflow jets remained in nine (50%) aneurysms in the incompletely occluded group and nine (11.8%) in the completely occluded group (P = 0.001). In the incompletely occluded group, regions with inflow jets after treatment corresponded with the patent areas shown on follow-up digital subtraction angiography. The mean reduction ratios of velocity in the whole aneurysm and on the neck plane were lower in the incompletely occluded than in the completely occluded group (P = 0.003; P = 0.017). Multivariate analysis revealed that the only independent risk factors for incomplete aneurysm occlusion were the reduction ratios of velocity (in the whole aneurysm, threshold 0.362, P = 0.005; on the neck plane, threshold 0.273, P = 0.015).
Conclusions
After pipeline embolisation device placement, reduction ratios of velocity in the whole aneurysm of less than 0.362 and on the neck plane of less than 0.273 are significantly associated with a greater risk of aneurysm incomplete occlusion. In addition, the persistence of inflow jets in aneurysms is associated with incomplete occlusion in the inflow jet area.
Keywords: Aneurysm, flow diversion, haemodynamics, pipeline embolisation device
Introduction
Pipeline embolisation devices (PEDs), which are dedicated flow diverters (FDs), received US Food and Drug Administration approval in 2011 for the endovascular treatment of large or giant wide-necked intracranial aneurysms of the internal carotid artery from the petrous to the superior hypophyseal segments. These devices are designed to create a low-flow haemodynamic state within an aneurysm that assists thrombosis and ultimate occlusion.1 Several studies have evaluated the safety and efficacy of PEDs, and the results are encouraging.2–4 A meta-analysis performed in 2013 that analysed the results of 1451 patients with 1654 aneurysms treated with FDs reported a complete aneurysm occlusion rate of 76%, and procedure-related morbidity and mortality rates of 5% and 4%.5 Nevertheless, some aneurysms remain unoccluded for a long time after PED treatment.6 To date, there are no reliable means of predicting thrombosis of an aneurysm, and it is unknown how much flow reduction is needed to induce progressive thrombosis.
Computational fluid dynamics (CFD) simulations have been used to study the flow disruption effects induced by flow-diverting stents. Previous studies have shown that dynamic factors play an important role in the prognosis of FD-implanted aneurysms;7–11 however, these studies were relatively small, which may have introduced bias, and multidimensional factors were not investigated. Ouared et al.12 showed that successful FD treatment in sidewall intracranial aneurysms requires a reduction in the post-stent average velocity by at least one-third from the initial pre-stent conditions; however, their study included 12 patients and analysed only haemodynamic factors. Mut et al.13 demonstrated that the pre and post-treatment inflow rates, post-treatment aneurysm average velocity and post-treatment shear rate significantly differ between occluded and non-occluded intracranial aneurysms at 6 months after FD treatment. However, it remains unclear whether these variables can predict the outcome of FD treatment. The aim of the present study was to determine which haemodynamic characteristics are associated with complete aneurysm occlusion in a large consecutive patient series.
Methods
The clinical and radiological data of 122 patients who had undergone PED treatment between January 2016 and October 2016 in our centre were retrospectively reviewed. The inclusion criteria were: at least one aneurysm that had initially been treated with a PED; sufficient imaging data for analysis; calculation of CFD. The exclusion criteria were: dissecting and fusiform aneurysm (15 patients); insufficient follow-up data (10 patients); and insufficient three-dimensional data for CFD analysis (14 patients). Thus 83 patients with 94 aneurysms were included in our research. The results of treatment were evaluated on the basis of the O’Kelly-Marotta scale14 for the degree of filling after flow diversion (A, total filling; B, subtotal filling; C, entry remnant; D, no filling), with categories A, B and C defined as incomplete occlusion. Initial and follow-up digital subtraction angiography (DSA) data were reviewed by two experienced neurointerventionalists (JL and XJY), and aneurysms were classified as incompletely occluded (ICO) or completely occluded (CO) in accordance with the above-mentioned O’Kelly-Marotta scale criteria. The CO and ICO groups were compared regarding clinical characteristics, namely patients’ baseline characteristics, morphological factors (e.g. aneurysm size, aneurysm height and neck width) and treatment-related factors (e.g. treatment strategy (with or without coils) and duration of follow-up) (Table 1).
Table 1.
Univariate analysis between CO and ICO groups.
| Characteristics (mean ± SD) | CO group (n = 76, 80.9%) | ICO group (n = 18, 19.1%) | P value |
|---|---|---|---|
| Age (years) | 51.9 ± 10.1 | 48.4 ± 17.3 | 0.420 |
| Male | 18 (23.7) | 2 (11.1) | 0.394 |
| Smoking | 7 (9.2) | 0 (0.0) | 0.401 |
| Hypertension | 25 (32.9) | 8 (44.4) | 0.356 |
| Anterior circulation | 65 (85.5) | 16 (88.9) | 1.000 |
| Treatment strategy (PED with coils) | 38 (50.0) | 9 (50.0) | 1.000 |
| Aneurysm size (mm) | 9.1 ± 6.5 | 12.6 ± 6.6 | 0.043* |
| Aneurysm height (mm) | 8.5 ± 6.2 | 11.7 ± 6.0 | 0.052 |
| Neck width (mm) | 7.6 ± 4.9 | 10.1 ± 5.7 | 0.068 |
| Aspect ratio | 1.2 ± 0.6 | 1.3 ± 0.5 | 0.516 |
| Parent artery size (mm) | 3.7 ± 1.1 | 3.5 ± 0.7 | 0.516 |
| High size ratio | 2.8 ± 2.4 | 3.8 ± 2.4 | 0.094 |
| Follow-up (months) | 12.0 ± 2.2 | 11.1 ± 2.6 | 0.155 |
| Inflow jets (pre) | 34 (44.7) | 13 (72.2) | 0.292 |
| Inflow jets (post) | 9 (11.8) | 9 (50.0) | 0.001* |
| Flow complexity (pre-com) | 44 (57.9) | 14 (77.8) | 0.119 |
| Flow complexity (post-com) | 23 (30.3) | 8 (44.4) | 0.250 |
| RR of velocity (AN) | 0.750 ± 0.263 | 0.283 ± 0.567 | 0.003* |
| RR of WSS (AN) | 0.538 ± 0.397 | –0.025 ± 1.018 | 0.033* |
| RR of pressure (AN) | –3.556 ± 29.648 | –0.214 ± 0.385 | 0.635 |
| RR of Velocity (neck plane) | 0.388 ± 0.355 | 0.076 ± 0.480 | 0.017* |
| RR of WSS (neck plane) | –0.037 ± 1.413 | –1.011 ± 1.833 | 0.046* |
P < 0.05.
AN: aneurysm; CO: completely occluded; com: complex; ICO: incompletely occluded; PED: pipeline embolisation device; RR: reduction ratio; SD: standard deviation; WSS: wall shear stress.
Patients were premedicated with dual antiplatelet therapy that consisted of a daily dose of 100 mg aspirin and 75 mg clopidogrel, both of which were initiated at least 5 days before the procedure. We used thromboelastography to identify the hyporesponders to clopidogrel; however, the test was not conducted in all patients in our series. Patients with an inhibition rate of less than 30% were deemed hyporesponsive to clopidogrel. Those patients were administered a booster dose of 300 mg clopidogrel. Aspirin was continued for 6 months, and clopidogrel was discontinued 3 months after the procedure if there were no other coronal or cerebral comorbidities necessitating the use of antiplatelet drugs.
PED and loose coiling
When treating large and giant aneurysms with a FD, additional coil placement may prevent postoperative aneurysm rupture.3,15 Morales et al.16 found that the first inserted coils (when the packing density is <12%) significantly reduce intra-aneurysmal flow velocity by more than 50%. Thus we chose a packing density cut-off value of 12% as the criterion for loose packing in the present study. Loose coil packing can aid in decreasing intra-aneurysmal flow velocity. The coils enable the slow washout of the aneurysmal wall, and stabilise large, rapidly accumulated intra-aneurysmal thrombi by changing the morphology of the aneurysmal cavity. Coils act similarly to breakwaters, and further mechanically buffer the intra-aneurysmal haemodynamic forces and decrease aneurysmal wall pulsation, which further promotes thrombosis and protects the flimsy aneurysmal wall.17
Computational modelling and haemodynamic simulations
Patient-specific aneurysm morphologies were reconstructed from three-dimensional rotational angiography images. CFD calculations were retrospectively calculated after each patient’s follow-up was completed. Recognised open computational modelling methods were used to calculate the haemodynamics.18,19 A virtual stenting technique was used to simulate the in vivo PED, and a porous media method was used to simulate the coil mass; these methods have been applied by many researchers.18,20,21 Packing density was defined as the ratio between the coil volume and the aneurysm volume. The porous media method is a realistic and computationally tractable representation of the coil mesh in haemodynamic studies.22,23 Briefly, the aneurysm dome was filled with porous medium corresponding to the coil mass, and the remaining vessel volume, including the residual neck and parent vessel, was filled with fluid to represent blood flow. The permeability (К) is a measure of the fluid conductivity through the porous medium.22,23 To approximate the permeability of a porous medium, we used a simplified expression based on the capillary theory of Kozeny. In this approach, the porous medium is approximated as a layer of solid material with straight parallel tubes of fixed cross-section intersecting the sample. The permeability is calculated using the formula К = φ3/cS2, where S is the specific surface area of coils, φ is the porosity of the medium, and c is the Kozeny coefficient (which is taken to be 2). The drag factor, CD, is determined by using standard CD versus Reynolds number diagrams, and was estimated to be 2.2.22,23
The deployed FD was merged with the aneurysm geometry in ICEM CFD version 14.0 (ANSYS, Inc., Canonsburg, PA, USA) to create finite volume tetrahedral elements for CFD simulation. The element size was set to 0.01 mm to represent the geometry of the FD sufficiently.24 Mesh sizes ranged from 3 to 5 million elements in cases without a FD, and from 20 to 40 million elements in cases with a FD. After meshing, haemodynamic variables based on CFD were calculated under pulsatile flow conditions using commercial software (ANSYS CFX 14.0; ANSYS, Inc.). The vessel wall was assumed to be rigid with a no-slip boundary condition. Blood was modelled as a homogenous, laminar, incompressible Newtonian fluid (attenuation 1060 kg/m3, viscosity 0.004 Pa/second). The governing equation underlying the calculation was the Navier–Stokes formulation. The pulsatile period velocity profile was obtained by transcranial Doppler from a normal subject, and was set as the inflow boundary condition. The flow waveforms were scaled to achieve a mean inlet wall shear stress (WSS) of 1.5 Pa under pulsatile conditions. As the physiological flow waveforms measured by transcranial Doppler were pulsatile, the flow data (velocity, flow rate and oscillatory shear stress) would actually change during the complete cardiac cycle. However, the patient-specific flow data were not always available in clinical practice, and so we had to use the representative population average value (1.5 Pa)25,26 to eliminate bias resulting from individual differences. Eight hundred time steps (0.001 s/step) were set for each cardiac cycle. Two cardiac cycles were simulated for numerical stability. Results from the second cardiac cycle were collected as output for post-processing of haemodynamic variables.
Computational findings at the aneurysmal neck and in the whole aneurysm were examined precisely. Mean flow velocity and mean WSS at peak systole before and after treatment were extracted and compared between the two groups. The haemodynamic reduction ratio (RR), defined as (pre-treatment value – post-treatment value)/pre-treatment value, was calculated for each patient-specific aneurysm. The following factors were calculated: mean RRs of velocity on the neck plane and in the whole aneurysm, mean RRs of WSS on the neck and on the aneurysm wall and mean RR of pressure. In addition to these quantitative variables, some qualitative variables relating to blood flow (flow complexity, inflow jet) were also evaluated using the method of Cebral et al.27 Pre and post-treatment haemodynamic factors were compared between the ICO and CO groups. In addition, haemodynamic changes were compared between patients with a PED alone versus those with a PED and coils to determine the effects of these treatment strategies (Table 2).
Table 2.
Univariate analysis between PED alone and PED with coils groups.
| Treatment strategy (mean ± SD) | PED with coils (n = 47, 50.0%) | PED alone (n = 47, 50.0%) | P value |
|---|---|---|---|
| RR of velocity (AN) | 0.763 ± 0.415 | 0.558 ± 0.327 | 0.009* |
| RR of velocity (neck plane) | 0.399 ± 0.494 | 0.257 ± 0.258 | 0.084 |
| Inflow jets (pre) | 23 (48.9) | 24 (51.0) | 0.312 |
| Inflow jets (post) | 9 (19.1) | 9 (19.1) | 1.000 |
| Flow complexity (pre-com) | 26 (55.3) | 32 (68.0) | 0.203 |
| Flow complexity (post-com) | 13 (27.7) | 18 (38.3) | 0.273 |
| Packing density, % | 9.84 ± 9 |
P < 0.05.
AN: aneurysm; com: complex; PED: pipeline embolisation device; RR: reduction ratio; SD: standard deviation.
Statistical analysis
Analyses were performed with statistical software (SPSS version 22.0; IBM, Chicago, IL, USA). The one-sample Kolmogorov–Smirnov test was used to determine whether the continuous variables were normally distributed. For normally distributed data, the independent-samples t-test was executed. The Mann–Whitney U test was used to compare non-parametric data, while the χ2 test was used for categorical variables. Data are presented as mean±standard deviation for continuous variables, and as frequency (percentage) for categorical variables. Risk factors with a P value of less than 0.1 in univariate analysis were entered into a multivariate logistic regression analysis to assess the independent risk factors and odds ratios with 95% confidence intervals of the ICO group and the CO group. A two-tailed P value of less than 0.05 was considered statistically significant in the multivariate logistic regression. Finally, we used receiver operating characteristic curves and the Youden index to identify the threshold values of haemodynamic variables and calculate the area under the receiver operating characteristic curve.
Results
Eighty-three patients (64 women and 19 men) harbouring 94 intracranial aneurysms and ranging in age from 8 to 73 years underwent PED treatment (PED alone or PED with loose packing coils) and were enrolled in our study. Angiographic follow-up occurred at an average of 12 months. On follow-up DSA, 76 (80.9%) aneurysms were found to be CO, while the remaining 18 (19.1%) were ICO (six with subtotal filling and 12 with entry remnants). Haemodynamic changes and important treatment-related factors are shown in Tables 1 and 2, and the results of multivariate logistic regression are shown in Table 3.
Table 3.
Multivariate logistic analysis between CO and ICO groups.
| Variables | OR# (95% CI) | P value | ROC area (1a,b,c) | Threshold value |
|---|---|---|---|---|
| RR of velocity (AN) | 16.77 (2.32–121.43) | 0.005* | 0.861 | 0.362 |
| RR of velocity (neck plane) | 6.42 (1.44–28.51) | 0.015* | 0.273 | |
| Aneurysm height | 1.05 (0.49–2.22) | 0.906 | ||
| Neck width | 1.14 (0.90–1.43) | 0.275 | ||
| High size ratio | 1.03 (0.70–1.52) | 0.886 | ||
| Aneurysm size | 0.79 (0.36–1.73) | 0.558 | ||
| RR of WSS (AN) | 2.04 (0.67–6.16) | 0.208 | ||
| RR of WSS (neck plane) | 1.30 (0.93–1.80) | 0.121 |
P < 0.05.
Under the non-parametric assumption.
Null hypothesis: true area = 0.5.
The area under the non-parametric assumption of the model including two index is 0.861.
AN: aneurysm; CO: completely occluded; ICO: incompletely occluded; RR: reduction ratio; WSS: wall shear stress.
Flow analysis
Qualitative analysis
Before treatment, aneurysmal flow patterns were similar in all cases, with flow entering the aneurysm and directly impinging on the distal wall (flow indicated by the black arrows in Figure 1 (d1), (g1), and Figure 2 (d1), (g1)). FD treatment resulted in some changes in blood flow. Before treatment, complex flow patterns were present in 77.8% of aneurysms in the ICO group and 57.9% in the CO group (P = 0.119, Table 1). After PED placement, complex flow patterns were present in 44.4% of aneurysms in the ICO group and 30.3% in the CO group (P = 0.250, Table 1). The reduction in the proportion of complex flow patterns after treatment was similar in the ICO and CO groups.
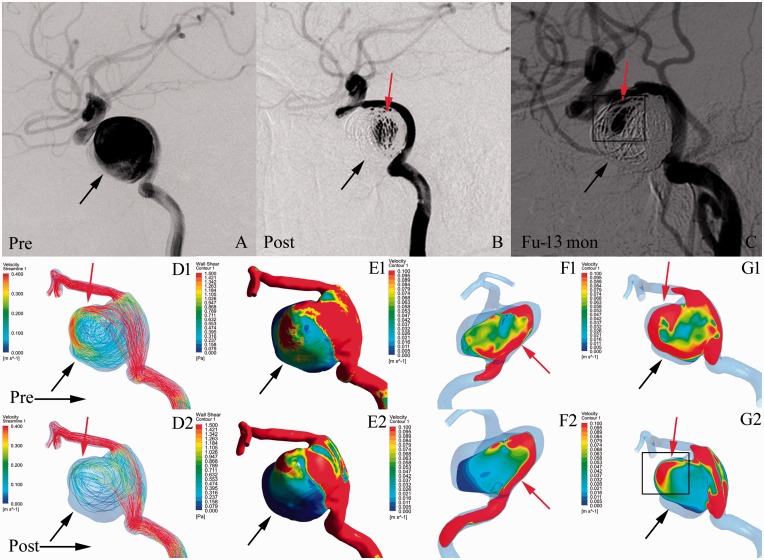
Figure 1.
Illustrations of the haemodynamics in an incompletely occluded aneurysm after treatment with a pipeline embolisation device (PED) with coils. Angiographic images at pre-treatment (a), post-treatment (b), and 13-month follow-up (c). (d) Velocity streamlines at pre-treatment (d1) and post-treatment (d2). (e) Wall shear stress (WSS) distribution at pre-treatment (e1) and post-treatment (e2). (f) Velocity distribution on the neck plane at pre-treatment (f1) and post-treatment (f2). (g) Velocity distribution on the lateral section of the aneurysm at pre-treatment (g1) and post-treatment (g2). In illustration (c), the red arrows indicate the patent area on follow-up. After treatment, the WSS on the aneurysm wall and velocity in the whole aneurysm were decreased (e1, e2; d1, d2; black arrows). In illustration (g2), the red arrows indicate the inflow jets in the aneurysm after treatment. Persistent inflow jets (red arrows in g2) were located in the areas of incomplete occlusion shown on follow-up (red arrows in c).
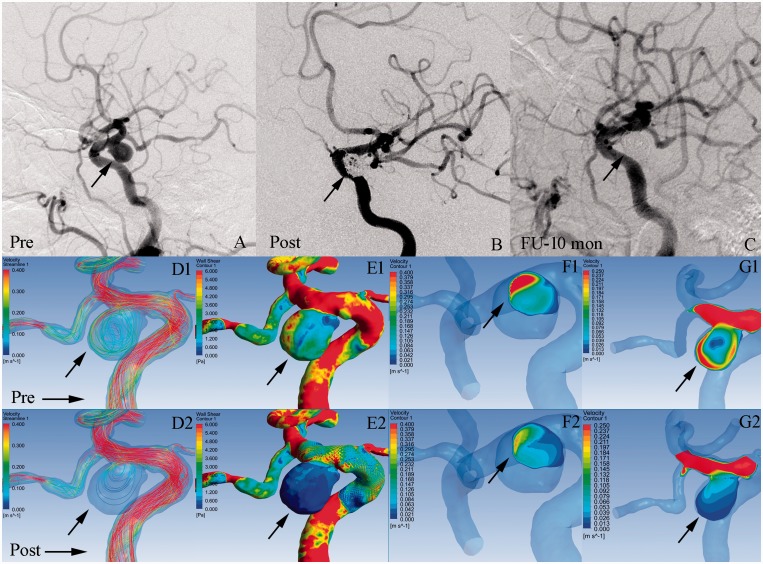
Figure 2.
Illustrations of the haemodynamics in a completely occluded aneurysm after treatment with a pipeline embolisation device (PED)-assisted coiling. Angiographic images at pre-treatment (a), post-treatment (b), and 10-month follow-up (c). (d) Velocity streamlines at pre-treatment (d1) and post-treatment (d2). (e) Wall shear stress (WSS) distribution at pre-treatment (e1) and post-treatment (e2). (f) Velocity distribution on the neck plane at pre-treatment (f1) and post-treatment (f2). (g) Velocity distribution on the lateral section of the aneurysm at pre-treatment (g1) and post-treatment (g2). After treatment, the WSS on the aneurysm wall, velocity in the whole aneurysm and velocity on the neck plane were obviously decreased (e1, e2; d1, d2; f1, f2; black arrows). There were no inflow jets in the aneurysm and on the neck plane after PED treatment (g2, f2).
Before treatment, inflow jets were found in 13 (72.2%) ICO aneurysms and 34 (44.7%) CO aneurysms (P = 0.250, Table 1). After treatment, inflow jets were still present in nine (50%) ICO aneurysms and nine (11.8%) CO aneurysms (P = 0.001, Table 1). The areas of high velocity blood flow were reduced (black arrows in g1 and g2, Figure 1). Interestingly, persistent inflow jets were located in the areas of incomplete occlusion shown on follow-up DSA (red arrows in g2 and c, Figure 1). In the CO group, 67 (88.2%) aneurysms had no inflow jets after treatment (black arrows in g1 and g2, Figure 2).
Quantitative analysis
According to univariate analysis, the CO group had a significantly greater mean RR of velocity in the whole aneurysm (0.750 vs. 0.283, P = 0.003, Table 1) and on the neck plane (0.388 vs. 0.076, P = 0.017, Table 1) than the ICO group. This is clearly demonstrated in Figures 2 (d1, d2, f1, f2) and 1 (d1, d2, f1, f2), respectively. Moreover, the final multivariate logistic analysis revealed that the only factors significantly associated with the outcomes of aneurysms were the mean RRs of velocity in the whole aneurysm (P = 0.005, Table 3) and on the neck plane (P = 0.015, Table 3). The threshold values for incomplete aneurysm occlusion were 0.362 for the RR of velocity in the whole aneurysm and 0.273 for the RR of velocity on the neck plane. The area under the curve for the non-parametric assumption of the model including the RRs of velocity (in the whole aneurysm and on the neck plane) was 0.861. Data on WSS and pressure are shown in Table 1.
PED alone versus PED with coils
Treatment comprising the PED with coils was implemented in nine aneurysms in the ICO group (50%) and 38 (50%) in the CO group; this intergroup difference was not significant (P = 1.0, Table 1). The mean packing density of the PED with coils was 9.84% (Table 2). For the comparison of cases treated with a PED alone versus a PED with coils, 47 aneurysms were treated with a PED alone, while 47 were treated with a PED with coils. The combined use of coils and a PED reduced velocity in the whole aneurysm by 76.3%, whereas use of a PED alone reduced velocity in the whole aneurysm by 55.8%; this was the only significant difference detected between treatment with a PED alone versus a PED with coils (P = 0.009, Table 2). The mean RR of velocity on the neck plane tended to be higher for aneurysms treated with a PED with coils than for those treated with a PED alone (0.399 vs. 0.257); however, this difference was not significant (P = 0.084, Table 2). The proportion of aneurysms with complex flow patterns was 68% in the PED alone group and 55.3% in the PED with coils group (P = 0.203, Table 2) before treatment, and 38.3% and 27.7%, respectively, after treatment (P = 0.273, Table 2). The proportions of aneurysms with inflow jets were similar in the PED alone and PED with coils groups both before and after treatment (51.0% and 48.9%, respectively, P = 0.312; and 19.1% and 19.1%, respectively, P = 1.0, Table 2).
Analysis of clinical factors
There were no significant differences in baseline characteristics between the ICO and CO groups. According to univariate analysis, the only morphological factor that significantly differed between the ICO and CO groups was aneurysm size; the mean aneurysm size was 12.6 mm in the ICO group and 9.1 mm in the CO group (P = 0.043, Table 1). The mean aneurysm height was 11.7 mm in the ICO group and 8.5 mm in the CO group; this index was closely related to aneurysm outcomes (P = 0.052, Table 1). The mean duration of follow-up was 11.1 months in the ICO group and 12 months in the CO group (P = 0.155, Table 1). All clinical characteristics are shown in Table 1.
Discussion
Increasing research has shown that PED treatment is more effective than conventional coils, especially for large and giant aneurysms.28–30 However, some aneurysms fail to occlude completely in the long term even after the use of a PED, and little is known about the effects of haemodynamic variables on the treatment outcome. In the present large series of patients, we evaluated the correlations between various haemodynamic variables and aneurysm occlusion after PED treatment. Our main findings are that the mean RRs of velocity in the whole aneurysm (threshold value 0.362) and on the neck plane (threshold value 0.273) are significant independent risk factors for aneurysm occlusion. Furthermore, the persistence of an inflow jet after this treatment may make it difficult to achieve complete occlusion in the long term.
Haemodynamics affect the outcome of aneurysms
The principal role of a PED is to guide the blood flow into the parent artery and divert it from the aneurysm. Therefore, flow patterns may play an important role in determining outcomes after FD treatment of aneurysms. Karmonik et al.31 found that inflow jets in aneurysms indicate potential risk zones for incomplete occlusion; however, their study was small. In the present larger study, we analysed the characteristics of blood flow in aneurysms and their necks. Our findings were similar in that the areas of aneurysms with strong inflow jets according to CFD analysis were identical to the patent areas on follow-up DSA. The incidence of post-treatment persistence of inflow jets was also significantly greater in the ICO group than in the CO group (50% vs. 11.8%, P = 0.001, Table 1). Inflow jets result in high velocity flow in areas of incomplete occlusion, as has been shown in animal studies. Cebral et al.9 analysed the haemodynamics after the occlusion of experimental aneurysms in 20 rabbits; the velocity, vorticity, and shear rates in non-occluded areas were about 2.8 times greater than those in occluded areas (P < 0.0001). Thus the flow pattern, especially when inflow jets are still present after treatment, can be used to predict the outcomes of aneurysms treated with PEDs. A persistent high velocity inflow jet after PED implantation may indicate that further intervention, such as the insertion of more coils or deployment of another PED, is necessary to ensure complete occlusion of the aneurysm in the long term. The outcomes of aneurysms are dependent on haemodynamic variables and the degree of similarity between haemodynamic and clinical images. In the present retrospective study, pre and post-treatment CFD simulations were performed at the end of each patient’s imaging follow-up. CFD simulations can not only evaluate the outcomes of aneurysms based on the flow velocity, but can also predict high-risk areas and improve treatment methods in accordance with the location of the inflow jet. Intraoperative rapid assessment of haemodynamics is the future direction of clinical practice. With the development of aneurysm identification using artificial intelligence and cloud computing, it only takes a short time to calculate intraoperative haemodynamics.32 Furthermore, the flow visualisation afforded by this technology can inform aneurysm treatment planning and potentially avoid poor outcomes.
In addition to the flow pattern, we also calculated and analysed quantitative variables to explore the factors affecting aneurysm outcome after PED treatment. We found that the mean RRs of velocity in the whole aneurysm and on the neck plane were independent risk factors for incomplete occlusion. Mut et al.13 compared two groups of patients with a total of 23 aneurysms treated with PED implantation: a fast occlusion group (CO at 3 months) and a slow occlusion group (aneurysm patent or ICO at 6 months). They found that aneurysms in the fast occlusion group had a significantly lower post-treatment mean velocity (fast 1.13 cm/s, slow 3.11 cm/s, P = 0.02), inflow rate (fast 0.47 mL/s, slow 1.89 mL/s, P = 0.004), and shear rate (fast 20.52 1/s, slow 32.37 1/s, P = 0.02) than those in the slow occlusion group.13 However, they only compared post-treatment haemodynamic conditions between the fast and slow occlusion groups, and did not compare post versus pre-treatment haemodynamic conditions within the same group.13 In the present study, the mean RR of velocity was 75% in the CO group and 28.3% in the ICO group (Table 1). We also found that a RR of velocity in the whole aneurysm of greater than 0.362 and a RR of velocity on the neck plane of greater than 0.273 indicated complete aneurysm occlusion after PED treatment. Ouared et al.12 showed that for successful FD treatment, the post-stent average velocity in sidewall intracranial aneurysms must be reduced by at least one-third (threshold value 0.353) from the initial pre-stent conditions. Our large cohort study shows that the intra-aneurysmal velocity RR (especially the threshold value of 0.362) can be used to predict whether FD treatment will result in aneurysm occlusion or non-occlusion at final follow-up. Our results may help physicians treating aneurysms with PED implantation to determine when to insert more coils or deploy another PED to reduce the risk of incomplete occlusion and thus improve outcomes. The performance of preoperative CFD simulation guided by specialists before endovascular treatment is the direction of the clinical application of CFD.
Supplementary loose coil packing is sometimes considered to help accelerate the process of thrombosis and provide immediate protection for the dome during the initial non-thrombosed phase after PED placement, and may constitute a safe and effective treatment for aneurysms at high risk of rupture (or re-rupture) or with complex anatomy.3,33 Adjunctive coiling is reportedly a predictor of aneurysm occlusion.34 Another study demonstrated that a single FD provides more reduction of the inflow rate than does low packing density coiling; however, there is less reduction in average velocity inside the aneurysm.24 These findings indicate that the main role of a FD is to divert inflow, whereas that of coils is to create stasis in the aneurysm. We found that adding coils reduced the velocity in the whole aneurysm significantly more than using a PED alone (76.3% vs. 55.8%, P = 0.009, Table 2). However, whether or not coils were used had no influence on the subsequent occlusion of aneurysms in the present study (of the 18 ICO aneurysms, nine were treated by PED placement, while nine were treated by a PED with coils, packing density 9.84%, Tables 1 and 2).
Antiplatelet therapy strategy
Although previous studies found an association between platelet testing results and neurovascular complications after stent/PED placement, there is no clear evidence that changing the antiplatelet therapy based on platelet test results improves the clinical outcome.35,36 Furthermore, some studies with large cohorts found that platelet function testing is not significantly associated with symptomatic events, and even has an association with significantly higher morbidity and complication rates in patients who undergo PED placement.37,38 The optimal medication regimen and duration of therapy are yet to be determined. Further prospective studies are needed to determine if and when platelet testing and correction of antiplatelet therapies are appropriate in patients who undergo PED placement.
Influence of aneurysm size on outcomes
A recent study found that aneurysm size is a predictor of aneurysm persistence after FD placement.34 In the present study, the mean aneurysm size was larger in the ICO group than in the CO group; however, although significant in univariate analysis, this difference was not significant according to multivariate regression analysis. Numerous other studies of FDs have shown a tendency towards decreasing occlusion rates with increasing aneurysm size;30,39,40 however, in most series, this tendency has not been statistically significant, as was the case in the present study. Large aneurysms take longer to thrombose and may have complex flow patterns as a result of pulsatile flow, resulting in coil changes.41 With longer follow-up, the complete occlusion rate increases significantly.30 In addition, one study has shown that changes in haemodynamic variables are more significant for smaller aneurysms.42 Aneurysm morphology is strongly correlated with WSS and the ratio between the aneurysm volume and inflow at the aneurysm neck.7 In the present study, haemodynamic factors were the only significant predictors of outcomes; thus, morphological factors may be less important than haemodynamic factors in the outcomes of aneurysms.
Limitations
This was a single-centre retrospective study, and was therefore subject to an inherent bias in patient selection. Furthermore, although the haemodynamic variables we identified have previously been reported to be useful for understanding flow haemodynamics in aneurysms, computational modelling of stents and coils and simplified CFD analyses do not very precisely reflect the actual situation after endovascular treatment. The CFD simulation had to make simplifying assumptions, such as assumed inlet flow waveforms, as patient-specific waveforms were not available. In addition, the average duration of follow-up in our study was 12 months. A longer follow-up is needed, especially for large aneurysms. Thus larger prospective studies with longer follow-up and more comprehensive assessment of possible prognostic factors are necessary in the future.
Conclusions
Overall, we found that low RRs of velocity in the whole aneurysm (threshold value 0.362) and on the neck plane (threshold value 0.273) are important for achieving incomplete occlusion after PED treatment of aneurysms. Persistent inflow jets indicate the potential risk areas for incomplete occlusion. Future prospective studies with larger sample sizes are necessary to validate these results.
Acknowledgements
The authors would like to thank Kelly Zammit from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Author contribution
JFC prepared the manuscript. YSZ and JL contributed equally to data collection. ZBT, WQL, QQZ and YZ performed clinical follow-up. JL and XJY performed the interventional procedures.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the research was supported by the National Key Research and Development Program of China (grant numbers 2016YFC1300800 and 2016YFC1300802), the National Natural Science Foundation of China (grant numbers 81220108007, 81371315, 81471167 and 81671139) and the Special Research Project for Capital Health Development (grant number 2018-4-1077). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jing L, Zhong J, Liu J, et al. Hemodynamic effect of flow diverter and coils in treatment of large and giant intracranial aneurysms. World Neurosurg 2016; 89: 199–207. [DOI] [PubMed] [Google Scholar]
- 2.Becske T, Potts MB, Shapiro M, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 2017; 127: 81–88. [DOI] [PubMed] [Google Scholar]
- 3.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm Study of Pipeline in an Observational Registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 6.Daou B, Atallah E, Chalouhi N, et al. Aneurysms with persistent filling after failed treatment with the Pipeline embolization device. J Neurosurg 2019; 130: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 7.Larrabide I, Geers AJ, Morales HG, et al. Effect of aneurysm and ICA morphology on hemodynamics before and after flow diverter treatment. J Neurointerv Surg 2015; 7: 272–280. [DOI] [PubMed] [Google Scholar]
- 8.Huang Q, Xu J, Cheng J, et al. Hemodynamic changes by flow diverters in rabbit aneurysm models: a computational fluid dynamic study based on micro-computed tomography reconstruction. Stroke; a journal of cerebral circulation 2013; 44: 1936–1941. [DOI] [PubMed] [Google Scholar]
- 9.Cebral JR, Mut F, Raschi M, et al. Analysis of hemodynamics and aneurysm occlusion after flow-diverting treatment in rabbit models. AJNR Am J Neuroradiol 2014; 35: 1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung B, Mut F, Kadirvel R, et al. Hemodynamic analysis of fast and slow aneurysm occlusions by flow diversion in rabbits. J Neurointerv Surg 2015; 7: 931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paliwal N, Damiano RJ, Davies JM, et al. Association between hemodynamic modifications and clinical outcome of intracranial aneurysms treated using flow diverters. Proc SPIE Int Soc Opt Eng 2017; 10135: 101352F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouared R, Larrabide I, Brina O, et al. Computational fluid dynamics analysis of flow reduction induced by flow-diverting stents in intracranial aneurysms: a patient-unspecific hemodynamics change perspective. J Neurointerv Surg 2016; 8: 1288–1293. [DOI] [PubMed] [Google Scholar]
- 13.Mut F, Raschi M, Scrivano E, et al. Association between hemodynamic conditions and occlusion times after flow diversion in cerebral aneurysms. J Neurointerv Surg 2015; 7: 286–290. [DOI] [PubMed] [Google Scholar]
- 14.O’Kelly C J, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol: journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences 2010; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 2010; 41: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 16.Morales HG, Kim M, Vivas EE, et al. How do coil configuration and packing density influence intra-aneurysmal hemodynamics? AJNR Am J Neuroradiol 2011; 32: 1935–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boecher-Schwarz HG, Ringel K, Kopacz L, et al. Ex vivo study of the physical effect of coils on pressure and flow dynamics in experimental aneurysms. AJNR Am J Neuroradiol 2000; 21: 1532–1536. [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Jing L, Wang C, et al. Effect of hemodynamics on outcome of subtotally occluded paraclinoid aneurysms after stent-assisted coil embolization. J Neurointerv Surg 2016; 8: 1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Tian Z, Liu J, et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with Enterprise stents and the Pipeline device. J Translat Med 2016; 14: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Meng Z, Zhang Y, et al. Phantom-based experimental validation of fast virtual deployment of self-expandable stents for cerebral aneurysms. Biomed Engng Online 2016; 15: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paliwal N, Yu H, Xu J, et al. Virtual stenting workflow with vessel-specific initialization and adaptive expansion for neurovascular stents and flow diverters. Comput Methods Biomech Biomed Engng 2016; 19: 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakalis NM, Mitsos AP, Byrne JV, et al. The haemodynamics of endovascular aneurysm treatment: a computational modelling approach for estimating the influence of multiple coil deployment. IEEE Trans Med Imag 2008; 27: 814–824. [DOI] [PubMed] [Google Scholar]
- 23.Mitsos AP, Kakalis NM, Ventikos YP, et al. Haemodynamic simulation of aneurysm coiling in an anatomically accurate computational fluid dynamics model: technical note. Neuroradiology 2008; 50: 341–347. [DOI] [PubMed] [Google Scholar]
- 24.Damiano RJ, Ma D, Xiang J, et al. Finite element modeling of endovascular coiling and flow diversion enables hemodynamic prediction of complex treatment strategies for intracranial aneurysm. J Biomech 2015; 48: 3332–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cebral JR, Castro MA, Putman CM, et al. Flow-area relationship in internal carotid and vertebral arteries. Physiol Measurement 2008; 29: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovblad KO, Yilmaz H, Chouiter A, et al. Intracranial aneurysm stenting: follow-up with MR angiography. J Magnet Reson Imag: JMRI 2006; 24: 418–422. [DOI] [PubMed] [Google Scholar]
- 27.Cebral JR, Mut F, Weir J, et al. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol 2011; 32: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalouhi N, Zanaty M, Whiting A, et al. Safety and efficacy of the Pipeline Embolization Device in 100 small intracranial aneurysms. J Neurosurg 2015; 122: 1498–1502. [DOI] [PubMed] [Google Scholar]
- 29.McAuliffe W, Wycoco V, Rice H, et al. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 2012; 33: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmonik C, Chintalapani G, Redel T, et al. Hemodynamics at the ostium of cerebral aneurysms with relation to post-treatment changes by a virtual flow diverter: a computational fluid dynamics study. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference 2013; 2013: 1895–1898. [DOI] [PubMed] [Google Scholar]
- 32.Xiang J, Varble N, Davies JM, et al. Initial clinical experience with AView – a clinical computational platform for intracranial aneurysm morphology, hemodynamics, and treatment management. World Neurosurg 2017; 108: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin N, Brouillard AM, Krishna C, et al. Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery 2015; 76: 142–149. [DOI] [PubMed] [Google Scholar]
- 34.Bender MT, Colby GP, Lin LM, et al. Predictors of cerebral aneurysm persistence and occlusion after flow diversion: a single-institution series of 445 cases with angiographic follow-up. J Neurosurg 2019; 130: 259–267. [DOI] [PubMed] [Google Scholar]
- 35.Fifi JT, Brockington C, Narang J, et al. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol 2013; 34: 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heller RS, Dandamudi V, Lanfranchi M, et al. Effect of antiplatelet therapy on thromboembolism after flow diversion with the pipeline embolization device. J Neurosurg 2013; 119: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 37.Brinjikji W, Lanzino G, Cloft HJ, et al. Platelet testing is associated with worse clinical outcomes for patients treated with the pipeline embolization device. AJNR Am J Neuroradiol 2015; 36: 2090–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skukalek SL, Winkler AM, Kang J, et al. Effect of antiplatelet therapy and platelet function testing on hemorrhagic and thrombotic complications in patients with cerebral aneurysms treated with the pipeline embolization device: a review and meta-analysis. J Neurointerv Surg 2016; 8: 58–65. [DOI] [PubMed] [Google Scholar]
- 39.Moshayedi H, Omofoye OA, Yap E, et al. Factors affecting the obliteration rate of intracranial aneurysms treated with a single pipeline embolization device. World Neurosurg 2017; 104: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu AH, Cheung AK, Wenderoth JD, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 2015; 36: 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004; 231: 653–658. [DOI] [PubMed] [Google Scholar]
- 42.Larrabide I, Aguilar ML, Morales HG, et al. Intra-aneurysmal pressure and flow changes induced by flow diverters: relation to aneurysm size and shape. AJNR Am J Neuroradiol 2013; 34: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]