Abstract
Background
Endovascular treatment of large complex morphology aneurysms is challenging. High recanalization rates have been reported with techniques such as stent-assisted coiling and balloon-assisted coiling. Flow diverter devices have been introduced to improve efficacy outcomes and recanalization rates. Thromboembolic complications and in-device stenosis are certainly more worrisome when treatment of bilateral internal carotid arteries has been performed. This study aimed to report our experience with mid-term imaging follow-up of staged bilateral Pipeline embolization device placement for the treatment of bilateral internal carotid artery aneurysms.
Methods
We reviewed the clinical, angiographic, and follow-up imaging data in all consecutive patients treated with bilateral internal carotid artery aneurysms who underwent elective Pipeline embolization.
Results
Six female patients were treated, harboring a total of 13 aneurysms. Of these, 60% were asymptomatic. Diplopia and headache were the most common symptoms. The most common location was the paraclinoid segment (6/13), including by cavernous segment (4/13) and ophthalmic segment (2/13). Successful delivery of the device was achieved in 12 cases. Difficult distal access precluded the deployment of the device in one case. The treatment was always staged with at least eight weeks' difference between the two procedures. All aneurysm necks were covered completely. There were no periprocedural complications. Angiographic follow-up ranged between 3 and 12 months, and computed tomography angiogram follow-up ranged between 2 and 24 months. Complete aneurysm occlusion was achieved in all cases.
Conclusion
In our series, Pipeline deployment for the treatment of bilateral internal carotid artery aneurysms in a staged fashion is safe and feasible. Mid-term imaging follow-up showed permanent occlusion of all the treated aneurysms.
Keywords: Complex intracranial aneurysms, flow diverter, Pipeline embolic device, endovascular treatment
Introduction
The primary aim of the treatment of intracranial aneurysms (IA) is to prevent rupture and subarachnoid hemorrhage (SAH). IA are the most common cause of SAH, occurring at an estimated incidence of 6–16 cases per 100,000 people per year.1 Mortality related to SAH has been reported to be as high as 67.5%.2 Full recovery after treatment is achieved in only one third of patients. Of those patients with unruptured intracranial lesions, 20% will have multiple aneurysms.3 Patients with multiple aneurysms have higher mortality and significantly poorer outcomes than those with a single aneurysm.4
The treatment methods for IA include microsurgical clipping and endovascular embolization. The latter has gained major acceptance over time due to progress in endovascular techniques and the expertise of operators, as well as technological advances that have allowed improvement in long-term outcomes. The randomized clinical International Subarachnoid Aneurysm Trial demonstrated the superior efficacy of endovascular treatment compared to microsurgical clipping. After one year post procedure, 23.5% of patients in the endovascular arm had died or were dependent compared to 30.9% in the surgical arm.5 Similarly, the International Study of Unruptured Intracranial Aneurysms indicated less common adverse outcomes in the endovascular treatment arm (9.3%) compared to the surgical treatment arm (13.7%).6
However, endovascular treatment of large complex morphology aneurysms is challenging. Bilateral aneurysms can make treatment even more challenging. High recanalization rates reported with techniques such as stent-assisted coiling and balloon-assisted coiling have resulted in the introduction of flow diverter devices to improve efficacy outcomes.7
Thromboembolic complications and in-device stenosis are certainly more worrisome when treatment of bilateral internal carotid arteries has been performed. Here, we report our experience with long-term imaging follow-up of staged bilateral Pipeline embolization device (PED) placement for the treatment of bilateral internal carotid artery (ICA) aneurysms.
Materials and methods
Patient population
The study was conducted under Institutional Review Board approval. From electronic medical records and the radiology PACS system, we reviewed the clinical, angiographic, and follow-up imaging data in all consecutive patients with bilateral ICA aneurysms who underwent elective pipeline embolization from March 2014 to January 2019.
The indications for endovascular treatment were unruptured wide-necked aneurysms and large or giant aneurysms. For each patient, information on demographics, risk factors, and clinical presentation were collected. There were six female patients with bilateral ICA aneurysms. The mean age was 46 years. These six patients harbored 13 aneurysms. Sixty percent of patients were asymptomatic, while 40% presented with headaches, facial numbness, and diplopia due to mass effect of the aneurysm (Table 1). The most common location was the paraclinoid segment (6/13), including cavernous (4/13) and ophthalmic segment aneurysms (2/13).
Table 1.
Aneurysm and device characteristics.
| Patient no. | Aneurysm | Age | Clinical presentation | Type | Location | Size (mm) | Pipeline device used (mm) | Follow-up CTA (months) | Follow-up DSA (months) | Outcome (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 31 | Incidental, previous TIA | Saccular wide neck | Right ventral paraclinoid segment | 8 | 4.25 × 18 | 2, 11, 21, 25 | 3, 6, 12 | Residual (3); occluded (6) |
| 2 | 31 | Left ophthalmic | 5.7 | 4.5 × 16 | 8, 18, 23 | 3, 6, 18 | Residual (3); occluded (8) | |||
| 2 | 3 | 37 | Incidental | Dysplastic | Left paraclinoid aneurysm | 16 | 4.25 × 20 | 3, 8 | 6 | Residual (3); occluded (8) |
| 4 | 37 | Dysplastic | Right large ventral paraclinoid | 14 | 4.25 × 18 | 1, 8 | 3, 6 | Residual (3); occluded (6, 8) | ||
| 5 | 37 | Saccular | Right ophthalmic | 5.8 | 4.25 × 18 | 3, 6, 12, 24 | 8 | Residual (3); occluded (8) | ||
| 3 | 6 | 70 | Diplopia, sixth cranial nerve palsy | Saccular | Left cavernous | 18 × 12 | 5 × 3 | 5,12 | Occluded (8) | |
| 7 | 70 | Saccular | Right cavernous | 2.12 × 8 | 4.7 × 18 | 1, 6, 12, 24 | 3, 6 | Occluded (5) | ||
| 4 | 8 | 56 | Incidental, headache, diplopia | Fusiform | Right cavernous | 12.4 × 11.1 | 4.5 × 30 | 6, 12 | Occluded (12) | |
| 9 | 56 | Saccular dysplastic multi-lobulated | Left cavernous | 37 | Attempted | |||||
| 5 | 10 | 36 | Headache, transient vision loss | Saccular | Left ophthalmic | 5.3 | 3.5 × 16 | 5, 21 | 3, 6 | Occluded (6) |
| 11 | 36 | Saccular | Right dorsal paraclinoid | 21 | 4 × 12 | 6 | 3, 21 | Occluded (6) | ||
| 6 | 12 | 36 | Asymptomatic, family history | Saccular | Right ophthalmic | 10 | 4 × 16 Coils | 4 | 2 | Occluded (2) |
| 13 | Saccular | Left cavernous | 18 | 4 × 12 | 2 | Occluded (2) |
CTA: computed tomography angiogram; DSA: digital subtraction angiography; ICA: internal carotid artery; TIA: transient ischemic attack.
Procedural information and aneurysm characteristics assessed included the morphology, location, distribution, and size. Aneurysm sizes were classified applying the definitions of small (<7 mm), large (≥7–≤25 mm), and giant (>25 mm). Wide-necked aneurysms were defined as a neck length of >4 mm. Complex aneurysms or aneurysms with branches originating from the aneurysm sac were included (see details in Table 1).
The PED
The PED is a flexible microcatheter-delivered self-expandable mesh cylinder, made of 25% platinum and 75% nickel–cobalt–chromium alloy, and has a porosity of 65–70%. The woven wires of the device provide approximately 30% metal coverage of the arterial wall surface. The PED implant is available in diameters from 2.5 to 5.0 mm and lengths from 0 to 35 mm. During delivery, the device may expand to its maximum size, which is 0.25 mm larger than nominal diameter when unconstrained across the aneurysm neck, or it may conform to the diameter of the vessel in which it is implanted.8
PED placement protocol
All patients were premedicated with aspirin 325 mg and Plavix 75 mg, starting 5 days before the procedure.
Femoral arterial access was obtained under ultrasound guidance and under general anesthesia. All patients had a triaxial approach with a Neuron Max catheter (Penumbra, Inc., Alameda, CA) in the common carotid artery, and a 5Fr Sofia 115 cm catheter (MicroVention, Inc., Tustin, CA) in the precavernous ICA. Contrast injections were performed, demonstrating the site and characteristics of the aneurysms. A Phenom microcatheter (Medtronic, Minneapolis, MN) was used to deploy the Pipeline device under continuous fluoroscopy. A single PED was used in all cases. The length of the stent used was determined by measurement of the caliber of the vessels. Coils were used in one case only. A single flow diverter was used in almost all cases, with the exception of one where three devices were deployed to achieve coverage of the neck of the aneurysm. A control injection was performed in every case to confirm complete apposition of the device to the vessel wall. Hemostasis was achieved with the Mynx closure device (AccessClosure, Mountain View, CA) and 5 minutes of manual pressure in most cases.
All patients were transferred to the intensive care unit for recovery overnight. Patients were discharged the next day on daily aspirin 325 mg and Plavix 75 mg antiplatelet therapy for 12 weeks and 81 mg aspirin thereafter.
Results
Successful delivery of the device was achieved in 10 cases. Difficult distal access precluded the deployment of the device in one case. Among the successful cases, immediate post-deployment angiography showed complete coverage of aneurysm neck, complete apposition of the distal device, and stagnation of contrast within the aneurysmal sac. Representative images can be seen in Figures 1 and 2.
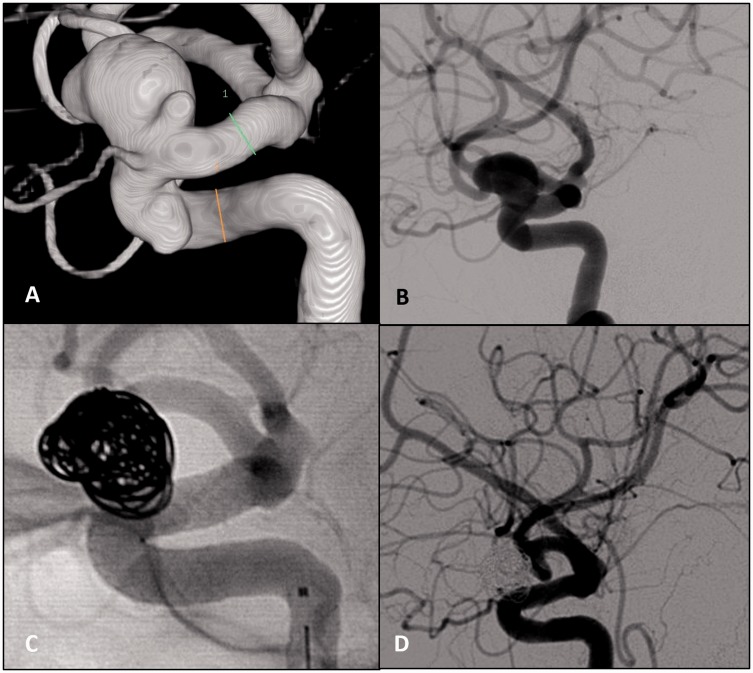
Figure 1.
(a) and (b) Three-dimensional and lateral digital subtraction angiography (DSA) of a complex carotid-ophthalmic aneurysm taken before treatment. (c) Lateral usubstracted catheter angiogram, lateral projection following placement of the Pipeline embolization device (PED). (d) Lateral DSA at two-month follow-up shows no evidence of aneurysm contrast filling.
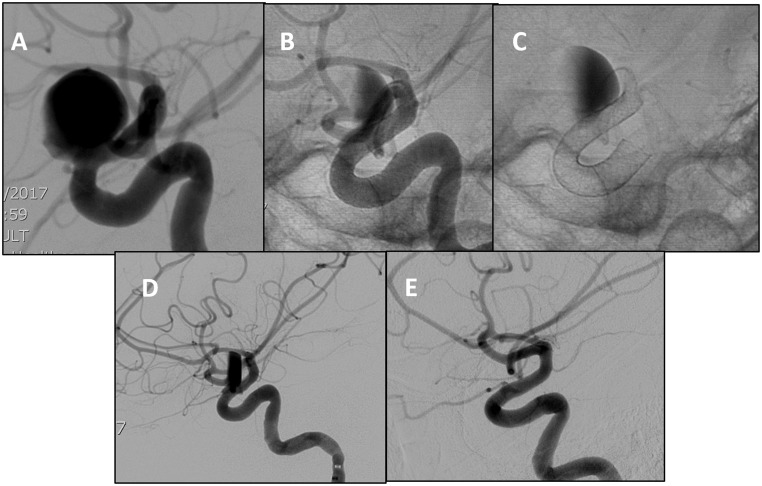
Figure 2.
(a)–(c) Lateral DSA of a large dorsal paraclinoid internal carotid artery (ICA) aneurysm. Unsubtracted images showing pre- and postoperative changes of PED embolization with contrast stagnation in the sac. (d) and (e) Lateral DSA taken immediately after surgery and at six-month follow-up. Image shows contrast stagnation and complete resolution of the aneurysm, respectively.
The treatment was staged with at least eight weeks' difference between the two procedures. One procedural complication consisting in distal stent migration occurred, requiring the deployment of a second and third flow-divert stents (Figure 3). There were no periprocedural complications.
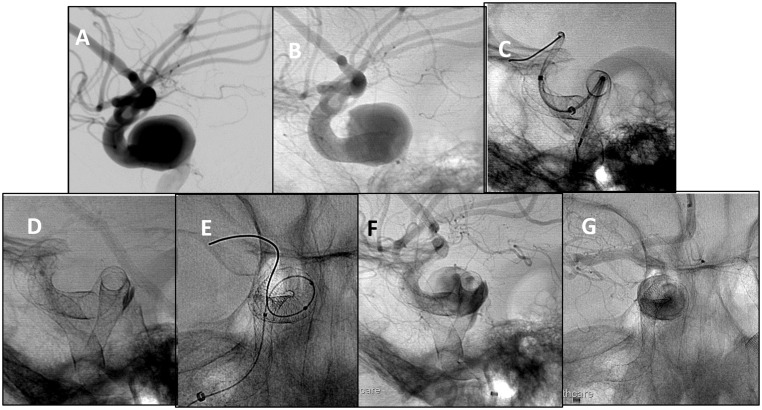
Figure 3.
(a)–(c) Lateral DSA of symptomatic large right cavernous aneurysm. The PED was placed. However, there was proximal migration in the aneurysmal sac due to severe vessel tortuosity. A second PED was deployed with proximal stent migration. (d)–(g) Lateral and AP unsubtracted angiographic images. A third PED was placed, completing the construct and covering the neck of the aneurysm. Satisfactory contrast stagnation in the aneurysmal sac is demonstrated.
Post-treatment clinical and imaging follow-up with computed tomography angiogram (CTA) or digital subtraction angiogram was performed in all cases. Follow-up in clinic was done for a mean period of 8.6 months (range 2–24 months). Among the symptomatic patients, all had improvement of their pre-existing symptoms. In all patients, pre-stent Rankin Scale scores were unchanged after the procedure.
Angiographic follow-up ranged between 3 and 12 months (M = 5.2 months), and CTA follow-up ranged between 2 and 24 months (M = 8.6 months). During follow-up, complete aneurysm occlusion was achieved in all cases (Figures 2 and 3). There were no parent artery occlusions. Asymptomatic severe in stent stenosis occurred in one patient. This patient underwent balloon angioplasty, with improvement of the stenotic segment (Figure 4).
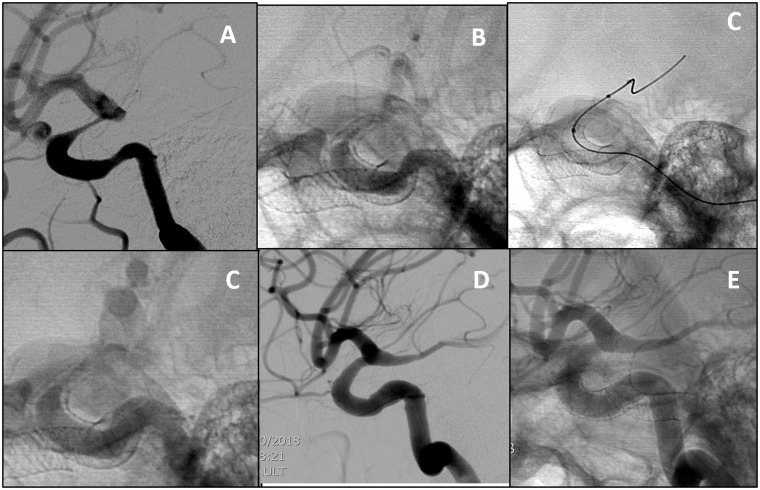
Figure 4.
Carotid ophthalmic aneurysm. (a)(b) Lateral DSA at the three-month follow-up and unsubtracted images showing severe symptomatic supraclinoid ICA stenosis due to intimal hyperplasia. (c)(d) Balloon angioplasty was performed with the Scepter XC balloon, with improvement in the caliber of the vessel at the end of the procedure. (e) and (f) Lateral DSA and unsubtracted images 12 months post procedure show only minimal narrowing and intimal hyperplasia. The patient is asymptomatic.
Discussion
The introduction of flow-diverting devices has transformed the treatment of complex aneurysms previously considered not amenable for endovascular treatment. By modifying flow dynamics, the flow diverter promotes thrombosis and intimal hyperplasia, leading to aneurysm occlusion and vessel reconstruction.9 The device's design has an important role in the hemodynamic effect. The porosity allows neck aneurysm coverage, while the pore density preserves the patency of branching vessels.10,11
The only flow diverter that has been approved by the Food and Drug Administration (FDA) for use in the USA is the PED (ev3/Covidien, Irvine, CA). Specifically, for the treatment of large and giant wide neck aneurysms of the ICA up to the superior hypophyseal segment.7,12 In this study, the complete aneurysm occlusion rate at 180 days was 73.6%, and the major complication rate (ipsilateral stroke or neurologic death) was 5.6%. The PED was successfully deployed in 99.1% of cases.13
Additional flow-diverter stents are currently available in the world and are Conformité Européenne (CE) marked, including SILK (SFD; Balt Extrusion, Montmorency, France), P64 flow modulation device (Phenox, Bochum, Germany), and the flow redirection endoluminal device system (FRED; MicroVention, Inc.).7
In 2011, the PED for the Intracranial Treatment of Aneurysms (PITA) trial evaluated PED use in unruptured aneurysms that were wide necked (>4 mm), had unfavorable dome/neck ratios (<1.5), or had failed previous therapy. The success rate was 96.8%, the aneurysm occlusion rate was 93.3% at 180 days, and the periprocedural complication rate was 6.5% with no mortality.14 Unfortunately, long-term effectiveness and safety data are lacking, as are data concerning the use of this device in bilateral or multiple aneurysms. More recently in December 2018, the FDA expanded the indications to small and medium wide-necked aneurysms in the ICA up to the terminus.
Our study demonstrates comparable outcomes to the PUFS and PITA trials. The PED was successfully placed in 90.1% of cases. During angiographic follow-up, the aneurysm occlusion progressively increased over time, and nearly all the aneurysms were occluded at six months. Retreatment rate was 10%, with no perioperative mortality or major complications. These results were similar to those reported by Becske et al. in the five-year follow-up of patients treated in the PUFS trial. The aneurysm rate occlusion at one-, three, and five-year follow-up increased over time to 86.8%, 93.4%, and 95.2%, respectively. The retreatment rate was 5.7%. Adverse events related to the device at one, three, and five years were noted in 1%, 3.5%, and 0% of subjects. The mortality rate was 3.7%.15
Thromboembolic events and in-device stenosis are well-known complications associated with flow diversion treatment. The International Retrospective Study of the PED (IntrePED), a multicenter aneurysm treatment study, reported a neurologic morbidity and mortality rate of 8.4%. Most adverse events were ischemic strokes from thromboembolic complications and were substantially more common in large, anterior circulation aneurysms.13
A strong association between the number of devices and complication rate has been described. Tan et al. identified multiple PED deployment as a significant risk factor for neurologic changes due to thromboembolic events.16 Therefore, this is more worrisome when treatment of bilateral internal carotid arteries has been performed.
Only one case of significant in-stent stenosis occurred at the 11- and 18-month angiographic follow-up, requiring retreatment with balloon angioplasty. The patient was asymptomatic. Regression of in-stent stenosis was also observed in our series (1/11). Transient in-stent stenosis has been reported previously in the literature with the use flow-diverter devices and is directly related to intimal hyperplasia relapse.9,17 Stent deployment constitutes a mechanical arterial injury promoting physiologic arterial healing with neo-intima formation and vessel reconstruction. This may be overbalanced by excessive neovascularization, leading to chronic inflammation, intimal hyperplasia, and subsequently impaired arterial healing.18,19 Intimal hyperplasia is the main contributing factor to in-stent stenosis, with dual antiplatelet therapy being the mainstay of treatment.20
The main limitations of this study include its single-arm, single-center design, with a lack of control groups and small number of patients.
Conclusion
In our series, Pipeline deployment for the treatment of bilateral ICA aneurysms in a staged fashion is safe and feasible. Mid-term imaging follow-up showed permanent occlusion of all the treated aneurysms. Further studies with larger populations are warranted.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Broderick JP, Brott TG, Duldner JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet 2017; 389: 655–666. [DOI] [PubMed] [Google Scholar]
- 3.Rinne J, Hernesniemi J, Puranen M, et al. Multiple intracranial aneurysms in a defined population: prospective angiographic and clinical study. Neurosurgery 1994; 35: 803–808. [DOI] [PubMed] [Google Scholar]
- 4.Andic C, Aydemir F, Kardes O, et al. Single-stage endovascular treatment of multiple intracranial aneurysms with combined endovascular techniques: is it safe to treat all at once? J Neurointerv Surg 2017; 9: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 5.Molyneux AJ, Birks J, Clarke A, et al. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015; 385: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RD, Torner JC, Capuano AW, et al. Aneurysm morphology and prediction of rupture : an international study of unruptured intracranial. Neurosurgery 2018; 82: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-mufti F, Amuluru K, Gandhi CD. Flow diversion for intracranial aneurysm management: a new standard of care. Neurotherapeutics 2016; 13: 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro M, Raz E, Becske T, et al. Variable porosity of the Pipeline embolization device in straight and curved vessels: a guide for optimal deployment strategy. AJNR Am J Neuroradiol 2014; 35: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buyukkaya R, Kocaeli H, Yildirim N. treatment of complex intracranial aneurysms using flow-diverting Silk® Stents: an analysis of 32 consecutive patients. Interv Neuroradiol 2014; 20: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vries J, Boogaarts J, Van Norden A, et al. New generation of flow diverter (Surpass) for unruptured intracranial aneurysms. Stroke 2013; 44: 1567–1577. [DOI] [PubMed] [Google Scholar]
- 11.Yavuz K, Geyik S, Saatci I, et al. Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the Pipeline embolization device. AJNR Am J Neuroradiol 2014; 35: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Galdámez M, Pérez S, Vega A, et al. Endovascular treatment of intracranial aneurysms using the Pipeline Flex embolization device: a case series of 30 consecutive patients. J Neurointerv Surg 2016; 8: 396–401. [DOI] [PubMed] [Google Scholar]
- 13.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 14.Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becske T, Potts MB, Kallmes DF, et al. Long-term clinical and angiographic outcomes following Pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or Failed Aneurysms trial. Neurosurgery 2017; 80: 40–48. [DOI] [PubMed] [Google Scholar]
- 16.Tan LA, Keigher KM, Munich SA, et al. Thromboembolic complications with Pipeline embolization device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 2015; 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 17.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the Pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilar Pérez M, Bhogal P, Henkes E, et al. In-stent stenosis after p64 flow diverter treatment. Clin Neuroradiol 2018; 28: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehmer GJ, Smith KJ. Drug-eluting coronary artery stents. Am Fam Physician 2009; 80: 1245–1251. [PubMed] [Google Scholar]
- 20.Berge J, Biondi A, Machi P, et al. Flow-diverter Silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. Am J Neuroradiol 2012; 33: 1150–1155. . [DOI] [PMC free article] [PubMed] [Google Scholar]