Abstract
The cancer-protective ability of hesperidin was investigated on 7, 12-dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced skin carcinogenesis in Swiss albino mice. Topical application of DMBA+TPA on mice skin led to 100% tumour incidence and rise in average number of tumours. Administration of different doses of hesperidin (HPD) before (pre) or after (post) and continuous (pre and post) DMBA application significantly reduced tumour incidence and average number of tumours in comparison to DMBA+TPA treatment alone. Topical application of DMBA+TPA increased oxidative stress as shown by significantly increased TBARS values and reduced glutathione contents, and glutathione-S-transferase, superoxide dismutase and catalase activities. Hesperidin treatment significantly reduced TBARS values and elevated glutathione concentration and glutathione-S-transferase, superoxide dismutase and catalase activities in the skin/tumors of mice treated with HPD+DMBA+TPA, HPD+DMBA+TPA+HPD or DMBA+TPA+HPD when compared to DMBA+TPA application alone. The study of molecular mechanisms showed that hesperidin suppressed expression of Rassf7, Nrf2, PARP and NF-κB in a dose dependent manner with a maximum inhibition at the level of 300 mg/kg body weight hesperidin. In conclusion, oral administration of hesperidin protected mice against chemical carcinogenesis by increasing antioxidant status, reducing DMBA+TPA induced lipid peroxidation and inflammatory response, and repressing of Rassf7, Nrf2, PARP and NF-κB levels.
Keywords: Cancer research, Biochemistry, Antioxidants, NF-κB, Mice, Nrf2, Hesperidin, Chemoprevention
Cancer research; Biochemistry; Antioxidants; NF-κB; Mice; Nrf2; Hesperidin; Chemoprevention
1. Introduction
Cancer is a multifaceted disease and varies in its presentation, development and outcome among patients, a fact that is attributed to the heterogeneity and variability of cancer cells both at molecular and cellular level. Carcinogenesis is a multistep, multipath and multifocal process encompassing a series of genetic and epigenetic changes in the cell leading to genomic instability, which finally ends up in cancer development [1]. During carcinogenesis cells experience several metabolic, behavioral and genetic alterations which lead to excessive and untimely proliferation. The cancerous cells are also able to avoid surveillance by the immune system, and finally acquire invasive characteristics leading into distant metastases [1].
The term chemoprevention was introduced by Michael Sporn in the year 1976, who defined it as the reversal of carcinogenesis process. The aim of chemoprevention is to arrest or reverse either the initiation or the progression phase of carcinogenesis into neoplastic cells [2]. Chemoprevention is the pharmacological intervention of the process of any disease so that the manifestation of the disease could be delayed or inhibited [3]. Chemoprevention in clinical oncology aims to address healthy individuals who are at higher risk of cancer production, subjects with precancerous lesions, and those patients who are at a greater risk for the induction of a second primary cancer [4]. Chemoprevention is an extensively used strategy, with great acceptance among physicians and patients similar to that of drugs that are used against cardiovascular diseases with the intention to reduce cholesterol levels and blood pressure. Chemoprevention may be also used in healthy subjects to inhibit or reduce the risk of cancer development using various pharmacological agents [5].
The pharmacological substances, which inhibit cancer by either suppressing or reversing neoplastic transformation through the restraint of one or more steps of carcinogenesis have been identified and systematically evaluated for their potential as chemopreventive agents [6]. The cancer related mortality rate could be reduced by pharmacological intervention before the onset of carcinogenesis, and the use of dietary or herbal products looks an attractive alternative due to their daily use and non-toxicity [3]. The dietary factors play an essential role in human health and provide protection against certain chronic diseases including cancer [7]. The possibility of diet modification with the intention to reduce cancer risk has recently attracted the attention of investigators worldwide, because of the correlation of the increased global human cancer mortality rates with the changes in lifestyle and dietary habits [8].
Mouse skin carcinogenesis model has become a very useful tool in studying the genetic and biological changes involved in tumour promotion [9]. The mouse skin has been used as an experimental model system to study the mechanisms of carcinogenesis and the modulation of sequential steps involved in this process for more than 50 years [10, 11]. The two-stage model of mouse skin carcinogenesis involves initiation step stimulated by topical application of a suboptimal dose of a carcinogen such as DMBA and promotion step requiring multiple treatments of a tumour promoting agent like TPA. Initiation process is irreversible and probably involves somatic mutation, whereas promotion phase is reversible at least in early stages and involves induction of altered gene expression [11]. There are various factors that accelerate the formation of skin cancer, one of them includes accumulation of unsaturated lipids. The accumulation of unsaturated lipids plays a key role in carcinogenesis as they are required in certain types of cancer [12]. Mouse skin carcinogenesis model is a very useful tool in studying the genetic and biological alterations involved in tumor promotion [13]. Many genetic changes involved in the chemical initiation of benign papillomas and their transition into squamous cell carcinoma have been well characterized in mouse skin carcinogenesis model [14].
Different natural and synthetic substances are already investigated to control carcinogen induced hyper cell proliferation in the target organ/s during the initiation and post-initiation phases of carcinogenesis [15]. A variety of beverages, vegetables, fruits, spices, medicinal plants and isolated single compounds have been examined for their chemopreventive activity in the last few decades in various models including humans [15]. The epidemiological surveys indicate that intake of citrus fruits had a conducive effect on the prevention of cancer. Moreover, polyphenolic grape seed fraction has been found to suppress tumour promotion in two stage mouse skin carcinogenesis model in a previous study [16].
Hesperidin (3,5,7 – trihydroxyflavanone 7- rhamnoglucoside) is a bioflavonoid that is a glycoside of hesperitin. French Chemist Lebreton isolated hesperidin in 1828 from the spongy inner portion of the peel (albedo) of oranges. It is also present in lemons and other citrus fruits [17]. Hesperidin is mainly used as antioxidant, since it attenuates the effects of oxidative stress by scavenging reactive oxygen species (ROS) and inhibiting cell oxidative injury [18]. In vitro application of hesperidin provides protection against the production of ROS and caspase-dependent apoptosis in cultured human polymorphonuclear neutrophils [19]. Hesperidin has been reported to possess antiallergic, antihypotensive, antimicrobial, antioxidant, vasodilator, anti-inflammatory, antihyperlipidemic, antihypertensive, hepatoprotective, nephroprotective, and cardioprotective properties [20, 21, 22, 23]. Dietary hesperidin supplementation suppressed tumour initiation and promotion and arrested the neoplastic transformation in C3H10T1/2 fibroblasts [24]. Topical and oral administration of hesperidin were effective in the healing of clean or infected wounds [25]. Hesperidin also accelerated repair and regeneration of irradiated excision wound in mice and was found to be non-toxic up to 5 g/kg body weight in mice [26, 27, 28, 29]. Dietary administration of hesperidin at the level of 5% for 13 weeks did not induce any toxic effects in different tissues [30]. It has also been reported to protect liver and kidney against the iron-induced oxidative stress [31, 32]. Hesperidin deficiency causes abnormal capillary leakage and leads to abnormal capillary permeability and fragility [33]. Therefore, the present investigation was implemented to elucidate the chemopreventive potential of hesperidin in a two stages 7, 12-dimethylbenz[a]anthracene and 12-O-tetradecanoyl phorbol-13-acetate-induced skin carcinogenesis model in Swiss albino mice.
2. Methods
2.1. Chemicals
Hesperidin (98%) (HPD) was procured from Himedia Ltd, Mumbai, India. 7,12-dimethylbenz[a]anthracene (DMBA), 12-O-tetradecanoyl-13-phorbol acetate (TPA), 1-chloro-2,4-dinitrobenzene (CDNB), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), reduced glutathione (GSH), triton X-100, ethylenediaminetetraacetic acid (EDTA), bovine serum albumin (BSA), sodium pyruvate, thiobarbituric acid (TBA), pyruvic acid, phenazine methosulphate (PMS), nitro blue tetrazolium (NBT), and reduced nicotinamide adenine dinucleotide (NADH) were obtained from Sigma Aldrich Chemical Co. (Bangalore, India). Sodium carbonate, carboxymethylcellulose (CMC), trichloroacetic acid, potassium chloride, potassium sodium-tartrate, sodium hydroxide, sodium chloride, sulphuric acid, hydrochloric acid and Tris buffer were supplied by Merck India Limited (Mumbai, India). Antibodies against Rassf7, Nrf2, PARP, NF-κB and β actin were procured from Elabscience Biotechnology Co. Ltd. (New Delhi, India).
2.2. Animal care and handling
Six to eight weeks old adult male Swiss albino mice with an average weight of 22 ± 5 g were selected from a pathogen free inbred colony maintained under a controlled environment; temperature (23 ± 2 °C), humidity (50 ± 5%) and light regimen (12 h of light and dark cycle). The animal care and handling were performed according to the guidelines issued by the World Health Organization, Geneva, Switzerland and the INSA (Indian National Science Academy, New Delhi, India). The animals had free access to sterile feed and water during the experiments. Usually five animals were kept in a polypropylene cage containing sterile paddy husk (procured locally) as bedding throughout the experiment. Institutional Animal Ethics Committee (IAEC) of the Mizoram University, Aizawl vide letter no. IAEC/4503, approved the study and all experiments were carried out in compliance with its regulations and guidelines.
2.2.1. Dissolution of hesperidin and administration
Hesperidin was weighed and dissolved in normal saline containing 0.5% carboxymethylcellulose (CMC). The animals were provided with 0, 10, 20, 30 or 40 mg/ml of freshly prepared hesperidin in drinking water daily during the experiment.
2.3. Experimental protocol
2.3.1. Induction of skin papillomas
Skin papillomas were induced in male Swiss albino mice using DMBA-TPA protocol [34]. Briefly, the hairs of mice dorsum were depilated with an electric mouse clipper (Wahl Clipper Corporation, Sterling, IL, USA) followed by the application of depilatory cream (Jolene India Ltd., Mumbai) 48 h before the application of DMBA to avoid hair regrowth. The mice that did not show signs of hair regrowth were selected for the experiments. 20 μg DMBA in 200 μl acetone/animal was topically applied on the shaved dorsum of animals twice a week with a gap of 72 hours between the two applications for two weeks. This procedure was followed by the application of 5.0 μg TPA in 200 μl acetone/animal twice a week until 24 weeks when the study was terminated.
The chemopreventive activity of hesperidin was determined by allocating the animals into the following treatment groups:
2.3.1.1. SPS control group
Animals of this group were topically applied with acetone on the skin of shaved dorsum and were provided 0.5% CMC in 0.9% sterile physiological saline (SPS) in drinking water daily during the experimental period and served as untreated control group.
2.3.1.2. SPS+DMBA+TPA group
The animals of this group received topical application of DMBA and TPA as described above (2.3.1.) for induction of skin papillomas and received no other treatment.
2.3.1.3. HPD +DMBA-TPA (Pretreatment) group
The animals of this group were given 100, 200, 300 or 400 mg/kg hesperidin in drinking water for two weeks before the 1st topical application of DMBA+TPA (2.3.1.).
2.3.1.4. DMBA+TPA+ HPD (Posttreatment) group
Animals of this group received 100, 200, 300 or 400 mg/kg hesperidin orally in drinking water continuously after 1st application of (2.3.1.) and until the termination of the experiment.
2.3.1.5. HPD +DMBA-TPA+HPD (Pre-Posttreatment) group
Animals of this group received 100, 200, 300 or 400 mg/kg hesperidin orally in drinking water continuously two weeks before DMBA+TPA application and also thereafter (2.3.1.3) until the termination of the experiment.
Usually 10 animals were used for each dose of hesperidin and a total of 140 animals were utilized to complete the entire study. Animals were initially weighed, then weekly and finally before autopsy. Papillomas appearing in the shaved area were recorded at weekly intervals and only papillomas with a diameter of more than 1 mm and if they persisted for 2 weeks or more were included in data analysis. Animals were sacrificed 24 weeks after the commencement of the treatments. The mice were euthanized under ketamine anesthesia, their dorsal skin/skin containing papillomas were removed surgically and washed in cold physiological saline. One part of skin/skin tumour was stored at -80 °C for biochemical analyses, whereas the other part was stored in 10% buffered formalin for histopathological assessment.
2.4. Histopathological assessment
The fixed tumours were dehydrated sequentially in 50%, 70%, 80%, 90% and 100% alcohol. They were kept in xylene and embedded in paraffin wax. The 5 μm thick sections were cut using a rotary microtome (Leica RM 2125 RTS, Germany) and mounted on to the glass microslides (Axiva, New Delhi, India). The slides containing sections were processed and stained with hematoxylin and eosin for histopathological examination. The slides were scored blindly by a pathologist under a transmitted light microscope (Leica DM 2500, Germany) and the tumours were classified [11].
2.5. Biochemical assays
2.5.1. Preparation of tissue homogenate
The animals from different groups were sacrificed by euthanasia after 24 weeks of 1st application of DMBA. For biochemical studies, a known amount of skin/tumours was washed in 0.9% ice cold saline, minced into small pieces with the help of scissors and forceps and 10% homogenate (W/V) was prepared in 50 mM phosphate buffer, pH 7.4 using a digital Sonicator (PCI 500F Analytics, Ahmedabad, India).
2.5.2. Estimation of reduced glutathione
The glutathione (GSH) in skin papillomas was determined by standard method [31, 32]. Briefly, 100 μl of homogenate (10% w/v) was mixed with 2.2 ml phosphate buffer (0.1 M, pH 7.4) and 0.4 ml DTNB (4 mg/1 ml) in a total volume of 3.0 ml. The yellow colour developed was read immediately at 412 nm using a UV-VIS spectrophotometer (SW 3.5.1.0. Biospectrometer, Eppendorf India Ltd., Chennai). The GSH concentration was calculated as μmol GSH/mg tissue from a standard curve.
2.5.3. Estimation of glutathione S-transferase activity
The glutathione-S-transferase (GST) activity was measured by standard method [31, 32]. Briefly, 100 μl sample homogenate, 2.4 ml phosphate buffer (0.1 M, pH 6.5), 0.2 ml reduced glutathione (1.0 mM), and 0.2 ml CDNB (1.0 mM) was added in a total volume of 3.0 ml. The changes in absorbance were recorded at 340 nm in a UV-VIS spectrophotometer and the enzymatic activity was calculated as nmol CDNB conjugate formed/min/mg protein using a molar extinction coefficient of 9.6 × 103 M−1 cm−1.
2.5.4. Estimation of catalase activity
The catalase (CAT) activity was measured according to previous studies [31, 32]. Briefly, the tissues were homogenized (10% w/v) in 50 mM phosphate buffer, pH 7.4, containing 1 mM EDTA and 1 mM PMSF. Catalase was estimated at 240 nm by monitoring the decrease of H2O2. In brief, the reaction mixture (1 ml) contained 0.02 ml of suitably diluted cytosolic sample in phosphate buffer (50 mM, pH 7.4) and 0.1 ml of 30 mM H2O2 in phosphate buffer. Changes in absorbance were recorded at 240 nm using a UV-VIS double beam spectrophotometer every 30 seconds. The catalase activity was calculated as international units (UI)/mg protein.
2.5.5. Estimation of superoxide dismutase activity
Total Superoxide dismutase (SOD) activity was measured as described earlier [31, 32]. Briefly, 900 μl buffer was mixed with 100 μl each of tissue homogenate, NBT, PMS and NADH. The control consisted of all the reagents apart from the homogenate, while the blank consisted of buffer and the homogenate without any reagents. The absorbance of sample, control and blank was read at 560 nm using a UV-Visible Spectrophotometer and the enzyme activity is expressed in units (1 U = 50% inhibition of NBT reduction).
2.5.6. Estimation of lipid peroxidation
The assay for lipid peroxidation (MDA) was performed according to standard protocol [31, 32]. Lipid peroxidation values were estimated spectrophotometrically as thiobarbituric acid reactive substances (TBARS) and are expressed in terms of malondialdehyde (MDA) formed per mg protein. In brief, 0.4 ml of homogenate was mixed with 1.6 ml of 0.15 M Tris KCl buffer, 0.5 ml of 30%TCA and 0.5 ml of 52 mM TBA and placed into a water bath for 45 min at 80 °C. The tubes were removed, cooled in ice and centrifuged at room temperature for 10 min at 3,000 rpm. The absorbance of the clear supernatant was measured against the blank of distilled water at 538 nm in UV-VIS spectrophotometer. The MDA concentration of the sample was calculated using the extinction coefficient of 1.56 × 106 M-1cm-1. The content of MDA has been expressed as nmol/mg protein.
2.5.7. Western blot analysis of Rassf7, Nrf2, PARP and NF-κB
The molecular mechanism of action of hesperidin was carried out on protein lysates for the expression of Rassf7, Nrf2, PARP and NF-κB according to the manufacturer's protocol in the tumour tissue (Elabscience Biotechnology Co. Ltd. New Delhi). Briefly, tissues were lysed in ice-cold lysis buffer. The samples were run on a 10% SDS-PAGE gel and proteins were transferred to PVDF membranes (BioRad Laboratories, Hercules, CA, USA). Membranes were probed with a 1:500 dilution of primary antibodies against Rassf7, Nrf2, PARP, NF-κB and β-actin (Elabscience Biotechnology Co. Ltd. New Delhi). The membranes were further incubated at room temperature for 1 h with horseradish peroxidase-conjugated secondary antibodies followed by reaction with ECL Plus (Amersham, St. Louis MO). Membranes were subsequently probed with a mouse monoclonal β-actin antibody (Elabscience Biotechnology Co. Ltd. New Delhi) as an internal protein loading control.
2.6. Statistical analyses
The level of significance in the alteration in the body weights after various treatments was determined using Student's t-test. The statistical significance for biochemical tests was carried out using one-way analysis of variance (ANOVA) with the application of Tukey's Post-hoc test for multiple mean comparison wherever necessary. The Origin 8 (Origin Lab Corporation, Northampton, MA, USA) and Graphpad Prism 5 (GraphPad Software, San Diego, CA, USA) statistical softwares were used for data analyses. The data are expressed as the mean ± standard error of the mean (SEM).
3. Results
3.1. Effect of various doses of HPD treatment on body weight
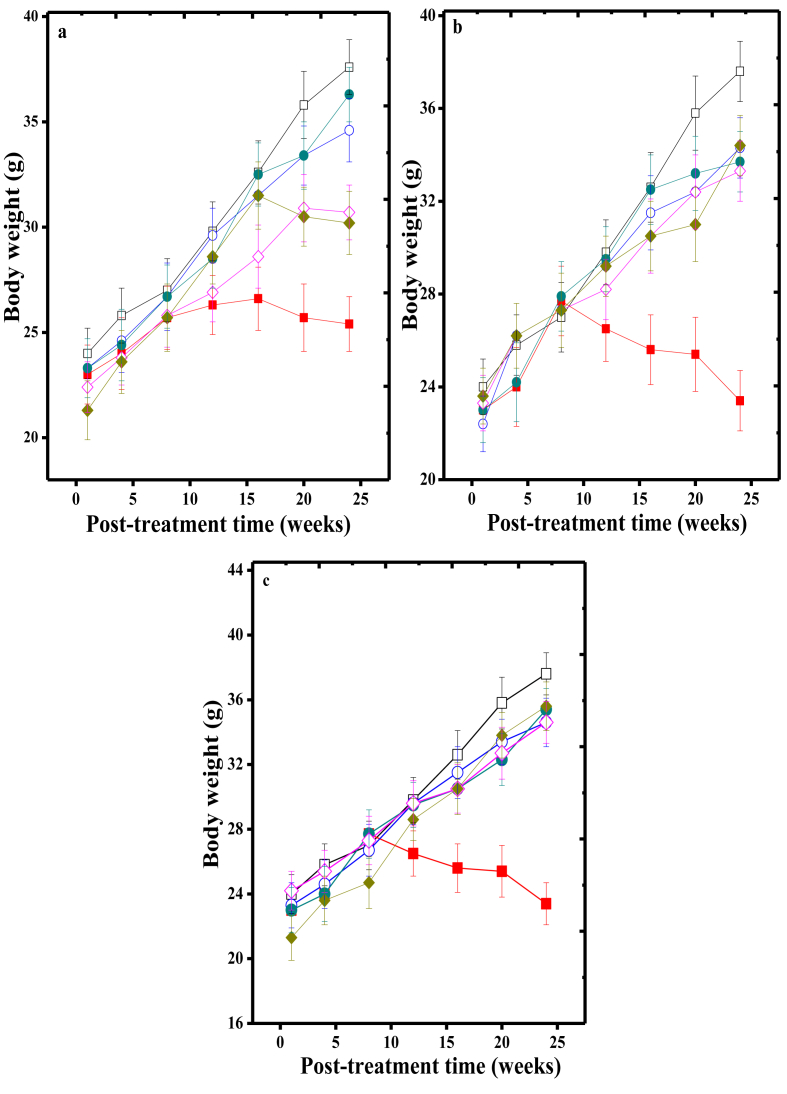
The body weights of mice ranged between 21.3 to 24.2 g at the beginning of the experiment. The average body weight was increased in all groups with time and the highest rise was observed at 24 weeks apart from SPS+DMBA+TPA group (25.4 ± 1.3 vs 37.6 ± 1.3 normal untreated), where a statistically significant decline was observed (p < 0.0001). The HPD treatment had an ameliorative effect as indicated by the increase in body weight when compared with the SPS+DMBA+TPA group (Fig. 1a,b,c).
Fig. 1.
Effect of the various doses of Hesperidin on body weight changes of albino mice receiving SPS+DMBA+TPA application for the induction of skin papilloma. a: Pre-treatment; b: Post-treatment and c: Pre-post treatment. Open squares: Sterile physiological saline (normal); Closed squares: SPS+DMBA-TPA (carcinogen treatment alone); Open circle: 100 mg/kg body weight Hesperidin; Closed circles: 200 mg/kg body weight Hesperidin; Open diamonds: 300 mg/kg body weight Hesperidin and Closed diamonds: 400 mg/kg body weight Hesperidin. The data are expressed as Mean ± Standard error of the mean
3.2. Effect of various doses of HPD treatment on tumour induction
The chemopreventive effect of HPD on DMBA+TPA induced tumours in mice is depicted in Fig. 2 a,b,c,d). Application of DMBA+TPA caused appearance of skin papilloma after 6 weeks of first DMBA application. The evaluation of tumour incidence at the end of the experiment (24 week) mice showed 100% tumour incidence in SPS+DMBA+TPA group (Fig. 2a), whereas HPD treatment after cancer initation reduced the tumor incidence in a dose dependent manner by 15% (100 mg/kg), 27.37% (200 mg/kg), 45% (300) and 31% (400 mg/kg) and the lowest incidence was observed for 300 mg/kg followed by 400 mg/kg in the DMBA+TPA+HPD group (Fig. 2a). The analysis of data on linear and linear quadratic models did not show any clear fitting on either equation. However, when the data of 400 mg/kg HPD was excluded from the analysis a clear linear dose response (r2 = 0.99, p < 0.01) was observed. A similar effect was observed in HPD+DMBA+TPA+HPD treatments; HPD oral administration reduced the average number (tumor multiplicity) of tumours in mice in a dose dependent manner and a maximum reduction (p < 0.01) was observed for 300 mg/kg body weight HPD in HPD+DMBA+TPA, DMBA+TPA+HPD and HPD+DMBA+TPA+HPD groups (Fig. 2b,c,d). The fitting of data of tumor multiplicity on linear model revealed the linear dose response for HPD+DMBA+TPA (r2 = 0.90, p < 0.02), DMBA+TPA+HPD (r2 = 0.97, p < 0.01) and HPD+DMBA+TPA+HPD (r2 = 0.66, NS) groups. The maximum reduction in tumor incidence and multiplicity was observed for the group receiving HPD after DMBA+TPA treatment (Fig. 2a,c).
Fig. 2.
Induction of skin papilloma after application of DMBA+TPA or oral administration of various doses of Hesperidin treatment in Swiss albino mice. a: Tumor incidence in Hesperidin post-treated group; b: Average number of tumors scored in Hesperidin pretreated group; c: Average number of tumors scored in Hesperidin post-treated group and d: Average number of tumors scored in pre and post-treated Hesperidin treated group. Open squares: SPS+DMBA+TPA (carcinogen treatment alone); Closed squares: 100 mg/kg body weight Hesperidin; Open circles: 200 mg/kg body weight Hesperidin; Closed circles: 300 mg/kg body weight Hesperidin and Closed stars: 400 mg/kg body weight Hesperidin. The data are expressed as Mean ± Standard error of the mean. N = 10; p < 0.01 when HPD+DMBA+TPA, DMBA+TPA+HPD and HPD+DMBA+TPA+HPD groups compared with SPS+DMBA+TPA group at all times. SPS: Sterile physiological saline; DMBA: 7,12-dimethylbenzanthracene and 12-O-tetradecanoyl-13-phorbol acetate (TPA).
3.3. Histopathology
The histopathological examination of papilloma confirmed the cancerous nature of DMBA-induced papilloma in mice (Fig. 3). Application of DMBA+TPA caused transformation of normal cells into neoplastic cells indicated by hyperplasia in the skin that led to the development of skin papilloma, whereas HPD treatment reduced the neoplastic transformation in the skin topically applied with DMBA+TPA (Fig. 3).
Fig. 3.
Cross section of normal skin and skin papilloma on 24th week post SPS+DMBA+TPA application representing malignant transformation in skin of Swiss albino mice. a: Normal skin, Note thin epidermis and normal dermis; b: SPS+DMBA+TPA; Note thickening of epidermis and proliferantion of neoplastic cells and tumor stroma; c: DMBA+TPA, Hyperplastic neoplasia; d: formation of papilloma; e: 100 mg/kg hesperidin treatment; f: 200 mg/kg Hesperidin treatment; g: 300 mg/kg Hesperidin treatment and h: 400 mg/kg Hesperidin treatment, Note the reduced neoplastic transformation after hesperidin treatment (e–f). SPS: Sterile physiological saline; DMBA: 7,12-dimethylbenzanthracene and 12-O-tetradecanoyl-13-phorbol acetate (TPA).
3.4. Determination of antioxidant status
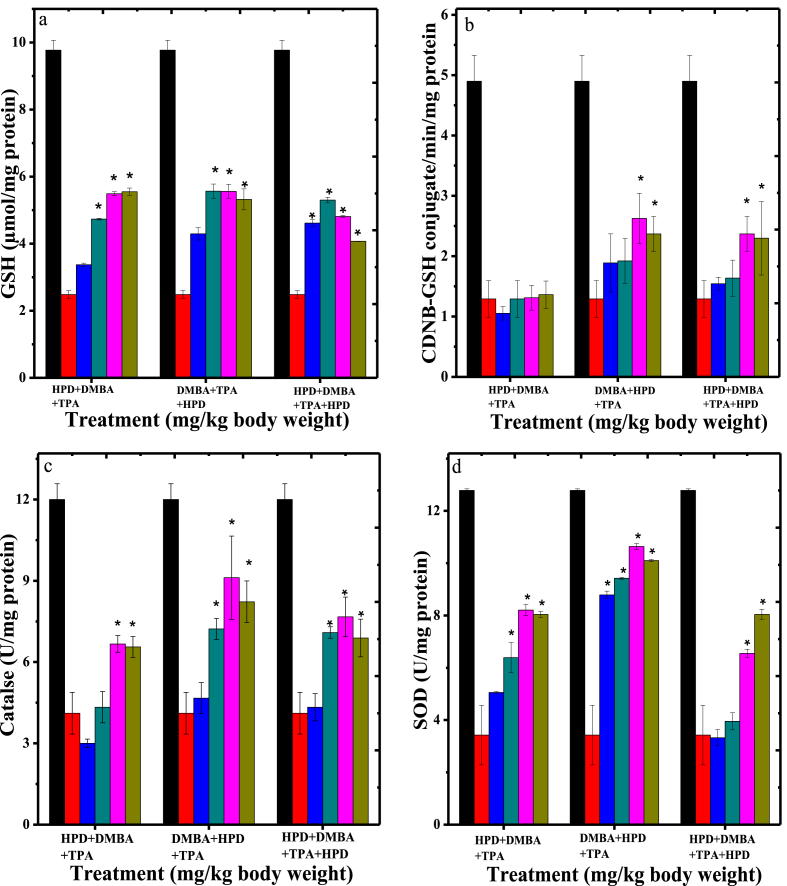
The effect of HPD treatment on DMBA+TPA induced oxidative stress has been determined by estimating various antioxidants in tumour tissue including GSH, GST, catalase, SOD and lipid peroxidation (Figs. 4 and 5).
Fig. 4.
Alteration in the Glutathione contents (a), activities of Glutathione-s-transferase (b), Catalase (c) and Superoxide dismutase (d) in the skin papilloma of mice treated with different doses of Hesperidin. Black bars: Sterile physiological saline (SPS); Red bars: SPS+DMBA+TPA; Blue bars: 100 mg/kg body weight Hesperidin; Cyan bars: 200 mg/kg body weight Hesperidin; Pink bars: 300 mg/kg body weight Hesperidin and Olive-green bars: 400 mg/kg body weight Hesperidin. The data represent Mean± Standard error of the mean. N = 10; *p < 0.01 when Hesperidin treatment groups are compared to SPS+DMBA+TPA group. SPS: Sterile physiological saline; DMBA: 7,12-dimethylbenzanthracene and 12-O-tetradecanoyl-13-phorbol acetate (TPA).
Fig. 5.
Alteration in the lipid peroxidation in the skin papilloma of mice treated with different doses of hesperidin. Black bars: Sterile physiological saline (SPS); Red bars: SPS+DMBA+TPA; Blue bars: 100 mg/kg body weight Hesperidin; Cyan bars: 200 mg/kg body weight Hesperidin; Pink bars: 300 mg/kg body weight Hesperidin and Olive-green bars: 400 mg/kg body weight Hesperidin. The data represent Mean ± Standard error of the mean. N = 10; *p < 0.01 when hesperidin treatment groups are compared to SPS+DMB+TPA group. SPS: Sterile physiological saline; DMBA: 7,12-dimethylbenzanthracene and 12-O-tetradecanoyl-13-phorbol acetate (TPA).
3.4.1. Glutathione
Induction of chemical carcinogenesis resulted in an approximate 3.2 folds reduction in the GSH concentration in the skin/tumours (Fig. 4a). This decline in GSH concentration was significantly higher (p < 0.01) when compared with non-drug-non-carcinogen treated control group (Fig. 4a). Administration of various doses of HPD to the animals receiving topical application of chemical carcinogens significantly increased the GSH levels in a dose dependent manner in all groups when compared to carcinogen treatment alone (Fig. 4a). The GSH contents increased approximately by 1.5 folds (p < 0.01) for 100, and 200 mgkg, whereas this increase was 2.2 folds for 300 and 400 mg/kg body weight HPD in HPD+DMBA+TPA group (Fig. 4a). The administration of 100, 200, 300 and 400 mg/kg HPD enhanced the GSH approximately by 2.2 folds (p < 0.01) in DMBA+TPA+HPD group. The continuous treatment (pre-post) with 100, 200, 300 and 400 mg/kg body weight HPD in drinking water resulted in an increase in GSH concentration approximately by 1.8, 2, 3.5 and 3.2 folds, respectively (Fig. 4a).
3.4.2. Glutathione S-transferase activity
Application of chemical carcinogens on to mouse skin resulted in an approximate 3.8 folds (p < 0.01) reduction in the GST activity in mouse skin tumours at 24 weeks post carcinogen treatment (Fig. 4b). HPD treatment resulted in an elevation in the GST activity when compared to the SPS group. Pre-treatment of HPD in drinking water did not alter GST activity at 24 weeks post-carcinogen treatment, when compared to SPS+DMBA+TPA group (Fig. 4b). The HPD post treatment at a dose of 100, 200, 300 and 400 mg/kg body weight in drinking water revealed a significant rise (p < 0.01) in the GST activity when compared to SPS+DMBA+TPA treatment alone, which was approximately 1.7 folds higher for this group, whereas the continuous treatment also enhanced GST activity (p < 0.01) approximately by 1.5 folds (Fig. 4b).
3.4.3. Catalase activity
Application of DMBA+TPA on mouse skin resulted in a decline in the catalase activity approximately by 2.9 folds when compared to untreated group (Fig. 4c). However, administration of different doses of HPD to carcinogen treated mice led to a significant (p < 0.01) elevation in the catalase activity in skin tumours at 24 weeks post-carcinogen treatment (Fig. 4c). The maximum elevation in the catalase activity was observed for 300 mg/kg body weight of HPD in all the groups and this rise at this dose was approximately 1.6, 2.2 and 1.9 folds for pre, post and pre-post HPD treatment when compared to carcinogen treatment alone (Fig. 4c).
3.4.4. Superoxide dismutase activity
Chemical carcinogenesis significantly (p < 0.01) reduced the SOD activity in the skin/tumours of mice at 24 weeks post-carcinogen treatment (Fig. 4d). Treatment of mice with different doses of HPD during carcinogenesis elevated SOD activity significantly (p < 0.01) in the skin tumours at 24 weeks post-carcinogen treatment in all the groups. Administration of different doses of HPD caused a dose dependent elevation in the SOD activity in all the groups up to 300 mg/kg body weight, where the SOD activity was greatest (Fig. 4d). This increase in SOD activity was approximately 2.4, 3 and 1.9 folds higher at 300 mg/kg in pre, post and pre-post HPD treated groups when compared to SPS+DMBA+TPA treatment alone.
3.4.5. Lipid peroxidation
Application of DMBA followed by TPA on mouse skin for the induction of chemical carcinogenesis caused a time dependent elevation in MDA that were approximately 23 folds (p < 0.01) higher in the skin tumours after 24 weeks post-carcinogen treatment (Fig. 5). Administration of 100, 200, 300 and 400 mg/kg body weight HPD in drinking water significantly inhibited the induction of MDA in the skin tumours of mice in a dose dependent manner in pre, post and pre-post HPD treated groups (Fig. 5) and the lowest lipid peroxidation was observed at 400 mg/kg in all the groups, except pre-post HPD group where it was greater than 300 mg/kg HPD (Fig. 5). The decline in lipid peroxidation was 1.7, 2 and 1.24 folds lower than SPS+DMBA+TPA treatment in pre, post and pre-post HPD treatment at 400 mg/kg HPD (Fig. 5).
3.4.6. Molecular markers
The western blot analysis of Rassf7, Nrf2, PARP and NF-κB showed a rise in the activity of these proteins after carcinogen treatment whereas hesperidin administration reduced the expression of Rassf7, Nrf2, PARP and NF-κB genes in a dose dependent manner and a maximum suppression was observed at 300 mg/kg body weight (Fig. 6).
Fig. 6.
Western blot analysis (Top) of Nrf2, Rassf7, PARP and NF-κB in the skin papillomas of mice treated with different doses of Hesperidin (HPD) immediately after DMBA+TPA application. The tumors were removed at 24 weeks after DMBA+TPA application for protein expression. a: SPS+DMBA+TPA alone, b: DMBA+TPA+100 mg/kg body weight HPD, c: DMBA+TPA+200 mg/kg body weight HPD, d: DMBA+TPA+ 300 mg/kg body weight HPD, e: DMBA+TPA+ 400 mg/kg body weight HPD. Bottom: relative expression of different proteins after treatment with 100, 200, 300 and 400 mg/kg body weight Hesperidin immediately after DMBA+TPA application. Black bars: β-actin; Red bars: RassF7; Blue bars: Nrf-2; Cyan bars: PARP and Pink bars: NF-κB. DMBA: 7,12-dimethylbenzanthracene and 12-O-tetradecanoyl-13-phorbol acetate (TPA). Full blot images of RassF7, Nrf-2, PARP and NF-κB are not available since the hard disk storing digital images has crashed and data are beyond retrieval.
4. Discussion
The increased rates of cancer induced mortality indicate that strategies are required to reduce the occurrence of cancer. Skin cancer is emerging as one of the common malignancies throughout the world and its incidence is constantly rising. The experimental models of skin carcinogenesis provide a tool to understand the process of cancer development and devise strategies to control it in a preclinical setting [10, 36]. In fact, vegetarian diet and several phytoceuticals have been found to reduce the risk of cancer development [37]. It may be worthwhile to explore the possible chemopreventive action of dietary ingredients in preclinical experimental setting. Therefore, the present study was conducted to investigate the chemopreventive effect of hesperidin, a bioflavonoid present in various citrus fruits in DMBA+TPA induced two stage skin carcinogenesis in the Swiss albino mice.
The topical application of DMBA+TPA on mouse skin induced skin papillomas efficiently, since tumours were developed in all the mice. DMBA+TPA application has been reported to induce skin papillomas in various strains of mouse earlier [10, 35]. Administration of different doses of HPD in drinking water to carcinogen treated mice significantly reduced tumour incidence and average number of tumours. A recent study has reported suppression in benzo-a-pyrene induced lung carcinoma in mice administered with hesperitin [36]. Hesperidin and hesperitin also inhibited neoplastic transformation of C3H 10T1/2 fibroblasts completely in a previous study [24]. In an experiment with rats treated with orange juice, a 22% reduction in colon cancer was observed [37]. Similarly, (−)-epi-gallocatechin-3-gallate, baicalein, genistein, oroxylin A, galangin and quercetin have been reported to be active against hepatocarcinogenesis [38]. Another flavonoid, isorhamnetin has been found to inhibit colorectal cancer in mouse [39]. Many other fruits, plant extracts and phytochemicals have been reported to exert chemopreventive action in vitro and in vivo [40, 41].
The chemopreventive effect of HPD seems to be due to the inhibition of initiation and promotion phases of carcinogenesis. The majority of the vivo studies on chemopreventive agents that reported significant reduction in the tumour formation, have been carried out until 20 weeks [42], whereas our study lasted 24 weeks. The analysis of 21- and 24-week data showed that the percentage of tumour inhibition was statistically significant up to 21 weeks (47.4%; p < 0.05) and the differences between these two time points were statistically non-significant indicating that the maximum effect was already attained by 21 weeks.
In multistep carcinogenesis, reactive oxygen species are indispensable mostly in the promotion phase to sustain inflammation required for cell proliferation and tumour progression [43]. The inflammatory response induced by TPA changes the fidelity of genome by inducing additional mutations in the cell and triggers the initiated cell to proliferate and form tumours [44]. The development of tumours after DMBA+TPA application in the present study may be an effect of the increased oxidative stress and sustained inflammatory response as indicated by a sharp reduction in GSH and GST, CAT and SOD activities accompanied by increased MDA values. HPD treatment inhibited the DMBA+TPA-induced reduction in GSH, GST, CAT and SOD and reduced MDA values, a fact that may be directly linked to the reduction in the tumour incidence and tumour multiplicity indicating that HPD activity was crucial in ameliorating oxidative stress-mediated carcinogenesis observed in this study. The GSH is a biological antioxidant present in high concentrations, and offers protection against oxidative damage [34, 45, 46]. The reduced levels of GST have been correlated with tumour induction provoked by DMBA/TPA treatment [34]. DMBA/TPA treatment has been reported to attenuate antioxidant enzymes including SOD and CAT in mice as well as squamous cell carcinomas [47]. It is well known that the antioxidant action of GSH is greater than that of SOD or CAT [48]. The lipoxygenase and cyclooxygenase are involved in tumorigenesis whose activities are changed by GSH [49]. The function of GSH has been found to be highly inconsistent and contradictory, depending on the cell type, nature of the carcinogen and pathways modulated by it [47]. The chemopreventive action of many flavonoids and phytochemicals has been attributed to their ability to elevate GSH in mouse skin [49].
The negative alteration in the antioxidant enzyme(s) leads into a shift in the intracellular oxidation/reduction balance favoring oxidative stress, which changes cell and organ sensitivity to tumorigenesis induced by physical or chemical agents. Oxidative stress stimulates the production of MDA and/or other aldehydes in the biological systems. These oxidative products can react with amino acids and/or DNA introducing cross linkages between proteins and nucleic acids, and may also induce alterations in DNA replication and transcription leading to tumour formation [34, 50]. Lipid peroxidation induced by reactive oxygen species (ROS) might be involved in tumour progression and promotion of carcinogenesis [51]. DMBA+TPA induced oxidative stress plays a pivotal role in mouse skin carcinogenesis is consistent with our findings, where topical application of DMBA+TPA led to a significant rise in MDA in skin/tumours of animals. An identical effect has been previously observed in the mouse skin carcinogenesis [45, 46]. Treatment of HPD in DMBA+TPA applied mice significantly lowered the oxidative stress as shown by the reduced MDA levels that may have caused reduction in tumour production. Certain flavonoids and Opuntia humifusa did lower MDA levels in mouse skin/skin tumours in previous studies [45, 52]. The alleviated levels of MDA may be directly correlated to the increase of the antioxidant enzymes' levels in our study. The use of antioxidants has been reported to act as protective agents against cancer [7, 53, 54].
The exact mechanism of action of hesperidin against DMBA+TPA induced carcinogenesis is not fully understood. Its action could be mediated through multiple putative mechanisms to inhibit DMBA+TPA induced carcinogenesis. Hesperidin is known to inhibit free radical formation and this action of hesperidin may have inhibited DMBA/TPA induced free radicals that may have subsequently reduced the mutagenic effect of latter reducing the tumour incidence. The lipid peroxidation is known to induce different kind of DNA damages leading to mutagenesis and neoplastic transformation [51]. The decrease of lipid peroxidation values and the increase in the GSH contents, GST, catalase and SOD activity may have reduced the DMBA/TPA induced oxidative stress and inflammation, which are directly involved in the process of carcinogenesis and thereby reducing the tumour incidence. The activation of NF-κB signaling has been reported to play a crucial role in DMBA+TPA induced skin carcinogenesis [55]. The investigation of the molecular mechanisms revealed that hesperidin reduced DMBA+TPA induced expression of NF-κB, RassF7, PARP, and Nrf2, which are hyperactivated in inflammation, cell proliferation and carcinogenesis, alleviating tumour incidence in the present study. Hesperidin and naringin have been found to inhibit the NF-κB, and COX-II activation earlier [56, 57, 58].
5. Conclusions
The chemopreventive action of HPD may be due to its ability to suppress DMBA+TPA induced free radicals, increase antioxidant enzymes and reduce oxidative stress as revealed by attenuated lipid peroxidation. This may have reduced neoplastic transformation and lowered the tumour incidence in the HPD treated groups. At molecular level hesperidin inhibited DMBA+TPA induced PARP, NF-κB, RassF7 and Nrf2 activation that seems to suppress sustained inflammation leading to reduction in tumour induction.
Declarations
Author contribution statement
Mathipi Vabeiryureilai: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Khawlhring Lalrinzuali: Performed the experiments; Analyzed and interpreted the data.
Ganesh Chandra Jagetia: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Council of Scientific and Industrial Research in the form of Junior Research Fellowship to MV, and University Grants Commission, Government of India, New Delhi, in the form of financial assistance to carry out this study to GCJ vide Grant No. F4-10/2010(BSR) UGC.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Sporn M.B., Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21(3):525–530. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 3.Lippman S.M., Hawk E.T. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69(13):5269–5284. doi: 10.1158/0008-5472.CAN-09-1750. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U., Bonanni B. Chemoprevention: from research to clinical oncology. Eur. J. Cancer. 2005;41(13):1833–1841. doi: 10.1016/j.ejca.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald P. Science, medicine, and the future: cancer chemoprevention. Br. Med. J. 2002;24(7339):714–718. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang M.T. Inhibitory effects of curcumin on tumorigenesis in mice. J. Cell. Biochem. 1997;27:26–34. [PubMed] [Google Scholar]
- 7.Kotecha R., Takami A., Espinoza J.L. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7(32):52517–52529. doi: 10.18632/oncotarget.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMarco-Crook C., Xiao H. Diet-based strategies for cancer chemoprevention: the role of combination regimens using dietary bioactive components. Ann. Rev. Food Sci. Technol. 2015;6:505–526. doi: 10.1146/annurev-food-081114-110833. [DOI] [PubMed] [Google Scholar]
- 9.Holden P.R., McGuire B., Stoler A., Balmain A., Pitts J.D. Changes in gap junctional intercellular communication in mouse skin carcinogenesis. Carcinogenesis. 1997;18(1):15–21. doi: 10.1093/carcin/18.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Yuspa S.H. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis—thirty-third GHA Clowes Memorial Award Lecture. Cancer Res. 1994;54:1178–1189. [PubMed] [Google Scholar]
- 11.Abel E.L., Angel J.M., Kiguchi K., DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat. Protoc. 2009;4(9):1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black H.S. Utility of skin/UV carcinogenesis model for evaluating the role of nutritional lipids in cancer. In: Roe D.A., editor. Diet, Nutrition and Skin Cancer: from Basic Research to Policy Implications. Alan R. Liss, Inc; New York: 1983. pp. 49–60. [Google Scholar]
- 13.Huang P.Y., Balmain A. Modeling cutaneous squamous carcinoma development in the mouse. Cold Spring Harbor Perspect. Med. 2014;4(9):a013623. doi: 10.1101/cshperspect.a013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong W.K., Sporn M.B. Recent advances in chemoprevention of cancer. Science. 1997;278(5340) doi: 10.1126/science.278.5340.1073. 1073-1037. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T., Tanaka T., Tanaka M., Kuno T. Cancer chemoprevention by citrus pulp and juices containing high amounts of β-cryptoxanthin and hesperidin. BioMed Res. Int. 2011;24:2012. doi: 10.1155/2012/516981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Wang J., Chen Y., Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20(9):1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 17.Garg A., Garg S., Zaneveld L.J., Singla A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res. 2001;15(8):655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 18.Pires Das Neves R.N., Carvalho F., Carvalho M., Fernandes E., Soares E., Bastos M.D., Pereira M.D. Protective activity of hesperidin and lipoic acid against sodium arsenite acute toxicity in mice. Toxicol. Pathol. 2004;32(5):527–535. doi: 10.1080/01926230490502566. [DOI] [PubMed] [Google Scholar]
- 19.Zielińska-Przyjemska M., Ignatowicz E. Citrus fruit flavonoids influence on neutrophil apoptosis and oxidative metabolism. Phytother Res. 2008;22(12):1557–1562. doi: 10.1002/ptr.2449. [DOI] [PubMed] [Google Scholar]
- 20.Tripoli E., La Guardia M., Giammanco S., Di Majo D., Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104(2):466–479. [Google Scholar]
- 21.Jain D.P., Somani R.S. Antioxidant potential of hesperidin protects gentamicin induced nephrotoxicity in experimental rats Austin. J. Pharmacol. Ther. 2015;3(2):1071. [Google Scholar]
- 22.Vabeiryureilai M., Lalrinzuali K., Jagetia G.C. Determination of anti- inflammatory and analgesic activities of a citrus bioflavanoid, hesperidin in mice. Immunochem. Immunopathol. Open Access. 2015;1(107):2. [Google Scholar]
- 23.Jagetia G.C. A review on the Anti-inflammatory activity of hesperidin, a bioflavonoid synthesized by citrus fruits. J. Immunol. Inflamm. Dis. Ther. 2018;1:10037. [Google Scholar]
- 24.Franke A.A., Cooney R.V., Custer L.J., Mordan L.J., Tanaka Y. Inhibition of neoplastic transformation and bioavailability of dietary flavonoid agents. Adv. Exp. Med. Biol. (1998) 2007;439:237–248. doi: 10.1007/978-1-4615-5335-9_17. [DOI] [PubMed] [Google Scholar]
- 25.Hasanoglu A., Ara C., Ozen S., Kali K., Senol M., Ertas E. Efficacy of micronized flavonoid fraction in healing of clean and infected wounds. Int. J. Angiol. 2001;10(1):41–44. doi: 10.1007/BF01616343. [DOI] [PubMed] [Google Scholar]
- 26.Jagetia G.C., Rao K.V.N.M. Topical application of hesperidin, a citrus bioflavanone accelerates healing of full thickness dermal excision wounds in mice exposed to 6 Gy of whole body γ-radiation. Clin. Res. Dermatol. 2017;4(3):1–8. [Google Scholar]
- 27.Jagetia G.C., Rao K.V.N.M. Hesperidin a citrus bioflavonoid potentiates repair and regeneration of deep dermal excision wounds of mice whole body exposed to different doses of 60Co γ-radiation. Clin. Dermatol. J. 2018;3(2) [Google Scholar]
- 28.Jagetia G.C., Rao K.V.N.M. Hesperidin treatment abates radiation-induced delay in healing of deep cutaneous excision wound of mice hemi-body exposed to different doses of γ-Radiation. Clin. Dermatol. Dermatitis. 2018;1(1):104. [Google Scholar]
- 29.Jagetia G.C., Rao K.V.N.M. Acceleration in the repair and regenerative responses by different doses of hesperidin in the deep full thickness cutaneous wound of mice whole body exposed to 6 Gy of γ -radiation. Nurs. Hlth. Care Int. J. 2018;2(3) [Google Scholar]
- 30.Kawabe M., Tamano S., Shibata M.A., Hirose M., Fukushima S., Ito N. Subchronic toxicity study of methyl hesperidin in mice. Toxicol. Lett. 1993;69:37–44. doi: 10.1016/0378-4274(93)90143-l. [DOI] [PubMed] [Google Scholar]
- 31.Jagetia G.C. Lalramthari. Attrition of iron-induced biochemical injury in mice kidney by a citrus bioflavonoid, hesperidin. Biochem. Physiol. 2018;7:240. [Google Scholar]
- 32.Jagetia G.C., Lalrinpuii T. Hesperidin a citrus bioflavonoid attenuates iron-induced biochemical oxidative stress in mouse liver Biomed. J. Sci. Tech. Res. 2018;8(1) BJSTR MS.ID.001602. [Google Scholar]
- 33.Selsman G.J., Horoschak S. The treatment of capillary fragility with a combination of hesperidin and vitamin C. Am. J. Dig. Dis. 1950;17(3):92–94. [Google Scholar]
- 34.Dhawan D., Balasubramanian S., Amonkar A.J., Singh N. Chemopreventive effect of 4′-demethyl epipodophyllotoxin on DMBA/TPA-induced mouse skin carcinogenesis. Carcinogenesis. 1999;20(6):997–1003. doi: 10.1093/carcin/20.6.997. [DOI] [PubMed] [Google Scholar]
- 35.Neagu M., Caruntu C., Constantin C., Boda D., Zurac S., Spandidos D.A., Tsatsakis A.M. Chemically induced skin carcinogenesis: updates in experimental models. Oncol. Rep. 2016;35:2516–2528. doi: 10.3892/or.2016.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodduluru L.N., Kasala E.R., Barua C.C., Karnam K.C., Dahiya V., Ellutla M. Antiproliferative and antioxidant potential of hesperetin against benzo (a) pyrene induced lung carcinogenesis in Swiss albino mice. Chem. Biol. Interact. 2015;242:345–352. doi: 10.1016/j.cbi.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Miyagi Y., Om A.S., Chee K.M., Bennink M.R. Inhibition of azoxymethane-induced colon cancer by orange juice. Nutr. Cancer. 2000;36(2):224–229. doi: 10.1207/S15327914NC3602_12. [DOI] [PubMed] [Google Scholar]
- 38.Leão M.B., Pavão A.C., Espinoza V.A., Taft C.A., Bulnes E.P. A multivariate model of chemical carcinogenesis. J. Mol. Struct. Theochem. 2005;719:129–135. [Google Scholar]
- 39.Saud S.M., Young M.R., Jones-Hall Y.L., Ileva L., Evbuomwan M.O., Wise J., Colburn N.H., Kim Y.S., Bobe G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013;73(17):5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan M.H., Ho C.T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008;37(11):2558–2574. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- 41.Xiao J., Ni X., Kai G., Chen X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013;53(5):497–506. doi: 10.1080/10408398.2010.548108. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y.P., Lou Y.R., Yen P., Newmark H.L., Mirochnitchenko O.I., Inouye M., Huang M.T. Enhanced skin carcinogenesis in transgenic mice with high expression of glutathione peroxidase or both glutathione peroxidase and superoxide dismutase. Cancer Res. 1997;57(8):1468–1474. [PubMed] [Google Scholar]
- 43.Closa D., Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56(4):185–191. doi: 10.1080/15216540410001701642. [DOI] [PubMed] [Google Scholar]
- 44.Rundhaug J.E., Fischer S.M. Molecular mechanisms of mouse skin tumor promotion. Cancers. 2010;2(2):436–482. doi: 10.3390/cancers2020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagetia G.C., Reddy T.K. The grape fruit flavonone naringin protects mice against doxorubicin-induced cardiotoxicity. J. Mol. Biochem. 2014;3(1) [Google Scholar]
- 46.Lushchak V.I. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberley T.D., Oberley L.W. Oxygen radicals and cancer. In: Yu B.P., editor. Free Radicals in Aging. CRC Press; Boca Raton, FL: 1993. pp. 247–267. [Google Scholar]
- 48.Artali R., Beretta G., Morazzoni P., Bombardelli E., Meneghetti F. Green tea catechins in chemoprevention of cancer: a molecular docking investigation into their interaction with glutathione S-transferase (GST P1-1) J. Enzym. Inhib. Med. Chem. 2009;24:287–295. doi: 10.1080/14756360802177282. [DOI] [PubMed] [Google Scholar]
- 49.Capdevila J.H., Morrow J.D., Belosludtsev Y.Y., Beauchamp D.R., DuBois R.N., Falck J.R. The catalytic outcomes of the constitutive and the mitogen inducible isoforms of prostaglandin H2 synthase are markedly affected by glutathione and glutathione peroxidase (s) Biochemistry. 1995;34(10):3325–3337. doi: 10.1021/bi00010a023. [DOI] [PubMed] [Google Scholar]
- 50.Perchellet J.P., Perchellet E.M. Antioxidants and multistage carcinogenesis in mouse skin. Free Radic. Biol. Med. 1989;7(4):377–408. doi: 10.1016/0891-5849(89)90124-x. [DOI] [PubMed] [Google Scholar]
- 51.Zhong H., Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J.A., Jung B.G., Lee B.J. Inhibitory effects of Opuntia humifusa on 7, 12-dimethylbenz[a] anthracene and 12-O-tetradecanoylphorbol-13-acetate induced two-stage skin carcinogenesis. Asian Pac. J. Cancer Prev. APJCP. 2012;13(9):4655–4660. doi: 10.7314/apjcp.2012.13.9.4655. [DOI] [PubMed] [Google Scholar]
- 53.Elangovan V., Balasubramanian S., Sekar N., Govindasamy S. Studies on the chemopreventive potential of some naturally-occurring bioflavonoids in 7, 12- dimethylbenz (a) anthracene-induced carcinogenesis in mouse skin. J. Clin. Biochem. Nutr. 1994;17(3):153–160. [Google Scholar]
- 54.Jagetia G.C. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J. Clin. Biochem. Nutr. 2007;40(2):74–81. doi: 10.3164/jcbn.40.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim C., Pasparakis M. Epidermal p65/NF- κB signalling is essential for skin carcinogenesis. EMBO Mol. Med. 2014;6(7):970–983. doi: 10.15252/emmm.201303541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirata A., Murakami Y., Shoji M., Kadoma Y., Fujisawa S. Kinetics of radical scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005;25(5):3367–3374. [PubMed] [Google Scholar]
- 57.Ghorbani A. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur. J. Nutr. 2012;51(1):39–46. doi: 10.1007/s00394-011-0187-2. [DOI] [PubMed] [Google Scholar]
- 58.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti- inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]