Abstract
The prime issue derived from prostate cancer (PCa) is its high prevalence to metastasize to bone. MicroRNA-204-5p (miR-204-5p) has been reported to be involved in the development and metastasis in a variety of cancers. However, the clinical significance and biological functions of miR-204-5p in bone metastasis of PCa are still not reported yet. In this study, we find that miR-204-5p expression is reduced in PCa tissues and serum sample with bone metastasis compared with that in PCa tissues and serum sample without bone metastasis, which is associated with advanced clinicopathological characteristics and poor bone metastasis-free survival in PCa patients. Moreover, upregulation of miR-204-5p inhibits the migration and invasion of PCa cells in vitro, and importantly, upregulating miR-204-5p represses bone metastasis of PCa cells in vivo. Our results further demonstrated that miR-204-5p suppresses invasion, migration, and bone metastasis of PCa cells via inactivating nuclear factor κB (NF-κB) signaling by simultaneously targeting TRAF1, TAB3, and MAP3K3. In clinical PCa samples, miR-204-5p expression negatively correlates with TRAF1, TAB3, and MAP3K3 expression and NF-κB signaling activity. Therefore, our findings reveal a new mechanism underpinning the bone metastasis of PCa, as well as provide evidence that miR-204-5p might serve as a novel serum biomarker in bone metastasis of PCa.
This study identifies a novel functional role of miR-204-5p in bone metastasis of prostate cancer and supports the potential clinical value of miR-204-5p as a serum biomarker in bone metastasis of PCa.
Introduction
Prostate cancer (PCa) represents one of the leading causes of cancer-related deaths in males worldwide and its high rate of mortality from this disease is associated with widespread metastasis to distant sites, particularly to bone.1 PCa metastasizes to bone with a high rate of 90% with advanced disease.2 The associated pain, pathological fracture, hypercalcemia, and nerve compression syndromes are consequences of the bone destruction, which can be devastating. Despite these observations, the mechanisms underlying the bone metastasis predilection of PCa are not completely understood.
The nuclear factor κB (NF-κB) signaling pathway as a mechanism pivotal to inflammation and cancer development has been reported to be constitutively activated in various types of cancers.3, 4 Since its documentation, ubiquitin modification has emerged as an important regulatory mechanism contributing to the activation of NF-κB signaling.5, 6 Besides simple degradation of the natural inhibitors of NF-κB (IκBs) via ubiquitination,7, 8 ubiquitin modification has been reported to occur in a growing number of proteins in the NF-κB signal transduction pathway, serving as a positive regulator in NF-κB activation.6, 9 After binding to the respective ligands, the receptors recruit multiple receptor-associated factors, including the adaptor protein TRADD, tumor necrosis factor receptor (TNFR)-associated factors (TRAFs), cellular inhibitor of apoptosis proteins c-IAP1 and c-IAP2, and receptor-interacting protein kinase (RIP1), which results in autoubiquitination of TRAF2 and/or cIAP1 and K63-linked polyubiquitination of RIP1.10, 11 The K63 polyubiquitination of RIP1 further serve as scaffolds to facilitate the recruitment of transforming growth factor β (TGF-β)-activated kinase-1 (TAK1)/TAB2/3 and inhibitor of NF-κB kinase (IKK)-α/β/γ complexes, leading to TAK1 activation and TAK1-mediated activation of the IKK complex, which promotes nuclear translocation and activation of NF-κB via triggering K48-linked polyubiquitination and proteasomal degradation of IκBs.12, 13 Notably, ubiquitin conjugation/deconjugation in NF-κB signaling has been demonstrated to be frequently deregulated in numerous human cancer types.5, 14
By coordinately regulating repertoires of target genes, microRNAs (miRNAs) have been reported to potentially modulate multiple steps of cancer development, progression, and metastasis.15, 16, 17, 18, 19, 20, 21 A growing body of studies has showed that miRNAs play crucial roles in sustaining NF-κB activity by ubiquitination-mediated mechanism.22, 23 Importantly, NF-κB activation was associated with the metastatic phenotype of PCa progression,24 even in the development of PCa bone metastasis.25, 26 However, how multiple ubiquitination-associated proteins in the NF-κB signal transduction are simultaneously disrupted in the bone metastasis of PCa, leading to constitutive activation of NF-κB signaling, is not clarified yet.
In this study, we found that miR-204-5p inhibited the activation of NF-κB signaling via simultaneously targeting TRAF1, TAB3, and MAP3K3 in bone metastatic PCa cells. miR-204-5p expression was downregulated in bone metastatic PCa tissues and serum samples compared with that non-bone metastatic PCa tissues and serum samples, which positively correlated with the clinicopathological characteristics and poor bone metastasis-free survival in PCa patients. Furthermore, upregulating miR-204-5p repressed bone metastasis of PCa cells in vivo, and invasion and migration abilities of PCa cells in vitro. Importantly, our results further demonstrated that activity of NF-κB signaling was essential for pro-metastasis ability of anti-miR-204-5p in PCa cells. Taken together, our findings provide a novel mechanism underlying the constitutive activation of NF-κB signaling in bone metastasis of PCa, as well as demonstrate that miR-204-5p functions as a tumor-suppressive miRNA in bone metastasis of PCa by inhibiting NF-κB signaling.
Results
miR-204-5p Is Downregulated in PCa Tissues with Bone Metastasis
To determine the miR-204-5p expression levels in PCa, we first analyzed the miRNA sequencing dataset of PCa from The Cancer Genome Atlas (TCGA), and found that miR-204-5p expression was downregulated in separate and paired PCa tissues compared with the adjacent normal tissues (ANT) (Figures 1A and 1B). Interestingly, miR-204-5p expression was further decreased in PCa tissues with bone metastasis (PCa/BM) compared with that in PCa tissues without bone metastasis (PCa/nBM) (Figure 1C), and the percentage of low expression of miR-204-5p was higher in PCa tissues with bone metastasis than that in PCa tissues without bone metastasis (Figure 1D). Consistent with these findings, the expression level of miR-204-5p in our 183 individual and 10 paired PCa tissues was downregulated compared with that in benign prostate lesions, including benign prostate hyperplasia and prostatitis, and ANT (Figures 1E and 1F), particularly in PCa tissues with bone metastasis (Figures 1G and 1H). We further examined the expression levels of miR-204-5p in normal prostate epithelial cells RWPE-1 and 6 PCa cells. As demonstrated in Figure 1I, miR-204-5p expression was differentially decreased compared with RWPE-1, including three bone metastatic PCa cell lines (VCaP, PC-3, and C4-2B). Thus, our results imply that low expression of miR-204-5p may be implicated in the bone metastasis of PCa.
Figure 1.
miR-204-5p Is Reduced in Bone Metastatic PCa Tissues
(A) miR-204-5p expression level was decreased in PCa tissues compared with that in adjacent normal tissues (ANT) as assessed by analyzing the TCGA PCa miRNA sequencing dataset (fold change of miR-204-5p median expression in tumor tissues/miR-204-5p median expression in ANT was 0.66) (ANT, n = 52; tumor, n = 498). (B) miR-204-5p expression level was decreased in 52 paired PCa tissues compared with that in the matched ANT as assessed by analyzing the TCGA PCa miRNA sequencing dataset (fold change of miR-204-5p median expression in paired tumor tissues/miR-204-5p median expression in the matched ANT was 0.71). (C) miR-204-5p expression level was decreased in bone metastatic PCa tissues (PCa/BM) compared with that in non-bone metastatic PCa tissues (PCa/nBM) as assessed by analyzing the TCGA PCa miRNA sequencing dataset (PCa/nBM, n = 11; PCa/BM, n = 9) (fold change of miR-204-5p median expression in PCa/BM /miR-204-5p median expression in PCa/nBM was 0.44). (D) Percentages and number of samples showed high or low miR-204-5p expression in bone metastatic and non-bone metastatic PCa tissues in PCa dataset from TCGA. (E) Real-time PCR analysis of miR-204-5p expression in 26 benign prostate lesions tissues and 183 PCa tissues (fold change of miR-204-5p median expression in tumor tissues/miR-204-5p median expression in benign lesions was 0.84). Transcript levels were normalized to U6 expression. Lines represent median and lower/upper quartiles. (F) Real-time PCR analysis of miR-204-5p expression ration in 10 paired PCa tissues. Transcript levels were normalized to U6 expression (fold change of miR-204-5p average expression in 10 paired tumor tissues/miR-204-5p average expression in the matched ANT was 0.58). (G) Real-time PCR analysis of miR-204-5p expression in 151 primary PCa tissues without bone metastasis (PCa/nBM) and 32 primary PCa tissues with bone metastasis (PCa/BM) (fold change of miR-204-5p median expression in PCa/BM/miR-204-5p median expression in PCa/nBM was 0.82). Transcript levels were normalized to U6 expression. (H) Percentages and number of samples showed high or low miR-204-5p expression in bone metastatic and non-bone metastatic PCa tissues in our PCa tissues. (I) Real-time PCR analysis of miR-204-5p expression levels in normal prostate epithelial cell (RWPE-1), primary PCa cell 22RV1, bone metastatic PCa cell lines (PC-3, C4-2B, and VCaP), and brain metastatic cell line DU145 and lymph node metastatic cell line LNCaP (fold changes of relative expression of miR-204-5p in 22RV1, LNCaP, DU145, VCaP, PC-3, and C4-2B/RWPE-1 are 0.23, 0.17, 0.66, 0.57, 0.31, and 0.42, respectively). Transcript levels were normalized to U6 expression. *p < 0.05.
Low Expression of miR-204-5p Correlates with Poor Bone Metastasis-free Survival in PCa Patients
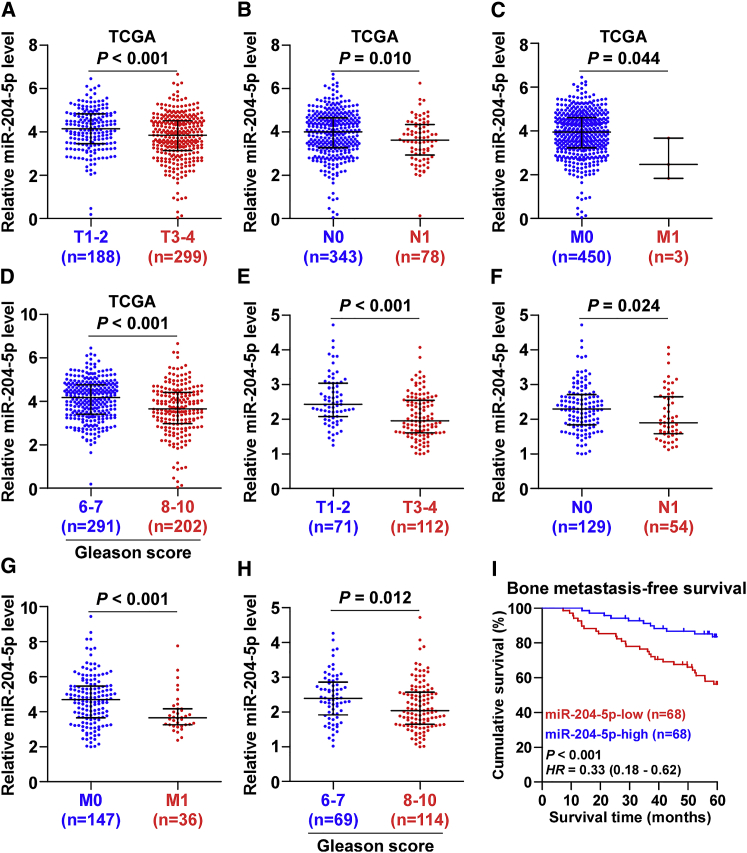
The clinical correlation analysis of miR-204-5p expression levels with clinicopathological characteristics in PCa patients from TCGA was performed and the results showed that low expression of miR-204-5p positively correlated with T classification, N classification, M classification, and Gleason grade in PCa patients (Figures 2A–2D). Consistently, our results demonstrated that miR-204-5p expression was inversely associated with the advanced clinicopathological characteristics in PCa patients (Figures 2E–2H). Statistical analysis resulted revealed that low expression of miR-204-5p positively correlated with serum PAS levels, Gleason grade, T classification, N classification, M classification, and bone metastasis status in PCa patients (Table S1). Kaplan-Meier survival analysis indicated that PCa patients with low miR-204-5p expression correlated with shorter bone metastasis-free survival compared with PCa patients with high miR-204-5p expression (Figure 2I). Taken together, our results indicate that low levels of miR-204-5p are positively associated with poor bone metastasis-free and advanced clinicopathological characteristics in PCa patients.
Figure 2.
Low Expression of miR-204-5p Correlates with Poor Clinicopathological Characteristics and Bone Metastasis-free Survival in PCa Patients
(A) miR-204-5p expression levels in PCa tissues with different tumor volume as assessed by TCGA (fold change of miR-204-5p median expression in T3-4 PCa tissues/miR-204-5p median expression in T1-2 PCa tissues was 0.81). (B) miR-204-5p expression levels in PCa tissues with different lymph node metastasis status as assessed by TCGA (fold change of miR-204-5p median expression in N1 PCa tissues/miR-204-5p median expression in N0 PCa tissues was 0.80). (C) miR-204-5p expression levels in PCa tissues with different distant metastasis status as assessed by TCGA (fold change of miR-204-5p median expression in M1 PCa tissues/miR-204-5p median expression in M0 PCa tissues was 0.36). (D) miR-204-5p expression levels in PCa tissues with different Gleason score as assessed by TCGA (fold change of miR-204-5p median expression in PCa tissues with Gleason score 8–10/miR-204-5p median expression in PCa tissues with Gleason score 6–7 was 0.70). (E) miR-204-5p expression levels in PCa tissues with different tumor volume (fold change of miR-204-5p median expression in T3-4 PCa tissues/miR-204-5p median expression in T1-2 PCa tissues was 0.80). (F) miR-204-5p expression levels in PCa tissues with different lymph node metastasis status (fold change of miR-204-5p median expression in N1 PCa tissues/miR-204-5p median expression in N0 PCa tissues was 0.82). (G) miR-204-5p expression levels in PCa tissues with different distant metastasis status (fold change of miR-204-5p median expression in M1 PCa tissues/miR-204-5p median expression in M0 PCa tissues was 0.79). (H) miR-204-5p expression levels in PCa tissues with different Gleason score (fold change of miR-204-5p median expression in PCa tissues with Gleason score 8–10/miR-204-5p median expression in PCa tissues with Gleason score 6–7 was 0.85). (I) Kaplan-Meier analysis of bone metastasis-free survival curves of PCa patients with high miR-204-5p expression (n = 68) versus low miR-204-5p expression (n = 68).
Upregulating miR-204-5p Represses Bone Metastasis of PC-3 Cells In Vivo
To investigate the effect of miR-204-5p on the bone metastasis of PCa in vivo, we stably overexpressed miR-204-5p via virus transduction in PC-3 cells that expressed the relative low expression level of miR-204-5p in all three bone metastatic PCa cell lines as shown in Figure 1I (Figure S1). A mouse model of bone metastasis was used, in which the luciferase-labeled vector or miR-204-5p-overexpressing PC-3 cells were inoculated respectively into the left cardiac ventricle of male nude mice to monitor the progression of bone metastasis by bioluminescence imaging (BLI) and X-rays. As shown in Figures 3A and 3B, mice inoculated with the miR-204-5p-overexpressing PC-3 cells displayed lower bone metastasis ability compared with the control group by X-ray and BLI. H&E staining of the bone tumor sections revealed that upregulating miR-204-5p reduced the tumor burden in bone (Figure 3C). Moreover, upregulating miR-204-5p decreased bone metastatic score and osteolytic area of metastatic tumors, but prolonged survival and bone metastasis-free survival compared to the control group (Figures 3D–3G). Gene Set Enrichment Analysis (GSEA) based on miR-204-5p expression data from TCGA was performed, and the result showed that low expression of miR-204-5p significantly and positively correlated with metastatic avidity in multiple cancer types (Figures S2A and S2B). Therefore, invasion and migration assays were carried out. As shown in Figures S3A and S3B, upregulating miR-204-5p suppressed the invasion and migration abilities in PCa cells. Conversely, silencing miR-204-5p yielded an opposite effect on PCa cells (Figure S3B). In addition, western blot analysis showed that upregulating miR-204-5p increased the expression of epithelial marker E-cadherin, but reduced mesenchymal markers vimentin and fibronection expression in PCa cells; by contrast, silencing miR-204-5p upregulated vimentin and fibronection, and decreased E-cadherin expression (Figure S3C). Collectively, these finding demonstrate that upregulating miR-204-5p represses the bone metastasis of PCa in vivo and EMT, invasion, and migration in vitro.
Figure 3.
Upregulating miR-204-5p Represses Bone Metastasis of PC-3 Cells In Vivo
(A) Representative BLIs signal of bone metastasis of a mouse from the vector or miR-204-5p-overexpressing groups of mice at 5 min and 8 weeks, respectively. (B) Representative radiographic images of bone metastases in the indicated mice (arrows indicate osteolytic lesions). (C) Representative H&E-stained sections of tibias from the indicated mouse. Scale bars, 500 μm (40×) and 100 μm (200×). (D) The sum of bone metastasis scores for each mouse in tumor-bearing mice inoculated with vector (n = 8) or miR-204-5p-overexpressing (n = 9) cells. (E) Quantification of the BLI signaling in the vector and miR-204-5p-overexpressing groups at 6, 7, and 8 weeks, respectively. *p < 0.05. (F and G) Kaplan-Meier analysis of mouse bone metastasis-free survival (F) and survival (G) in the vector and miR-204-5p-overexpressing groups.
miR-204-5p Targets Multiple Important Regulatory Components for the Activation of NF-κB Signaling
By analyzing several publicly available algorithms, including TargetScan, miRWalk, and miRanda, we found that several important regulatory components family for the activation of NF-κB signaling, including TNFR-associated factors (TRAF1, 2, and 7), TRAF3 interacting protein (TRAF3IP1 and 2), TGF-β activated kinase 1 binding protein (TAB2 and 3) and mitogen-activated protein kinase kinase kinase (MAP3K2, 3, and 5), may be potential targets of miR-204-5p (Figure 4A; Figure S4A). Microribonucleoprotein (miRNP) immunoprecipitation (IP) assay was first performed to investigate the association of miR-204-5p with these potential target transcripts, where hemagglutinin (HA)-Ago2 plasmid were cotransfected into miR-204-5p-overexpressing or vector PCa cells followed by HA-Ago2 IP using anti-HA-antibody. As shown in Figure 4B, our results showed that upregulating miR-204-5p significantly and differentially enhanced the enrichment of TRAF1, TAB3, and MAP3K3 in HA-Ago2 complexes in three independent PCa cells, respectively, compared with vector PCa cells. However, enrichments of TRAF2 and TAB2 were only increased in PC-3 cells, TRAF7, MAP3K2, and MAP3K5 were only in C4-2B cells, and TRAF3IP1 and TRAF3IP2 were only in VCaP cells (Figure 4B). Therefore, these findings revealed a direct and consistent association of miR-204-5p with TRAF1, TAB3, and MAP3K3 transcripts in all three PCa cell lines. Real-time PCR and western blotting analysis showed that upregulating miR-204-5p reduced, while silencing miR-204-5p increased the expression levels of TRAF1, TAB3, and MAP3K3 in PCa cells (Figure 4C; Figure S4B–S4D). Luciferase assay revealed that upregulating miR-204-5p repressed while silencing miR-204-5p elevated the reporter activity driven by the 3′UTRs of TRAF1, TAB3, and MAP3K3 in PCa cells (Figures 4D–4F; Figure S4A). Moveover, individual upregulating TRAF1, TAB3, and MAP3K3 rescued the invasion and migration abilities repressed by miR-204-5p overexpression in PCa cells (Figures S5A and S5B). Therefore, our results demonstrate that TRAF1, TAB3, and MAP3K3 are bona fide targets of miR-204-5p in PCa cells.
Figure 4.
miR-204-5p Targets TRAF1, TAB3, and MAP3K3
(A) Predictive target genes of miR-204-5p from TargetScan, miRanda, and miRWalk. (B) Heatmap shows the miRNP IP result of the association between miR-204-5p and TRAF1, TAB3, and MAP3K3 transcripts in PCa cells. Pseudo-color scale values were fold changes in miR-204-5p versus vector. Transcript levels were normalized by GAPDH expression. (C) Western blotting analysis of TRAF1, TAB3, and MAP3K3 expression in the indicated cells. α-tubulin served as the loading control. (D–F) Luciferase assay of cells transfected with pmirGLO-3′UTR reporter of TRAF1, TAB3, and MAP3K3 in the miR-204-5p overexpressing or silencing PCa cells: VCap (D), PC-3 (E), and C4-2B (F). Error bars represent the mean ± SD of three independent experiments. *p < 0.05.
miR-204-5p Inhibits NF-κB Activity in PCa Cells
Several lines of evidence have reported that TRAF1, TAB3, and MAP3K3 play important roles in sustaining the activity of NF-κB signaling.12, 13, 27 Thus, we further investigated the effects of miR-204-5p on the activity of NF-κB signaling. GSEA results showed that low levels of miR-204-5p were positively associated with the activity of NF-κB signaling (Figure S6A). Luciferase reporter assays demonstrated that upregulating miR-204-5p repressed while silencing miR-204-5p increased NF-κB-dependent luciferase activity in PCa cells (Figure 5A). Cellular fractionation and western blotting analysis revealed that overexpression of miR-204-5p decreased while silencing miR-204-5p promoted nuclear accumulation of NF-κB/p65 (Figure 5B). Real-time PCR analysis showed that upregulating miR-204-5p reduced whereas silencing miR-204-5p enhanced the expression levels of multiple downstream metastasis-associated target genes of NF-κB signaling in PCa cells, except for MMP9 in VCaP cells, MMP12 in PC-3 cells, and VEGFA in C4-2B cells (Figures 5C–5E). Thus, these results reveal that miR-204-5p inhibits NF-κB signaling pathway in PCa cells.
Figure 5.
miR-204-5p Suppresses the Activity of NF-κB Signaling in PCa Cells
(A) NF-κB transcriptional activity was assessed by luciferase reporter constructs in the indicated cells. Error bars represent the mean ± SD of three independent experiments. *p < 0.05. (B) Western blotting of nuclear NF-κB/p65 expression in the indicated cells. The nuclear protein p84 was used as the nuclear protein marker. (C–E) Real-time PCR analysis of multiple downstream metastasis-associated targeted genes of NF-κB signaling, Vimentin, SNAIL2, TWIST1, IL11, VEGFA, MMP1, MMP6, MMP9, MMP10, MMP12, and MMP13 in VCap (C), PC-3 (D), and C4-2B (E) cells. Transcript levels were normalized to U6 expression. Error bars represent the mean ± SD of three independent experiments. *p < 0.05. (F) NF-κB signaling inhibitors LY2409881 (10 μM) and JSH-23 (10 μM) attenuated the stimulatory effect of anti-miR-204-5p on NF-κB transcriptional activity in VCaP cells. Error bars represent the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01. (G and H) NF-κB signaling inhibitors LY2409881 (10 μM) and JSH-23 (10 μM) attenuated the stimulatory effect of anti-miR-204-5p on invasion (G) and migration (H) abilities in VCaP cells. Error bars represent the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01.
NF-κB Activation Is Essential for the Pro-metastasis Role of anti-miR-204-5p in PCa Cells
We further explored whether the pro-metastasis role of anti-miR-204-5p is dependent on NF-κB signaling activity in PCa cells via applying the inhibitors of NF-κB signaling, LY2409881 and JSH-23, in miR-204-5p-silencing VCaP cells, respectively. As shown in Figure S6B, LY2409881 and JSH-23 showed gradient inhibition of the NF-κB reporter activity in a dose-dependent manner in VCaP cells. The stimulatory effects of anti-miR-204-5p on NF-κB activity were attenuated by LY2409881 and JSH-23 in PCa cells (Figure 5E). In addition, LY2409881 and JSH-23 attenuated the migration and invasion abilities in miR-204-5p-silenced VCaP cells (Figures 5F and 5G). These results indicate that activation of NF-κB signaling is essential for the pro-metastasis role of anti-miR-204-5p in PCa cells.
miR-204-5p Is a Valuable Serum Biomarker in PCa Patients with Bone Metastasis
miR-204-5p has been reported to serve as a serum marker in several cancer or non-cancer disease types.28, 29, 30, 31 Therefore, we further examined whether miR-204-5p may be used as a novel bone metastasis serum marker in PCa patients. As shown in Figure 6A, miR-204-5p expression in the serum of PCa patients with bone metastasis was dramatically reduced compared with that in PCa patients without bone metastasis. Receiver operating characteristic (ROC) curve analysis of miR-204-5p in the serum of PCa patients exhibited an AUC of 0.79 (95% CI = 0.70–0.87, p < 0.001) (Figure 6B). Furthermore, miR-204-5p expression in the serum of PCa patients positively correlated with expression levels of miR-204-5p in PCa tissues (Figure 6C). Importantly, Kaplan-Meier analysis revealed that PCa patients with low serum expression of miR-204-5p showed shorter bone metastasis-free survival compared with that in PCa patients with high serum expression of miR-204-5p (Figure 6D). We further analyzed the clinical correlation of serum miR-204-5p expression with clinicopathological characteristics in PCa patients and found that miR-204-5p expression in the serum of PCa patients was downregulated in T3/4, N1, M1, or higher Gleason grade (>7) PCa patients, respectively (Figures 6E–6H). The clinical correlation analysis of miR-204-5p expression with clinicopathological characteristics revealed that low serum levels of miR-204-5p positively correlated with serum PSA levels, Gleason grade, T classification, N classification, M classification, and bone metastasis status in PCa patients (Table S2). Therefore, our results indicated that miR-204-5p may serve as a valuable serum prognostic biomarker in the bone metastasis of PCa.
Figure 6.
Low Serum Level of miR-204-5p Positively Correlates with Poor Bone Metastasis-free Survival
(A) Real-time PCR analysis of serum miR-204-5p level in 151 non-bone metastatic and 32 bone metastatic PCa serum samples. Transcript levels were normalized to U6 expression. (B) ROC curve for miR-204-5p in the serum of the PCa patients with bone metastasis and patients without bone metastasis. (C) Correlation of expression levels of miR-204-5p between the PCa tissues and their respective serum samples. (D) Kaplan-Meier analysis of bone metastasis-free survival curves of PCa patients with high serum miR-204-5p levels (n = 68) versus low serum miR-204-5p levels (n = 68). (E) Serum miR-204-5p expression levels in PCa tissues with different tumor volume. (F) Serum miR-204-5p expression levels in PCa tissues with different lymph node metastasis status. (G) Serum miR-204-5p expression levels in PCa tissues with different distant metastasis status. (H) Serum miR-204-5p expression levels in PCa tissues with different Gleason score.
Deletion of miR-204-5p Occurs in a Small Portion of PCa Patients
To clarify the underlying mechanism responsible for miR-204-5p downexpression in PCa tissues, we analyzed the miR-204-5p dataset in PCa from TCGA from a genetic perspective and found that deletion occurred in 3.1% of PCa tissues (Figure S7A). However, miR-204-5p expression level in PCa tissues with deletion had no significant difference compared with that in PCa tissues without deletion (Figure S7B). Consistently, our results revealed that deletion was found in 10/183 PCa tissues (approximately 5.5%) (Figure S7C), and there was no obvious difference between the expression level of miR-204-5p in PCa tissues with the deletion and those without deletion (Figure S7D). Collectively, these results indicate that deletion is not responsible for miR-204-5p downexpression in PCa tissues, suggesting that some other unknown regulatory mechanism contributes to the miR-204-5p downexpression phenomenon in PCa tissues.
Clinical Correlation of miR-204-5p with TRAF1, TAB3, MAP3K3, and NF-κB Activity in Human PCa Tissues
To further investigate the clinical significance of miR-204-5p with TRAF1, TAB3, and MAP3K3, and the activity of NF-κB signaling in PCa tissues, we measured miR-204-5p expression and the protein expression levels of TRAF1, TAB3, MAP3K3, and nuclear p65 in 4 random PCa tissues with bone metastasis (T1-4) and 4 PCa tissues without bone metastasis (T5-8). As shown in Figure 7A, miR-204-5p expression was reduced in PCa tissues with bone metastasis compared with that in PCa tissues without bone metastasis. By contrast to the miR-204-5p expression pattern, protein expression of TRAF1, TAB3, MAP3K3, and p65 expression was elevated in PCa tissues with bone metastasis compared with that in PCa tissues without bone metastasis (Figure 7A). Pearson analysis revealed that miR-204-5p expression inversely correlated with TRAF1, TAB3, MAP3K3, and nuclear p65 expression in clinical PCa tissues (Figures S8A–S8D). Taken together, our results indicate that overexpression of miR-204-5p inhibits NF-κB signaling by targeting TRAF1, TAB3, and MAP3K3, which further represses the bone metastasis of PCa (Figure 7B).
Figure 7.
Clinical Relevance of miR-204-5p with TRAF1, TAB3, MAP3K3, and NF-κB Signaling Activity in Human PCa and Bone Tissues
(A) Analysis of miR-204-5p expression with TRAF1, TAB3, MAP3K3, and nuclear p65 in 4 bone metastatic PCa tissues (T1-4) and 4 non-bone metastatic PCa tissues (T5-8). U6 was used as the control for RNA loading. miR-204-5p expression levels were normalized to that miR-204-5p expression of sample eight. Each bar represents the mean ± SD of three independent experiments. Loading controls were α-tubulin and p84 for the cytoplasmic and nuclear fractions. ***p < 0.001. (B) Hypothetical model illustrating that inhibition of the NF-κB pathway by miR-204-5p epigenetic disruption of several important regulatory components for the activation of NF-κB signaling inhibits bone metastasis of PCa.
Discussion
miR-204-5p has been reported to be downexpressed in several human cancer types, such as renal clear cell carcinoma, prostate cancer, hepatocellular carcinoma, and glioblastoma, and downexpression of miR-204-5p was implicated in the progression and metastasis via a variety of mechanisms.32, 33, 34, 35 In contrast to its well-documented tumor-suppressive role in cancer, Díaz-Martínez et al. have found that miR-204-5p was upregulated in vemurafenib-resistant melanoma. Similar effects were elicited by MEK and ERK inhibitors but not AKT or Rac inhibitors.36 This finding suggests that miR-204-5p may act a dual yet opposite role dependent on tumor types. Furthermore, several lines of evidence have demonstrated that miR-204-5p was downregulated in PCa tissues, and low levels of miR-204-5p were involved in chemotherapeutic resistance of PCa.33, 37 It is worth noting that dysregulation of miR-204-5p has been reported in metastatic PCa cells in vitro,38 suggesting that aberrant expression of miR-204-5p may be implicated in the metastatic phenotypes of PCa. However, the clinical significance and biological roles of miR-204-5p in bone metastasis of PCa remain not studied yet. In this study, our results demonstrated that miR-204-5p expression was downregulated in bone metastatic PCa tissues and serum samples, which positively associated with advanced clinicopathological characteristics and more importantly predicted poor bone metastasis-free survival in PCa patients. Our results further revealed that miR-204-5p reduced bone metastasis of PCa cells by inhibiting the NF-κB signaling via simultaneously targeting TRAF1, TAB3, and MAP3K3. Collectively, our findings reveal that miR-204-5p functions as a tumor-suppressive miRNA in the bone metastasis of PCa.
Several lines of evidence have shown that miR-204-5p was involved in the tumorigenesis and metastasis of various types of cancers via regulating varying signaling. In breast cancer, miR-204-5p has been found to be significantly downregulated, and overexpression of miR-204-5p repressed growth and metastasis of breast cancer by inhibiting PI3K/Akt pathway via targeting PIK3CB.39 In addition, Díaz-Martínez et al. have found that miR-204-5p contributed to cell growth and vemurafenib resistance via durably stimulating both Ras and MAPK signaling pathways.36 In the current study, our results showed that downexpression of miR-204-5p was significantly and positively associated with the activity of NF-κB signaling by GSEA and several publicly available algorithms. Our findings further demonstrated that miR-204-5p inhibited NF-κB signaling via simultaneously targeting TRAF1, TAB3, and MAP3K3. Importantly, activation of NF-κB signaling was required for the pro-metastasis role of anti-miR-204-5p in PCa cells. Therefore, our results uncover a novel mechanism by which miR-204-5p suppressed bone metastasis via inhibiting NF-κB signaling in PCa.
Since it was identified in several decades ago,40 the pivotal role of NF-κB signaling in various physiologic and pathologic diseases has been extensively reported.41, 42 Extensive studies have shown that NF-κB signaling was constitutively activated in a various types of human cancer and was involved in multiple process of cancer, including tumor initiation, progression, and metastasis.41, 43 Several lines of evidence have reported that activation of NF-κB signaling is crucial for in the development of bone metastasis in cancers.44, 45 Importantly, unrestrained activity of NF-κB signaling has been reported to contribute to the metastatic phenotype of PCa progression,24 even in the bone metastasis of PCa.25, 26 Therefore, further clarifying the specific mechanisms responsible for constitutive activation of NF-κB signaling in the bone metastasis of PCa is of great urgency. In the current study, our results revealed that miR-204-5p was downregulated in bone metastatic PCa tissues, which in turn activated NF-κB signaling via simultaneously upregulating multiple important regulatory components of NF-κB signaling, including TRAF1, TAB3, and MAP3K3 in PCa. Importantly, inhibition of NF-κB signaling activity by LY2409881 and JSH-23 blocked the pro-metastasis roles of anti-miR-204-5p in invasion and migration abilities of PCa cells. Hence, our results provide a novel regulatory mechanism contributing to constitutive activation of NF-κB signaling in bone metastasis of PCa.
Accumulating studies have reported that aberrant expression of important regulatory component proteins, such as TRAFs protein family, and MAP3K protein family and TAK1 binding protein family, play important roles in the activation of NF-κB signaling.46, 47, 48, 49 As an adaptor protein of NF-κB signaling cascades, TRAFs protein family transducer signal via binding to TNFR cytoplasmic domains and mediating TNF-induced activation of NF-κB signaling.12, 13 Overexpression of TRAFs have been reported in several human cancer types, and they contribute to the constitutively activated NF-κB signaling.50 On the other hand, another essential component of the NF-κB pathway, TAK1 binding protein, such as TAB3, is involved in the progression of cancers through activation of the NF-κB pathway.49 In addition, MAP3K3, the key factor for the activation of NF-κB signaling, has been demonstrated to promote the activation of NF-κB signaling through IκB kinase-α and IκB kinase-β when overexpressed.46, 51 Deficiency of MAP3K3 was absent in TNF-induced NF-κB activation.27 However, how these critical regulatory component proteins for the activation of NF-κB signaling are simultaneously dysregulated remains to be further elucidated. In this study, our results found that TRAF1, TAB3, and MAP3K3 were simultaneously upregulated due to miR-204-5p downexpression, which further constitutively activated NF-κB signaling and promoted the development of bone metastasis in PCa. Therefore, our findings clarify the underlying mechanism by which miR-204-5p inhibits NF-κB signaling via simultaneously targeting several regulatory components of NF-κB signaling in bone metastasis of PCa.
It has been widely documented that miRNAs have the potential to serve as a potential marker for the diagnosis and prognosis in various types of cancer.52, 53, 54 Several studies have shown that miR-204-5p was identified as a serum marker in several cancer or non-cancer disease types.28, 29, 30, 31 Notably, a study from Daniel et al. has revealed that expression levels of a panel of seven miRNAs, including miR-204-5p, in the blood of PCa patients may be used as diagnostic biomarkers for the identification of PCa.55 However, the relevance between serum miR-204-5p level and bone metastasis in PCa has not been reported yet. In this study, our results demonstrated that miR-204-5p level in the serum of PCa patients with bone metastasis was much lower than that in the serum of PCa patients without bone metastasis. Importantly, low serum levels of miR-204-5p showed shorter bone metastasis-free survival in PCa patients. Thus, our findings suggest that miR-204-5p holds promise as a novel serum prognostic marker for bone metastasis of PCa.
As mentioned above, miR-204-5p has been reported to be downregulated in the majority of cancer types. Therefore, it is tempting to further investigate, although it remains to be not elucidated yet, the underlying mechanisms responsible for miR-204-5p downexpression in cancers. With this question, our results in combination with the analysis result from TCGA revealed that deletion of MIR204 occurred in a small portion of PCa tissues. However, the expression level of miR-204-5p with deletion had no significant difference compared with those without deletion in PCa tissues. This finding indicates that deletion is not responsible for miR-204-5p downexpression in PCa tissues. Evidence has reported that miRNA expression could be controlled at a transcriptional level.56 Therefore, we further investigated whether some transcriptional factor may be involved in miR-204-5p downexpression in bone metastatic PCa tissues. Through analyzing the UCSC bioinformatics, we found two potential transcriptional factors, JunD Proto-Oncogene, AP-1 transcription factor subunit (JUND) and Spi-1 proto-oncogene (SPI1), with the potent binding ability in the promoter region of MIR204 (Figure S9). Numerous studies have reported that JUND and SPI1 function as oncogenes to be implicated in tumor carcinogenesis, progression, and metastasis.57, 58, 59, 60 In PCa, JUND has been reported to promote prostate carcinogenesis and PCa progression and aggression,61, 62, 63 and the literatures about the biological role of SPI1 in PCa are lacking. Taken together, the possibility that whether JUND or SPI1 has a functional role in bone metastasis of PCa, and if so, whether JUND or SPI1 promotes bone metastasis of PCa via transcriptionally inhibiting miR-204-5p is worth further investigation in the following work.
In summary, our results demonstrate that miR-204-5p represses the bone metastasis of PCa by inhibiting NF-κB signaling via simultaneously targeting TRAF1, TAB3, and MAP3K3. Importantly, low serum level of miR-204-5p predicts poor bone metastasis-free survival in PCa patients. Thus, our results provide evidence for miR-204-5p as a serum bone metastasis biomarker in PCa patients and clarify the underlying mechanism by which miR-204-5p inhibits bone metastasis of PCa, which will facilitate the identification of bone metastasis predictive factor in PCa and development of novel therapeutic strategy in the treatment of bone metastasis of PCa.
Materials and Methods
Cell Culture
The human PCa cell lines 22RV1, PC-3, VCaP, DU145, LNCaP, and normal prostate epithelial cells RWPE-1 were obtained from Shanghai Chinese Academy of Sciences cell bank (China). RWPE-1 cells were grown in defined keratinocyte-SFM (1×) (Invitrogen). PC-3, LNCaP, and 22Rv1 cells were cultured in RPMI-1640 medium (Life Technologies, Carlsbad, CA, US) supplemented with penicillin G (100 U/mL), streptomycin (100 mg/mL), and 10% fetal bovine serum (FBS, Life Technologies). DU145 and VCaP cells were grown in DMEM (Invitrogen) supplemented with 10% FBS. The C4-2B cell line was purchased from the MD Anderson Cancer Center and maintained in T-medium (Invitrogen) supplemented with 10% FBS. All cell lines were grown under a humidified atmosphere of 5% CO2 at 37°C. All cell lines were authenticated by short tandem repeat fingerprinting at Guangzhou Cellcook Biotech on June 19, 2017.
Plasmid, Small Interfering RNA, and Transfection
The human miR-204-5p gene was PCR-amplified from genomic DNA and cloned into a pMSCV-puro retroviral vector (Clontech, Japan). The pNF-κB-luc and control plasmids (Clontech, Japan) were used to examine the activity of transcription factor quantitatively. The 3′UTR regions of the human TRAF1, TAB3, and MAP3K3 were PCR-amplified from genomic DNA and cloned into pmirGLO vectors (Promega, USA), and the list of primers used in cloning reactions is presented in Table S3. Anti-miR-204-5p, plasmids for the TRAF1, TAB3, and MAP3K3 overexpression were synthesized and purified by RiboBio. Transfection of miRNA and plasmids was performed using Lipofectamine 3000 (Life Technologies, USA) as previously described.64
RNA Extraction, Reverse Transcription, and Real-Time PCR
Total RNA from tissues or cells was extracted using the RNA Isolation Kit (QIAGEN, USA) according to the manufacturer’s instructions. mRNA and miRNA were reverse transcribed from total mRNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, USA) according to the manufacturer’s protocol. cDNA was amplified and quantified on the CFX96 system (BIO-RAD, USA) using iQ SYBR Green (Bio-Rad, USA). The primers are provided in Table S4. Real-time PCR was performed according to a standard method, as described previously.65 Primers for U6 and miR-204-5p were synthesized and purified by RiboBio (Guangzhou, China). U6 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous controls. Relative fold expressions were calculated with the comparative threshold cycle (2-ΔΔCt) method as previously described.66
Western Blotting
Nuclear/cytoplasmic fractionation was separated using the Cell Fractionation Kit (Cell Signaling Technology, USA) according to the manufacturer’s instructions, and the whole cell lysates were extracted with RIPA Buffer (Cell Signaling Technology). Western blotting was performed according to a standard method, as described previously.67 Antibodies against TRAF1, TAB3, and MAP3K3 were purchased from Cell Signaling Technology, p65 from Proteintech, and p84 from Invitrogen. The membranes were stripped and reprobed with an anti-α-tubulin antibody (Sigma-Aldrich, USA) as the loading control.
Luciferase Assay
Cells (4 × 104) were seeded in triplicate in 24-well plates and cultured for 24 h and performed as previously described.68 Cells were transfected with 100 ng of the pNF-κB reporter luciferase plasmid, or pmirGLO-TRAF1-3′UTR, -TAB3-3′UTR, or -MAP3K3-3′UTR luciferase plasmid, plus 5 ng pRL-TK the Renilla plasmid (Promega) using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s recommendations. Luciferase and Renilla signals were measured 36 h after transfection using a Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol.
miRNA Immunoprecipitation
Cells were co-transfected with HA-Ago2, followed by HA-Ago2 immunoprecipitation using anti-HA-antibody, as previously described.69 Real-time PCR analysis of the IP material was performed to test the association of the mRNA of TRAF1, TAB3, and MAP3K3 with the RISC complex.
Invasion and Migration Assays
The invasion and migration assays were performed using Transwell chamber consisting of 8-mm membrane filter inserts (Corning) with or without coated Matrigel (BD Biosciences) respectively as described previously.70 Briefly, the cells were trypsinized and suspended in serum-free medium. Then, 1.5 × 105 cells were added to the upper chamber, and lower chamber was filled with the culture medium supplemented with 10% FBS. After incubation for 24–48 h, cells passed through the coated membrane to the lower surface, where cells were fixed with 4% paraformaldehyde and stained with hematoxylin. The cell count was performed under a microscope (×100).
Animal Study
All mouse experiments were approved by The Institutional Animal Care and Use Committee of Sun Yat-sen University (the approval number was L102012016110D) and were housed as previously described.71 For the bone metastasis study, BALB/c-nu mice (5–6 weeks old, 18–20 g) were anesthetized and inoculated into the left cardiac ventricle with 1 × 105 PC-3 cells in 100 μL of PBS. Bone metastases were monitored by BLI as previously described.72 Osteolytic lesions were identified on radiographs as radiolucent lesions in the bone. The area of the osteolytic lesions was measured using the Metamorph image analysis system and software (Universal Imaging Corporation), and the total extent of bone destruction per animal was expressed in square millimeters. Each bone metastasis was scored based on the following criteria: 0, no metastasis; 1, bone lesion covering <1/4 of the bone width; 2, bone lesion involving 1/4∼1/2 of the bone width; 3, bone lesion across 1/2∼3/4 of the bone width; and 4, bone lesion >3/4 of the bone width. The bone metastasis score for each mouse was the sum of the scores of all bone lesions from four limbs. For survival studies, mice were monitored daily for signs of discomfort and were either euthanized all at one time or individually when presenting signs of distress, such as a 10% loss of body weight, paralysis, or head tilting.
Patients, Tumor Tissues, and Serum Samples
A total of 183 individual and 10 paired PCa tissues and 26 benign prostate lesions tissues were obtained during surgery or needle biopsy between January 2010 and October 2013. Furthermore, 183 serum samples, including 151 primary PCa tissues without bone metastasis and 32 primary PCa tissues with bone metastasis, were also obtained in the PCa patients. miRNA was extracted from serum using miRNeasy Serum/Plasma Kit (Cat# 217184, QIAGEN) according to the manufacturer’s protocol. Patients were diagnosed based on clinical and pathological evidence, and the specimens were immediately snap-frozen and stored in liquid nitrogen tanks. For the use of these clinical materials for research purposes, prior patient consents and approval from the Institutional Research Ethics Committee were obtained. The clinicopathological features of the patients are summarized in Table S5. The expression levels of miR-204-5p were examined in our 183 clinical PCa samples by real-time PCR. Then, the expression level of miR-204-5p in each PCa tissue was further normalized with the one with the lowest expression level miR-204-5p, and the relative expression of miR-204-5p of all PCa tissues was used and analyzed in this study. The median of relative miR-204-5p expression in PCa tissues was used to stratify high and low expression of miR-204-5p.
Statistical Analysis
All values are presented as the mean ± SD. Significant differences were determined using the GraphPad 5.0 software (USA). Student’s t test was used to determine statistical differences between two groups. The chi-square test was used to analyze the relationship between miR-204-5p expression and clinicopathological characteristics. p < 0.05 was considered significant. All experiments were repeated three times.
Author Contributions
Conception and design, C.Z., and Shuai Huang; Development of methodology, Q.W., Sheng Huang, and J.P.; Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.), Y.T., S. He, and X.F.; Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis), X.P., and X.C.; Writing, review, and/or revision of the manuscript, C.Z, Sheng Huang, C.Y., and D.R.; Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases), Y.H., and Z.L.; Study supervision, C.Z., and Shuai Huang.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81872172, 81660362, 81503281, 81702671, and 81502219), the Science and Technology Planning Project of Health and Family Planning Commission of Jiangxi Province (20181026), and the Talent Grant of Sun Yat-Sen University (17ykpy30).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2019.09.008.
Contributor Information
Shuai Huang, Email: huang-shuai@hotmail.com.
Changye Zou, Email: zouchy@sysu.edu.cn.
Supplemental Information
References
- 1.Chiarodo A. National Cancer Institute roundtable on prostate cancer: future research directions. Cancer Res. 1991;51:2498–2505. [PubMed] [Google Scholar]
- 2.Weinfurt K.P., Li Y., Castel L.D., Saad F., Timbie J.W., Glendenning G.A., Schulman K.A. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 2005;16:579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 3.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 4.Perry J.A., Sinclair-Davis A.N., McAllaster M.R., de Graffenried C.L. TbSmee1 regulates hook complex morphology and the rate of flagellar pocket uptake in Trypanosoma brucei. Mol. Microbiol. 2018;107:344–362. doi: 10.1111/mmi.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wertz I.E., Dixit V.M. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harb. Perspect. Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harhaj E.W., Dixit V.M. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2011;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez M.S., Wright J., Thompson J., Thomas D., Baleux F., Virelizier J.L., Hay R.T., Arenzana-Seisdedos F. Identification of lysine residues required for signal-induced ubiquitination and degradation of I kappa B-alpha in vivo. Oncogene. 1996;12:2425–2435. [PubMed] [Google Scholar]
- 8.Wertz I.E., O’Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D.L. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 9.Liu S., Chen Z.J. Expanding role of ubiquitination in NF-κB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Deng L., Hong M., Akkaraju G.R., Inoue J., Chen Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 12.Adhikari A., Xu M., Chen Z.J. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z.J. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skaug B., Jiang X., Chen Z.J. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 15.Guo W., Ren D., Chen X., Tu X., Huang S., Wang M., Song L., Zou X., Peng X. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J. Cell. Biochem. 2013;114:1606–1615. doi: 10.1002/jcb.24502. [DOI] [PubMed] [Google Scholar]
- 16.Huang S., Wa Q., Pan J., Peng X., Ren D., Huang Y. Downregulation of miR-141-3p promotes bone metastasis via activating NF-kappaB signaling in prostate cancer. Journal of experimental & clinical cancer research. CR (East Lansing Mich.) 2017;36:173. doi: 10.1186/s13046-017-0645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren D., Wang M., Guo W., Zhao X., Tu X., Huang S., Zou X., Peng X. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR-145. Int. J. Oncol. 2013;42:1473–1481. doi: 10.3892/ijo.2013.1825. [DOI] [PubMed] [Google Scholar]
- 18.Jiang C., Yu M., Xie X., Huang G., Peng Y., Ren D., Lin M., Liu B., Liu M., Wang W., Kuang M. miR-217 targeting DKK1 promotes cancer stem cell properties via activation of the Wnt signaling pathway in hepatocellular carcinoma. Oncol. Rep. 2017;38:2351–2359. doi: 10.3892/or.2017.5924. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X., Ren D., Wu X., Lin X., Ye L., Lin C., Wu S., Zhu J., Peng X., Song L. miR-1266 Contributes to Pancreatic Cancer Progression and Chemoresistance by the STAT3 and NF-κB Signaling Pathways. Mol. Ther. Nucleic Acids. 2018;11:142–158. doi: 10.1016/j.omtn.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren D., Wang M., Guo W., Huang S., Wang Z., Zhao X., Du H., Song L., Peng X. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res. 2014;358:763–778. doi: 10.1007/s00441-014-2001-y. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y., Ren D., Yang Q., Cui Y., Guo W., Lai Y., Du H., Lin C., Li J., Song L., Peng X. The TGF-β signalling negative regulator PICK1 represses prostate cancer metastasis to bone. Br. J. Cancer. 2017;117:685–694. doi: 10.1038/bjc.2017.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L., Lin C., Gong H., Wang C., Liu L., Wu J., Tao S., Hu B., Cheng S.Y., Li M., Li J. miR-486 sustains NF-κB activity by disrupting multiple NF-κB-negative feedback loops. Cell Res. 2013;23:274–289. doi: 10.1038/cr.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L., Yu L., Zhang X., Lei F., Wang L., Liu X., Wu S., Zhu J., Wu G., Cao L. miR-892b Silencing Activates NF-κB and Promotes Aggressiveness in Breast Cancer. Cancer Res. 2016;76:1101–1111. doi: 10.1158/0008-5472.CAN-15-1770. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen D.P., Li J., Yadav S.S., Tewari A.K. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014;114:168–176. doi: 10.1111/bju.12488. [DOI] [PubMed] [Google Scholar]
- 25.Chen P.C., Cheng H.C., Tang C.H. CCN3 promotes prostate cancer bone metastasis by modulating the tumor-bone microenvironment through RANKL-dependent pathway. Carcinogenesis. 2013;34:1669–1679. doi: 10.1093/carcin/bgt103. [DOI] [PubMed] [Google Scholar]
- 26.Ren D., Yang Q., Dai Y., Guo W., Du H., Song L., Peng X. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-κB signaling pathway. Mol. Cancer. 2017;16:117. doi: 10.1186/s12943-017-0688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Lin Y., Guo Z., Cheng J., Huang J., Deng L., Liao W., Chen Z., Liu Z., Su B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat. Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 28.Montagnana M., Benati M., Danese E., Giudici S., Perfranceschi M., Ruzzenenete O., Salvagno G.L., Bassi A., Gelati M., Paviati E. Aberrant MicroRNA Expression in Patients with Endometrial Cancer. Int. J. Gynecol. Cancer. 2017;27:459–466. doi: 10.1097/IGC.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Liu X.S., Liu H.Y., Lu Y.Y., Li Y. Reduced expression of serum miR-204 predicts poor prognosis of gastric cancer. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15027702. gmr7702. [DOI] [PubMed] [Google Scholar]
- 30.Jia W., Wu Y., Zhang Q., Gao G., Zhang C., Xiang Y. Identification of four serum microRNAs from a genome-wide serum microRNA expression profile as potential non-invasive biomarkers for endometrioid endometrial cancer. Oncol. Lett. 2013;6:261–267. doi: 10.3892/ol.2013.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koga T., Migita K., Sato T., Sato S., Umeda M., Nonaka F., Fukui S., Kawashiri S.Y., Iwamoto N., Ichinose K. MicroRNA-204-3p inhibits lipopolysaccharide-induced cytokines in familial Mediterranean fever via the phosphoinositide 3-kinase gamma pathway. Rheumatology. 2017;57:718–726. doi: 10.1093/rheumatology/kex451. [DOI] [PubMed] [Google Scholar]
- 32.Mikhaylova O., Stratton Y., Hall D., Kellner E., Ehmer B., Drew A.F., Gallo C.A., Plas D.R., Biesiada J., Meller J., Czyzyk-Krzeska M.F. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell. 2012;21:532–546. doi: 10.1016/j.ccr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu Y., Ren L., Xie B., Liang Z., Chen J. MiR-204 enhances mitochondrial apoptosis in doxorubicin-treated prostate cancer cells by targeting SIRT1/p53 pathway. Oncotarget. 2017;8:97313–97322. doi: 10.18632/oncotarget.21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y.H., Tang W., Zhang X., Tan Z., Guo W.L., Zhao N., Pang S.M., Dang Y.W., Rong M.H., Cao J. Promising significance of the association of miR-204-5p expression with clinicopathological features of hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e7545. doi: 10.1097/MD.0000000000007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song S., Fajol A., Tu X., Ren B., Shi S. miR-204 suppresses the development and progression of human glioblastoma by targeting ATF2. Oncotarget. 2016;7:70058–70065. doi: 10.18632/oncotarget.11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Díaz-Martínez M., Benito-Jardón L., Alonso L., Koetz-Ploch L., Hernando E., Teixidó J. miR-204-5p and miR-211-5p Contribute to BRAF Inhibitor Resistance in Melanoma. Cancer Res. 2018;78:1017–1030. doi: 10.1158/0008-5472.CAN-17-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu G., Wang J., Chen G., Zhao X. microRNA-204 modulates chemosensitivity and apoptosis of prostate cancer cells by targeting zinc-finger E-box-binding homeobox 1 (ZEB1) Am. J. Transl. Res. 2017;9:3599–3610. [PMC free article] [PubMed] [Google Scholar]
- 38.Todorova K., Metodiev M.V., Metodieva G., Zasheva D., Mincheff M., Hayrabedyan S. miR-204 is dysregulated in metastatic prostate cancer in vitro. Mol. Carcinog. 2016;55:131–147. doi: 10.1002/mc.22263. [DOI] [PubMed] [Google Scholar]
- 39.Hong B.S., Ryu H.S., Kim N., Kim J., Lee E., Moon H., Kim K.H., Jin M.S., Kwon N.H., Kim S. Tumor Suppressor miRNA-204-5p Regulates Growth, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Cancer Res. 2019;79:1520–1534. doi: 10.1158/0008-5472.CAN-18-0891. [DOI] [PubMed] [Google Scholar]
- 40.Edbrooke M.R., Burt D.W., Cheshire J.K., Woo P. Identification of cis-acting sequences responsible for phorbol ester induction of human serum amyloid A gene expression via a nuclear factor kappaB-like transcription factor. Mol. Cell. Biol. 1989;9:1908–1916. doi: 10.1128/mcb.9.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karin M., Greten F.R. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 42.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park B.K., Zhang H., Zeng Q., Dai J., Keller E.T., Giordano T., Gu K., Shah V., Pei L., Zarbo R.J. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat. Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 45.Burnett R.M., Craven K.E., Krishnamurthy P., Goswami C.P., Badve S., Crooks P., Mathews W.P., Bhat-Nakshatri P., Nakshatri H. Organ-specific adaptive signaling pathway activation in metastatic breast cancer cells. Oncotarget. 2015;6:12682–12696. doi: 10.18632/oncotarget.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 47.Cai P.C., Shi L., Liu V.W., Tang H.W., Liu I.J., Leung T.H., Chan K.K., Yam J.W., Yao K.M., Ngan H.Y., Chan D.W. Elevated TAK1 augments tumor growth and metastatic capacities of ovarian cancer cells through activation of NF-κB signaling. Oncotarget. 2014;5:7549–7562. doi: 10.18632/oncotarget.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devin A., Cook A., Lin Y., Rodriguez Y., Kelliher M., Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 49.Jin G., Klika A., Callahan M., Faga B., Danzig J., Jiang Z., Li X., Stark G.R., Harrington J., Sherf B. Identification of a human NF-kappaB-activating protein, TAB3. Proc. Natl. Acad. Sci. USA. 2004;101:2028–2033. doi: 10.1073/pnas.0307314101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen R.R., Zhou A.Y., Kim E., O’Connell J.T., Hagerstrand D., Beroukhim R., Hahn W.C. TRAF2 is an NF-κB-activating oncogene in epithelial cancers. Oncogene. 2015;34:209–216. doi: 10.1038/onc.2013.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Q., Lee F.S. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J. Biol. Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- 52.Ren D., Lin B., Zhang X., Peng Y., Ye Z., Ma Y., Liang Y., Cao L., Li X., Li R. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget. 2017;8:49807–49823. doi: 10.18632/oncotarget.17971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L., Lin J., Ye Y., Oba T., Gentile E., Lian J., Wang J., Zhao Y., Gu J., Wistuba I.I. Serum MicroRNA-150 Predicts Prognosis for Early-Stage Non-Small Cell Lung Cancer and Promotes Tumor Cell Proliferation by Targeting Tumor Suppressor Gene SRCIN1. Clin. Pharmacol. Ther. 2017;103:1061–1073. doi: 10.1002/cpt.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manier S., Liu C.J., Avet-Loiseau H., Park J., Shi J., Campigotto F., Salem K.Z., Huynh D., Glavey S.V., Rivotto B. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129:2429–2436. doi: 10.1182/blood-2016-09-742296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daniel R., Wu Q., Williams V., Clark G., Guruli G., Zehner Z. A Panel of MicroRNAs as Diagnostic Biomarkers for the Identification of Prostate Cancer. Int. J. Mol. Sci. 2017;18:18. doi: 10.3390/ijms18061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang Y.S., Chen W.Y., Yin J.J., Sheppard-Tillman H., Huang J., Liu Y.N. EGF Receptor Promotes Prostate Cancer Bone Metastasis by Downregulating miR-1 and Activating TWIST1. Cancer Res. 2015;75:3077–3086. doi: 10.1158/0008-5472.CAN-14-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terol M., Gazon H., Lemasson I., Duc-Dodon M., Barbeau B., Césaire R., Mesnard J.M., Péloponèse J.M., Jr. HBZ-mediated shift of JunD from growth suppressor to tumor promoter in leukemic cells by inhibition of ribosomal protein S25 expression. Leukemia. 2017;31:2235–2243. doi: 10.1038/leu.2017.74. [DOI] [PubMed] [Google Scholar]
- 58.Wang C.C., Bajikar S.S., Jamal L., Atkins K.A., Janes K.A. A time- and matrix-dependent TGFBR3-JUND-KRT5 regulatory circuit in single breast epithelial cells and basal-like premalignancies. Nat. Cell Biol. 2014;16:345–356. doi: 10.1038/ncb2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rimmelé P., Esposito M., Delestré L., Guervilly J.H., Ridinger-Saison M., Despras E., Moreau-Gachelin F., Rosselli F., Guillouf C. The Spi1/PU.1 transcription factor accelerates replication fork progression by increasing PP1 phosphatase in leukemia. Oncotarget. 2017;8:37104–37114. doi: 10.18632/oncotarget.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seki M., Kimura S., Isobe T., Yoshida K., Ueno H., Nakajima-Takagi Y., Wang C., Lin L., Kon A., Suzuki H. Recurrent SPI1 (PU.1) fusions in high-risk pediatric T cell acute lymphoblastic leukemia. Nat. Genet. 2017;49:1274–1281. doi: 10.1038/ng.3900. [DOI] [PubMed] [Google Scholar]
- 61.Mehraein-Ghomi F., Basu H.S., Church D.R., Hoffmann F.M., Wilding G. Androgen receptor requires JunD as a coactivator to switch on an oxidative stress generation pathway in prostate cancer cells. Cancer Res. 2010;70:4560–4568. doi: 10.1158/0008-5472.CAN-09-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto H., Oue N., Sato A., Hasegawa Y., Yamamoto H., Matsubara A., Yasui W., Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 63.Zerbini L.F., Wang Y., Cho J.Y., Libermann T.A. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–2215. [PubMed] [Google Scholar]
- 64.Wu N., Ren D., Li S., Ma W., Hu S., Jin Y., Xiao S. RCC2 over-expression in tumor cells alters apoptosis and drug sensitivity by regulating Rac1 activation. BMC Cancer. 2018;18:67. doi: 10.1186/s12885-017-3908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang M., Ren D., Guo W., Huang S., Wang Z., Li Q., Du H., Song L., Peng X. N-cadherin promotes epithelial-mesenchymal transition and cancer stem cell-like traits via ErbB signaling in prostate cancer cells. Int. J. Oncol. 2016;48:595–606. doi: 10.3892/ijo.2015.3270. [DOI] [PubMed] [Google Scholar]
- 66.Wang X., Sun D., Tai J., Chen S., Yu M., Ren D., Wang L. TFAP2C promotes stemness and chemotherapeutic resistance in colorectal cancer via inactivating hippo signaling pathway. J. Exp. Clin. Cancer Res. 2018;37:27. doi: 10.1186/s13046-018-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X., Ren D., Guo L., Wang L., Wu S., Lin C., Ye L., Zhu J., Li J., Song L. Thymosin beta 10 is a key regulator of tumorigenesis and metastasis and a novel serum marker in breast cancer. Breast Cancer Res. 2017;19:15. doi: 10.1186/s13058-016-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X., Zhang L., Lin B., Chai X., Li R., Liao Y., Deng X., Liu Q., Yang W., Cai Y. Phospholipid Phosphatase 4 promotes proliferation and tumorigenesis, and activates Ca2+-permeable Cationic Channel in lung carcinoma cells. Mol. Cancer. 2017;16:147. doi: 10.1186/s12943-017-0717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X., Liu F., Lin B., Luo H., Liu M., Wu J., Li C., Li R., Zhang X., Zhou K., Ren D. miR-150 inhibits proliferation and tumorigenicity via retarding G1/S phase transition in nasopharyngeal carcinoma. Int. J. Oncol. 2017 doi: 10.3892/ijo.2017.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang S., Tang Y., Peng X., Cai X., Wa Q., Ren D., Li Q., Luo J., Li L., Zou X., Huang S. Acidic extracellular pH promotes prostate cancer bone metastasis by enhancing PC-3 stem cell characteristics, cell invasiveness and VEGF-induced vasculogenesis of BM-EPCs. Oncol. Rep. 2016;36:2025–2032. doi: 10.3892/or.2016.4997. [DOI] [PubMed] [Google Scholar]
- 71.Ma Y., Huang H., Jiang J., Wu L., Lin C., Tang A., Dai G., He J., Chen Y. AVE 0991 attenuates cardiac hypertrophy through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2016;474:621–625. doi: 10.1016/j.bbrc.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 72.Ren D., Dai Y., Yang Q., Zhang X., Guo W., Ye L., Huang S., Chen X., Lai Y., Du H. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J. Exp. Med. 2019;216:428–449. doi: 10.1084/jem.20180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.