Abstract
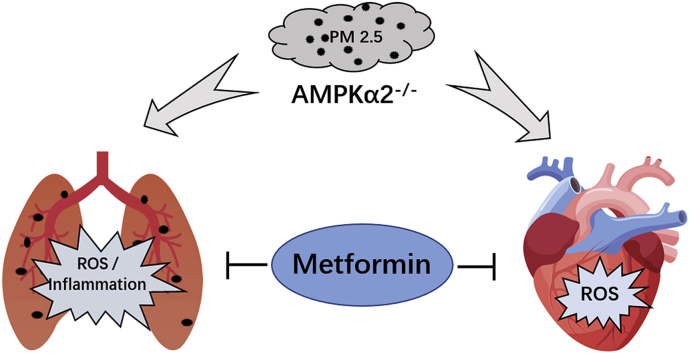
Fine particulate matter (PM2.5) airborne pollution increases the risk of respiratory and cardiovascular diseases. Although metformin is a well-known antidiabetic drug, it also confers protection against a series of diseases through the activation of AMP-activated protein kinase (AMPK). However, whether metformin affects PM2.5-induced adverse health effects has not been investigated. In this study, we exposed wild-type (WT) and AMPKα2−/− mice to PM2.5 every other day via intratracheal instillation for 4 weeks. After PM2.5 exposure, the AMPKα2−/− mice developed more severe lung injury and cardiac dysfunction than were developed in the WT mice; however the administration of metformin was effective in attenuating PM2.5-induced lung injury and cardiac dysfunction in both the WT and AMPKα2−/− mice. In the PM2.5-exposed mice, metformin treatment resulted in reduced systemic and pulmonary inflammation, preserved left ventricular ejection fraction, suppressed induction of pulmonary and myocardial fibrosis and oxidative stress, and increased levels of mitochondrial antioxidant enzymes. Moreover, pretreatment with metformin significantly attenuated PM2.5-induced cell death and oxidative stress in control and AMPKα2-depleted BEAS-2B and H9C2 cells, and was associated with preserved expression of mitochondrial antioxidant enzymes. These data support the notion that metformin protects against PM2.5-induced adverse health effects through a pathway that appears independent of AMPKα2. Our findings suggest that metformin may also be a novel drug for therapies that treat air pollution associated disease.
Keywords: Metformin, AMPKα2, PM2.5, Lung injury, Cardiac dysfunction
Graphical abstract
Highlights
-
•
Metformin protects against PM2.5 exposure-induced lung injury and cardiac dysfunction.
-
•
Metformin alleviates PM2.5-induced oxidative stress by regulating mitochondrial antioxidant enzymes.
-
•
Metformin attenuates PM2.5-induced cell death and oxidative stress in BEAS-2B and H9C2 cells.
-
•
Metformin confers protection in an AMPKα2 independent manner.
Abbreviation
- 3′-NT
3′-nitrotyrosine
- 4-HNE
4-hydroxynonenal
- AMPK
AMP sensitive protein kinase
- ANP
atrial natriuretic peptide
- ATF-3
transcription factor-3
- BCA
bicinchoninic acid
- DCFH-DA
2′, 7′-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidium
- DMEM
Dulbecco's modified eagle medium
- EF
ejection fraction
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- IL
interleukin
- LV
left ventricular
- mTOR
mammalian target of rapamycin
- PRDX
peroxiredoxin
- qPCR
quantitative real-time polymerase chain reaction
- ROS
reactive oxygen species
- SOD2
superoxide dismutase 2
- TGF-β
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
- TRX2
thioredoxin 2
- TRXR2
thioredoxin reductase 2
- WGA
germ agglutinin
1. Introduction
Fine particulate matter (PM2.5, aerodynamic diameter ≤ 2.5 μm) ambient pollution has become a major issue that threatens public health in China and other developing countries [1]. PM2.5 contains many toxic chemical compositions, including polycyclic aromatic hydrocarbons, transition metals, and endotoxins [2,3]. Because of the small size of its components, PM2.5 is easily inhaled into the airway and deposited in lung alveoli [4]. Particles that deposit in the alveolar tissue can translocate from the alveoli into the bloodstream to affect the cardiovascular system [5]. Epidemiological studies have demonstrated that long-term exposure to high concentrations of PM2.5 increases the risk of a number of cardiorespiratory illnesses, such as asthma [6], bronchitis [7], chronic obstructive pulmonary disease (COPD) [8], coronary artery disease [9] and heart failure [10]. As revealed by many toxicological studies, the adverse health effects of PM2.5 are associated with enhanced inflammation and oxidative stress [11,12]. Considering that ambient PM2.5 has maintained high levels for several years and air quality could not be improved in a short period, it is urgent to find effective therapeutic approaches for attenuating PM2.5 associated diseases.
AMP sensitive protein kinase (AMPK) is an important cellular energy sensor that is activated by an elevated AMP/ATP ratio [13]. Recently, it has been reported that PM2.5 exposure activated AMPK to promote autophagy in human lung epithelial A549 and BEAS-2B cells [14,15]. Using a whole-body exposure system, we recently demonstrated that AMPKα2 deficiency exacerbated long-term PM2.5-induced lung injury and cardiac dysfunction [16]. In contrast, the activation of AMPK by 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) ameliorates the ambient PM2.5-induced inflammatory response in healthy and diabetic mice [17].
Metformin, one of the most widely prescribed antidiabetic drugs, is a well-known AMPK activator. In experimental animal models, metformin was found to attenuate pulmonary and cardiovascular inflammation and/or oxidative stress through activation of AMPK [[18], [19], [20], [21], [22]]. Interestingly, a recent study showed that metformin prevents concentrated ambient PM2.5-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis [23]. Metformin also reduces the generation of mitochondrial reactive oxygen species (ROS) and interleukin 6 (IL-6) release in PM2.5-exposed alveolar macrophage cells, and prevents PM2.5-induced acceleration of carotid thrombosis [24]. However, whether metformin affects PM2.5-induced pulmonary and cardiovascular disorders remains unclear. Furthermore, we previously demonstrated that metformin was equally effective in attenuating transverse aortic constriction (TAC)-induced left ventricular hypertrophy and heart failure in both wild-type (WT) and AMPKα2−/− mice [25]. Thus, in this study, we treated PM2.5-exposed WT and AMPKα2−/− mice with metformin to determine whether metformin could protect against PM2.5-induced lung injury and cardiac dysfunction through an AMPKα2 dependent pathway.
2. Materials and methods
2.1. Antibodies and reagents
Antibodies against peroxiredoxin 3 (PRDX3), peroxiredoxin 5 (PRDX5), superoxide dismutase 2 (SOD2), thioredoxin reductase 2 (TRXR2), thioredoxin 2 (TRX2), Bcl-2 and β-tubulin, and ELISA kits for IL-6, tumor necrosis factor alpha (TNFα) and 3′-nitrotyrosine (3′-NT) were purchased from Abcam PLC (#ab73349, #ab180587, #ab13533, #ab180493, #ab185544, #ab194583, #ab6046, #ab100712, #ab108910, and #ab116691, Cambridge, UK). AMPKα1 and AMPKα2 antibodies were obtained from GeneTex (#GTX60403 and #GTX103487, Irvine, CA, USA). AMPKα and phosphor-AMPKαThr172 antibodies were purchased from Cell Signaling Technology (#5831 and #2535, Danvers, MA, USA). Galectin-3 and neutrophil antibodies were obtained from Bioss Biotechnology Co. Ltd. (#bs-20700R and #bs-6982R, Beijing, China). Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS), TRIzol and MTT were purchased from Invitrogen (Carlsbad, CA, USA). 2′, 7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) and dihydroethidium (DHE) were purchased from Sigma Chemical Co. (#D6883 and #D7008, St. Louis, MO, USA). An ELISA kit for 4-hydroxynonenal (4-HNE) was obtained from Donggeboye Biological Technology Co. Ltd (#DG30947 M, Beijing, China). Metformin and kits for TUNEL staining and bicinchoninic acid (BCA) assay were obtained from the Beyotime Institute of Biotechnology (#S1741, #C1038 and #P0010, Shanghai, China). Short hairpin RNA (shRNA) sequences targeting AMPKα2 (AATGGAATATGTGTCTGGAGG) or AMPKα1 (AGGCATCCTCATATAATTAAA or GCCTACCATCTCATAATAGAT) were constructed into the pLKO.1 lentiviral vector [26] (Addgene Plasmid #10878) for viral packaging. PM2.5 was collected using high-volume sampler particle collectors and the morphology, size distribution and component of the constituents have been described in a previous study [16]. All other chemicals made in China were of analytical grade.
2.2. Experimental animals
Animal studies were performed in accordance with the principles of laboratory animal care (NIH publication no. 85–23, revised 1985) and with approval from the University of Chinese Academy of Sciences Animal Care and Use Committee. Male C57BL/6J and AMPKα2−/− mice [27] (C57BL/6J background), at 8–10 weeks of age, were treated with 10 μl phosphate-buffered saline (PBS) or 10 mg/kg PM2.5 in 10 μl PBS every other day via intratracheal instillation and the mice were sacrificed at 4 weeks after the instillation treatment. The PM2.5-exposed mice were treated with metformin in drinking water (300 mg/kg/day) during the experimental period.
2.3. Histopathology staining
After perfusion with PBS, the mouse lung and heart tissues were harvested, washed, fixed with formalin and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E), Masson's trichrome stain kit (ScyTek Laboratories, Inc., UT, USA) or a TUNEL stain kit to assess fibrosis and apoptosis, respectively. As previously described [16], lung sections were also stained with monoclonal galectin-3 and neutrophil antibodies to identify macrophages and neutrophils, respectively. Frozen heart sections (4 μm) were stained with CF488A conjugated wheat germ agglutinin (WGA, #29022, Biotium Inc. Fremont, CA, USA) or DHE for 30 min to assess myocyte cross-sectional areas and superoxide levels, respectively. At least 4 mice per group were used for these experiments.
2.4. Echocardiographic measurement
As previously described [28], the mice were anesthetized with 1.5% isoflurane and echocardiographic images were obtained using a VisualSonics high resolution Veve 2100 system (Visual Sonics, Toronto, ON, Canada).
2.5. Cell culture and exposure of cells to PM2.5
The human bronchial epithelial BEAS-2B cell line and the rat cardio myoblast H9C2 cell line were obtained from the China Infrastructure of Cell Line Resource (Beijing, China) and maintained in DMEM supplemented with 10% FBS and 1% penicillin and streptomycin at 37 °C with 5% CO2. After being cultured for 24 h, the cells were pretreated with PBS or 1 mM metformin for 2 h. Then the culture medium was replaced with serum-free (for BEAS-2B) or 1% FBS (for H9C2) medium and the cells were exposed to freshly dispersed PM2.5 preparations for 24 h. Cell viability was measured with a MTT assay and intracellular ROS levels were determined by a Synergy H1 Hybrid Multi-Mode microplate reader (Biotek Instruments, Inc., Winooski, VT, USA) using DCFH-DA.
To generate a stable AMPKα2-knockdown cell line, 5 × 105 exponentially growing cells were transfected with AMPKα2 shRNA lentivirus for 24 h and then with puromycin (1 μg/mL) for 3 weeks for selection.
2.6. Quantitative real-time PCR and western blotting
Total RNA was extracted with TRIzol reagent and cDNA was synthesized using a PrimeScript RT reagent kit (#RR036B, TaKaRa, Otsu, Japan). A quantitative real-time polymerase chain reaction (qPCR) assay was performed using the SYBR Premix Ex Taq™ II Kit (#RR820DS, TaKaRa) and the results were normalized to 18 S ribosomal RNA. The primers used in the qPCR assay are listed in Table S1.
Proteins were extracted from the lung, heart or cultured cells using buffer (50 mM Tris-Cl, 150 mM NaCl, 100 μg/ml phenylmethylsulfonyl fluoride, protease and phosphatase inhibitor cocktail (#046931124001 and #4906837001, Roche, Basel, Switzerland), and 1% Triton X-100) on ice for 30 min. After 12000 g centrifugation at 4 °C for 20 min, the supernatant was used for Western blot analysis.
2.7. Data and statistical analysis
All values are expressed as the mean ± standard error of means (SEM). One- or two-way analysis of variance (ANOVA) was used to test each variable for differences among the treatment groups with StatView (SAS Institute Inc, Cary, NC, USA). If ANOVA demonstrated a significant effect, pairwise post hoc comparisons were made with the Fisher's least significant difference test. Statistical significance was defined as p < 0.05.
3. Results
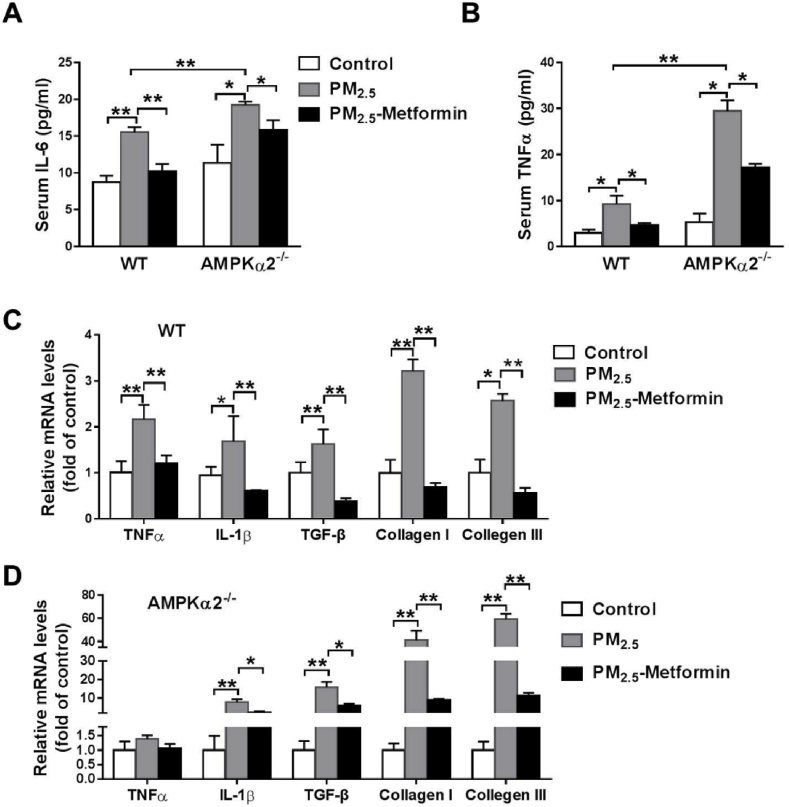
Metformin attenuates PM2.5-induced inflammation and fibrosis in both WT and AMPKα2−/− mice. Compared with the control mice, PM2.5-exposed mice exhibited significantly greater lung weight and higher lung weight to body weight ratio (Table S2). To investigate the protective effects of metformin on PM2.5-induced lung injury and cardiac dysfunction, we treated the PM2.5-exposed mice with metformin or a vehicle (untreated drinking water) for 4 weeks. PM2.5 exposure caused significant increases in serum TNFα and IL-6 levels in the WT and AMPKα2−/− mice, while the AMPKα2−/− mice exhibited higher serum TNFα and IL-6 levels than exhibited in the WT mice. However, metformin significantly attenuated the increase in serum TNFα and IL-6 levels in the PM2.5-exposed WT and AMPKα2−/− mice (Fig. 1A–B). Next, we performed qPCR to examine the effects of metformin on the mRNA levels of genes related to inflammation and fibrosis. In the WT mice, we found that PM2.5 exposure significantly increased the mRNA levels of TNFα, IL-1, transforming growth factor beta (TGF-β) and collagen I and III, and these increases were significantly attenuated by metformin treatment (Fig. 1C). Moreover, metformin also significantly attenuated the upregulation of TGF-β, and collagen I and III in the PM2.5-exposed AMPKα2−/− mice (Fig. 1D).
Fig. 1.
Metformin alleviates PM2.5-induced systemic and pulmonary inflammation. WT and AMPKα2−/− mice were administered PBS or 10 mg/kg PM2.5 every other day via intratracheal instillation for 4 weeks. During the entire experimental period, some of the PM2.5-exposed mice were treated with metformin in the drinking water (300 mg/kg/day). After PM2.5 exposure, serum IL-6 and TNFα levels (A–B), and mRNA levels of inflammatory and fibrotic genes in the lungs of WT and AMPKα2−/− mice were measured (C–D). N = 5; data are presented as the mean ± SEM; * indicates p < 0.05, ** indicates p < 0.01.
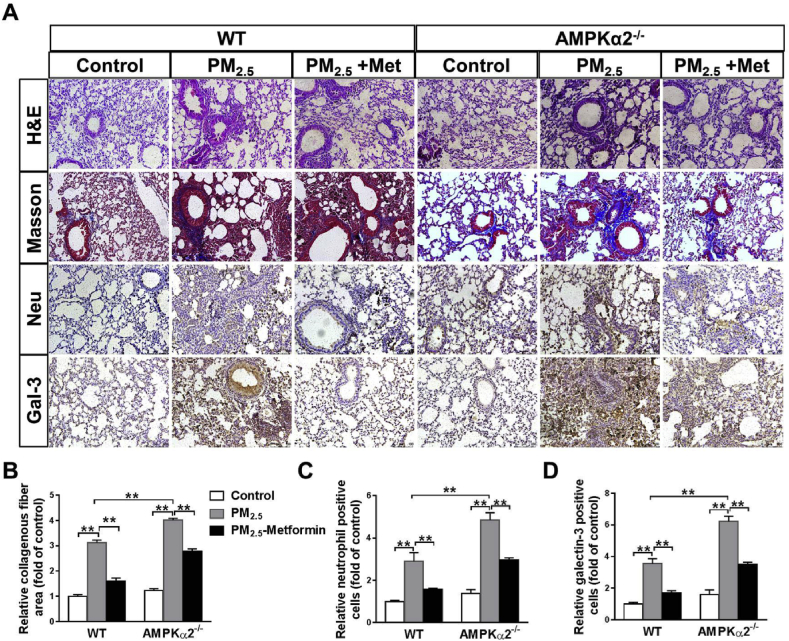
H&E and Masson's staining of the lung sections showed that PM2.5 exposure caused significant lung injury and fibrosis in the WT and AMPKα2−/− mice, as showed by alveoli collapse, airway epithelial thickening and collagen deposition. Immunohistochemical staining using antibodies against neutrophils and galectin-3 (a macrophage-specific marker) showed that PM2.5 exposure resulted in significant infiltration of neutrophils and macrophages in the lungs of WT and AMPKα2−/− mice. After PM2.5 exposure, the lungs of AMPKα2−/− mice exhibited more severe injury, fibrosis and infiltration of macrophages and neutrophils than the lungs of the WT mice. In both the WT and AMPKα2−/− mice, PM2.5-induced lung injury, fibrosis and inflammatory cell infiltration were significantly attenuated by metformin administration (Fig. 2). Taken together, these results indicate that metformin protects against lung fibrosis and inflammation in PM2.5-exposed mice in an AMPKα2 independent manner.
Fig. 2.
Metformin ameliorates PM2.5-induced pulmonary fibrosis and inflammation. (A) Representative lung sections from the PBS- and PM2.5-exposed WT and AMPKα2−/− mice with or without metformin (Met) treatment were stained with hematoxylin and eosin (H&E), Masson's trichrome, and antibodies specific for neutrophils and macrophages (galectin-3, Gal-3) (brown staining). Scale bar = 100 μm. The relative collagenous fiber area (B), and the number of Gal-3 positive cells (C) and neutrophils (D) were quantified. N = 4; ** indicates p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
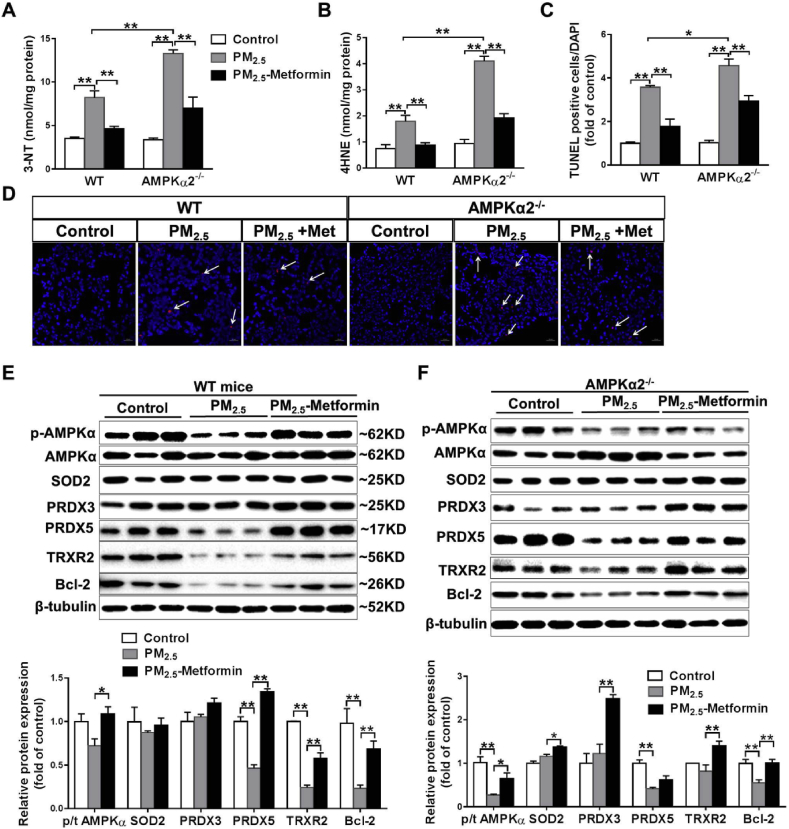
Metformin prevents PM2.5-induced oxidative stress and cell death in the lungs of WT and AMPKα2−/− mice. To determine whether metformin affects PM2.5-induced pulmonary oxidative stress, we measured the levels of two oxidative stress markers, 3′-NT and 4-HNE. PM2.5 exposure for 4 weeks significantly increased 3′-NT and 4-HNE levels in the lungs of the WT and AMPKα2−/− mice. Compared with the lungs of WT mice, those of the AMPKα2−/− mice exhibited significantly higher levels of 3′-NT and 4-HNE. Metformin administration significantly decreased pulmonary 3′-NT and 4-HNE levels in PM2.5-exposed WT and AMPKα2−/− mice (Fig. 3A–B). TUNEL staining revealed that PM2.5 exposure caused more TUNEL-positive cells in the lungs of AMPKα2−/− mice than in the lungs of the WT mice, while metformin treatment significantly decreased the apoptotic cells in the lungs of the PM2.5-exposed WT and AMPKα2−/− mice (Fig. 3C–D). Thus, these results indicated that the protective effect of metformin against PM2.5-induced oxidative stress and cell apoptosis is AMPKα2-independent.
Fig. 3.
Metformin attenuates PM2.5-induced pulmonary oxidative stress and cell death. After PM2.5 exposure, the levels of 3′-nitrotyrosine (3′-NT) (A) and 4-hydroxynonenal (4-HNE) (B) in lung tissue were measured. Lung sections from the control and PM2.5-exposed mice were stained with DAPI (blue) and TUNEL assay kit dye (red) (scale bar = 20 μm, arrows point to TUNEL-positive cells), and the TUNEL-positive cells were quantified (C, D). Lysates of lung tissue were examined by western blotting to determine the expression levels of total and phosphorylated AMPKα, superoxide dismutase 2 (SOD2), peroxiredoxin 3(PRDX3), PRDX5, thioredoxin reductase 2 (TRXR2) and Bcl-2. β-Tubulin was used as a loading control (E, F). N = 3–5; data are presented as the mean ± SEM; * indicates p < 0.05, ** indicates p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the lungs of the WT mice, the phosphorylation of AMPK was reduced in response to PM2.5 exposure, and this reduction in p-AMPK levels was diminished by metformin administration (Fig. 3E). PM2.5 exposure with or without metformin treatment did not affect the expression of AMPKα1 and AMPKα2 (Fig. S1). Because AMPKα2 deletion affects mitochondrial swelling and PRDX5 expression in PM2.5-exposed lungs [16], we performed Western blot analysis to determine the protein expression of pulmonary mitochondrial antioxidant enzymes. As shown in Fig. 3E, PM2.5 exposure had no obvious effect on SOD2 and PRDX3 expression, but significantly decreased the protein expression of PRDX5, TRXR2 and the anti-apoptotic protein Bcl-2. Metformin treatment significantly attenuated the PM2.5-induced reduction in PRDX5, TRXR2 and Bcl-2 expression. In the lungs of AMPKα2−/− mice, PM2.5 exposure resulted in significant decreases in p-AMPK, PRDX5 and Bcl-2 expression, and increases in AMPKα1 expression. The decreases in p-AMPK and Bcl-2 levels, as well as the induction of AMPKα1, were attenuated by metformin treatment. Moreover, metformin significantly increased the protein expression levels of SOD2, PRDX3 and TRXR2 in the lungs of the PM2.5-exposed AMPKα2−/− mice (Fig. 3F, Fig. S1).
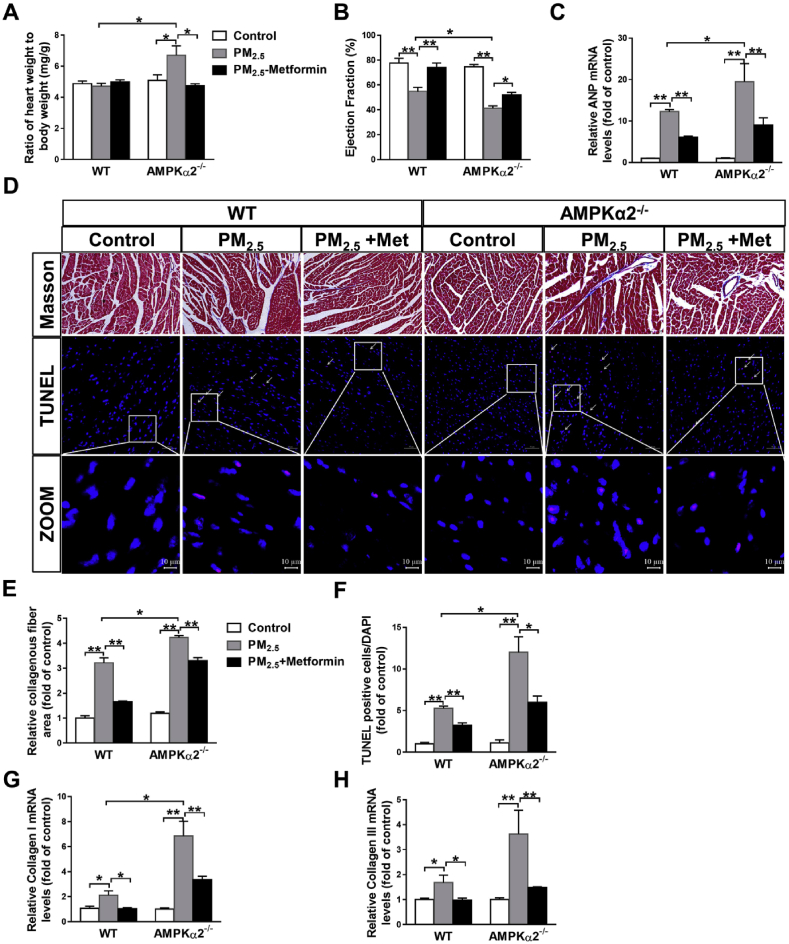
Metformin attenuates PM2.5-induced cardiac dysfunction and fibrosis. PM2.5 exposure had no obvious effect on body weight, heart weight or the ratio of heart weight to body weight in the WT mice, but significantly increased the heart weight, ratio of heart weight to body weight and cardiac myocyte cross-sectional area in the AMPKα2−/− mice. Metformin treatment significantly attenuated the increase in the ratio of heart weight to body weight and cardiac myocyte cross-sectional area in the PM2.5-exposed AMPKα2−/− mice (Tables S2–S3, Fig. 4A, and Fig. S2). Echocardiographic examination showed that PM2.5 exposure resulted in cardiac dysfunction in the WT and AMPKα2−/− mice, as indicated by the significant reduction in left ventricular (LV) ejection fraction (EF). In response to PM2.5 exposure, the AMPKα2−/− mice developed more severe LV dysfunction than the WT mice. Metformin significantly increased the LV EF values in the PM2.5-exposed WT and AMPKα2−/− mice (Fig. 4B). Furthermore, metformin also significantly attenuated PM2.5-induced upregulation of myocardial atrial natriuretic peptide (ANP; a marker for cardiac stress) (Fig. 4C). The results from Masson's staining and TUNEL assay demonstrated that the hearts from the WT and AMPKα2−/− mice developed obvious fibrosis and cardiomyocyte apoptosis after PM2.5 exposure, while metformin significantly decreased the collagenous fibrotic area and apoptotic cell numbers in the hearts of the PM2.5-exposed mice (Fig. 4D–F). As shown in Fig. 4G–H, metformin also attenuated PM2.5-induced increases in myocardial collagen I and III mRNA levels in WT and AMPKα2−/− mice. Together, these results indicated that metformin effectively attenuates PM2.5-induced cardiac dysfunction in both wild type and AMPKα2−/− mice.
Fig. 4.
Metformin ameliorates PM2.5-induced cardiac dysfunction, myocardial fibrosis and cardiomyocyte apoptosis. After PM2.5 exposure, the heart weight to body weight ratio (A), LV ejection fraction (B) and mRNA levels of atrial natriuretic peptide (ANP) (C) were measured. Representative heart sections from the control and PM2.5- or PM2.5 with metformin-treated WT and AMPKα2−/− mice were stained with Masson's trichrome (scale bar = 100 μm) and TUNEL assay kit dye (red) plus DAPI (blue) (scale bar = 50 μm) (D). The fibrotic area (E) and the TUNEL-positive cells were quantified (F) were quantified. The mRNA levels of myocardial collagen I and III were measured (G, H). N = 4–7; data are presented as the mean ± SEM; * indicates p < 0.05, ** indicates p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Metformin attenuates PM2.5-induced myocardial oxidative stress. PM2.5 exposure significantly increased myocardial 3′-NT and 4-HNE levels in the WT and AMPKα2−/− mice, while these increases were significantly attenuated by metformin treatment (Fig. 5A–B). To investigate the effects of PM2.5/metformin on myocardial superoxide generation, the heart sections were stained with Mitosox to evaluate the mitochondrial superoxide levels. As shown in Fig. 5C–D, PM2.5 exposure caused significant increases in the mitochondrial superoxide levels in the hearts of the WT and AMPKα2−/− mice. In both WT and AMPKα2−/− mice, PM2.5-induced myocardial superoxide overproduction was significantly attenuated by metformin administration (Fig. 5C–D). Similar results were also obtained from DHE staining (Fig. S3). In the hearts of the WT mice, PM2.5 exposure significantly decreased the protein expression of SOD2, PRDX3, PRDX5 and TRXR2, and this reduction was significantly attenuated by metformin treatment. PM2.5 exposure decreased AMPKα1 expression but had no significant effect on AMPKα2 or p-AMPKα expression levels. Metformin significantly increased the phosphorylation levels of AMPKα and TRX2 expression in the PM2.5-exposed hearts but did not affect AMPKα1 or AMPKα2 expression (Fig. 5E, Fig. S4). In hearts of AMPKα2−/− mice, PM2.5 exposure had no obvious effect on SOD2, PRDX3 or AMPKα1 expression, but significantly decreased p-AMPK levels and the protein expression of PRDX5, TRXR2 and TRX2, and these decreases were significantly attenuated by metformin (Fig. 5F, Fig. S4).
Fig. 5.
Metformin attenuates myocardial oxidative stress in PM2.5-exposed mice. After PM2.5 exposure, heart tissue was collected, and the myocardial 3′-nitrotyrosine (3′-NT) (A) and 4-HNE levels were measured. The heart sections were stained with Mitosox (scale bar = 20 μm), and the relative Mitosox fluorescence intensity was quantified (C, D). Lysates of the heart tissue were examined by western blotting for the expression of p-AMPKα, SOD2, PRDX5, PRDX3, TRXR2 and TRX2. β-Tubulin was used as a loading control (E, F). N = 4; data are presented as the mean ± SEM; * indicates p < 0.05, ** indicates p < 0.01.
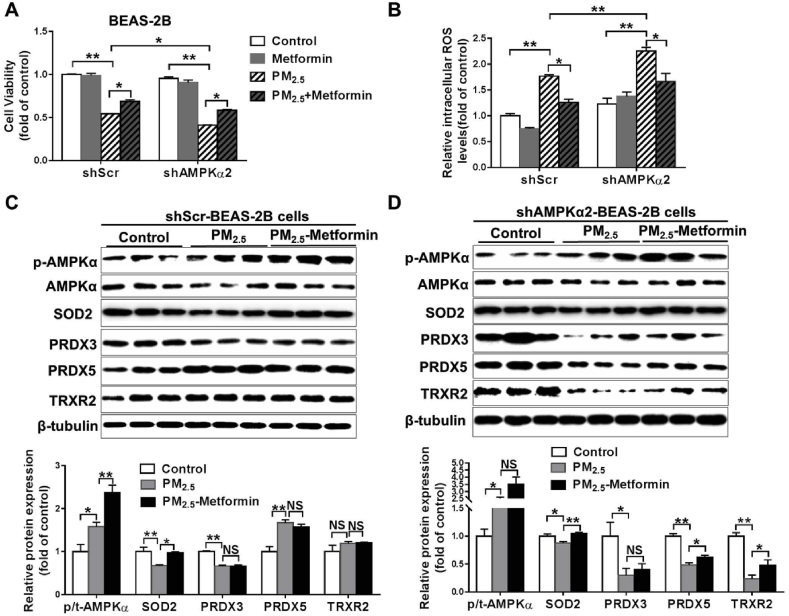
Metformin pretreatment attenuates PM2.5-induced cell death and oxidative stress. To determine whether metformin affects PM2.5-induced cell death in an AMPKα2-dependent manner, BEAS-2B and H9C2 cells were stably transfected with a shRNA lentiviral vector targeting scramble sequence (shScr) or AMPKα2 (shAMPKα2). The efficiency of knockdown was confirmed by Western blot analysis (Fig. S5). The cells were pretreated with 1 mM metformin for 2 h and were then replaced with fresh medium and exposed to 50 μg/ml PM2.5 for 24 h. After PM2.5 exposure, the shAMPKα2-BEAS-2B cells exhibited significantly lower cell viability and higher intracellular ROS levels than the shScr-BEAS-2B cells. Pretreatment with metformin significantly attenuated PM2.5-induced cell viability loss and increases in intracellular ROS levels in both shScr- and shAMPKα2-BEAS-2B cells (Fig. 6A–B). In the shScr-BEAS-2B cells, PM2.5 exposure had no obvious effect on the phosphorylation levels of AMPK or the expression of PRDX3 and TRXR2 but resulted in significant decreases in SOD2 expression and increases in PRDX5 expression. Metformin pretreatment increased p-AMPK and SOD2 expression in the PM2.5-exposed shScr-BEAS-2B cells (Fig. 6C). In the shAMPKα2-BEAS-2B cells, PM2.5 exposure significantly increased p-AMPK levels, whereas SOD2, PRDX3, PRDX5 and TRXR2 expression was decreased. Although metformin pretreatment did not significantly increase p-AMPK levels in PM2.5-exposed shAMPKα2-BEAS-2B cells, it significantly attenuated the reduction in SOD2, PRDX5 and TRXR2.
Fig. 6.
Metformin attenuates PM2.5-induced cell death and oxidative stress in BEAS-2B cells. Scramble shRNA (shScr)- and AMPKα2-specific shRNA (shAMPKα2)-stably transfected BEAS-2B cells were pretreated with PBS or 1 mM metformin for 2 h and then exposed to 50 μg/ml PM2.5 for 24 h. Cell viability (A) and intracellular ROS levels (B) were then determined. Lysates of the control and PM2.5-exposed shScr (C) or shAMPKα2 cells (D) with or without metformin pretreatment were examined by western blotting to determine the expression of phosphorylated AMPKα, SOD2, PRDX3, PRDX5 and TRXR2. β-Tubulin was used as a loading control. N = 3–4; data are presented as the mean ± SEM; * indicates p < 0.05, ** indicates p < 0.01, NS, not significant.
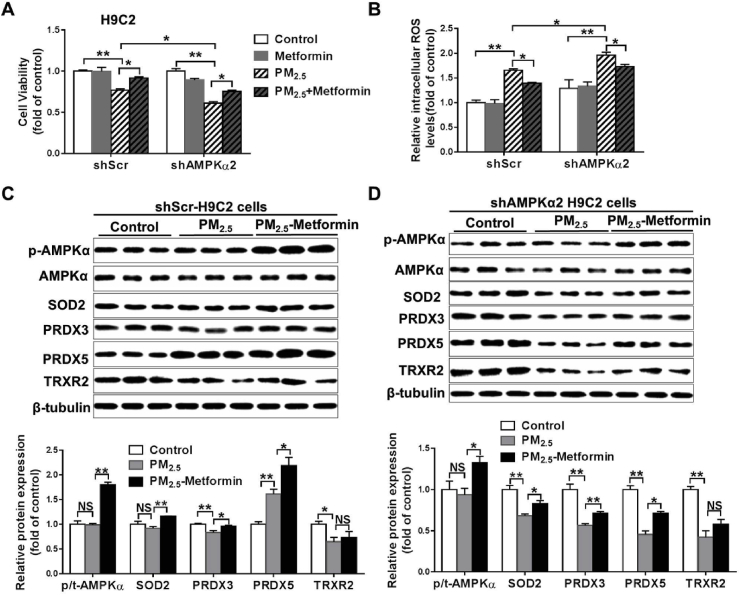
In the H9C2 cells, PM2.5 exposure significantly decreased cell viability, whereas it increased intracellular ROS levels in both the shScr and shAMPKα2 cells. However, the shAMPKα2-H9C2 cells exhibited significantly lower cell viability and higher intracellular ROS levels than the shScr-H9C2 cells. Pretreatment with 1 mM metformin significantly attenuated PM2.5-induced cell viability loss and oxidative stress in both the shScr and shAMPKα2 cells (Fig. 7A–B). In the shScr-H9C2 cells, PM2.5 exposure decreased PRDX3 and TRXR2 expression, whereas it increased PRDX5 expression. In shAMPKα2-H9C2 cells, PM2.5 exposure significantly decreased the expression of SOD2, PRDX3, PRDX5 and TRXR2. In these PM2.5-exposed cells, metformin pretreatment significantly increased p-AMPK, SOD2, PRDX3 and PRDX5 expression (Fig. 7C–D). We also stably transfected BEAS-2B and H9C2 cells with a shRNA lentiviral vector targeting AMPKα1 (shAMPKα1). In these shAMPKα1 stably transfect BEAS-2B and H9C2 cells, PM2.5-induced cell viability loss and oxidative stress could also be attenuated by pretreatment with metformin (Fig. S6). Taken together, these results indicate that metformin protects against PM2.5-induced cell death and oxidative stress in an AMPKα1 or AMPKα2 independent manner.
Fig. 7.
Metformin ameliorates PM2.5-induced cell death and oxidative stress in H9C2 cells. H9C2 cells stably transfected with shScr or shAMPKα2 were pretreated with PBS or 1 mM metformin for 2 h and then exposed to 50 μg/ml PM2.5 for 24 h. Cell viability (A) and intracellular ROS levels (B) were then determined. Lysates of the control and the PM2.5-exposed shScr- or shAMPKα2-H9C2 cells with or without of metformin pretreatment were examined by western blotting to determine the expression levels of phosphorylated AMPKα, SOD2, PRDX3, PRDX5 and TRXR2. β-Tubulin was used as a loading control (C-D). N = 3–4, data are mean ± SEM; * indicates p < 0.05, ** indicates p < 0.01, NS, not significant.
4. Discussion
The present study has two major new findings. First, we demonstrated that metformin was effective in attenuating PM2.5-induced lung injury and cardiac dysfunction in both WT and AMPKα2−/− mice, indicating that metformin protects mice from PM2.5 exposure in part, through an AMPKα2-independent pathway. Second, the protective effect of metformin in PM2.5-exposed mice was associated with the upregulation of mitochondrial antioxidant enzymes.
Metformin, a miracle drug, is well known for controlling hyperglycemia via regulation of hepatic glucose production. However, growing epidemiological and experimental studies have suggested that metformin has multiple additional health benefits in diabetic patients [[29], [30], [31]]. For example, metformin was found to decrease the risk of mortality from all causes and cardiovascular death in patients with type-2 diabetes mellitus [30]. Moreover, metformin is also known for its anti-aging [32] and anti-cancer effects [33]. Interestingly, two recent studies demonstrated that metformin prevents PM2.5-induced vascular insulin resistance [23] and acceleration of carotid thrombosis [24]. In addition, metformin can also alleviate endotoxemia-induced acute lung injury [18,34] and transverse aortic constriction (TAC)-induced heart failure [25,35]. Thus, the finding that metformin attenuated lung injury and cardiac dysfunction in PM2.5-exposed mice was fully anticipated.
PM2.5 exposure is always associated with excessive production of pro-inflammatory cytokines and remarkable pulmonary inflammatory responses [[36], [37], [38]]. The finding that metformin decreased serum TNFα and IL-6 levels and pulmonary TNFα and IL-1β mRNA levels in PM2.5-exposed mice suggested that metformin protects PM2.5-induced lung injury, at least partially, by suppressing inflammation. Our results were consistent with previous reports that showed that metformin alleviated systemic and pulmonary inflammation in lipopolysaccharide (LPS)-induced endotoxic mice [18,34]. The anti-inflammatory actions of metformin have also been found in different models, and the involved molecular mechanisms include suppression of mammalian target of rapamycin (mTOR) or toll-like receptor 4 signaling [18,34], induction of transcription factor-3 (ATF-3) [39], phosphorylation of histone deacetylase 5 (HDAC5) and restoration of Krüppel-like factor 2 (KLF2) [40]. Since AMPK can directly or indirectly affect the inflammatory response, these anti-inflammatory mechanisms of metformin were dependent on AMPK activation. Although AMPKα2 deletion exacerbated the PM2.5-induced pulmonary inflammatory response, in this study we found that metformin was effective in decreasing the systemic and pulmonary inflammatory responses in PM2.5-exposed AMPKα2−/− mice, indicating that metformin can also suppress inflammation through an AMPKα2-independent pathway.
PM2.5 has the ability to induce high levels of ROS [41], and the overproduction of intracellular ROS causes inflammatory cytokine release and DNA damage, which leads to lung injury [42]. PM2.5 exposure may also promote oxidative stress by decreasing the expression of antioxidant enzymes [16,37]. In the present study, we found that PM2.5 exposure significantly increased pulmonary 3′-NT and 4-HNE levels, which were associated with the reduction in mitochondrial antioxidant enzymes (PRDX5 and TRXR2). The finding that metformin attenuates PM2.5-induced pulmonary oxidative stress and the reduction in PRDX5 and TRXR2 expression suggested that metformin may act as a kind of antioxidant. Moreover, a similar antioxidative effect of metformin has also been described in other experimental models [[43], [44], [45]]. For example, metformin attenuates ischemia reperfusion-induced oxidative stress in fatty livers by increasing antioxidant enzyme activity [44]. Metformin also prevents PM2.5-induced mitochondrial ROS generation in alveolar macrophages, and mitochondrially targeted antioxidants mimic the effects of metformin in vitro and in vivo [24]. As AMPKα2 deletion exacerbated PM2.5-induced oxidative stress and the reduction in PRDX5 [16], it is possible that metformin may suppress ROS production through activating AMPKα. However, we did find that metformin decreased the 3′-NT or 4-HNE levels in the lungs of PM2.5-exposed AMPKα2−/− mice. Moreover, metformin also attenuated PM2.5-induced cell death and oxidative stress in AMPKα2-depleted BEAS-2B cells. Thus, it is likely that metformin inhibits PM2.5-induced pulmonary oxidative stress independent of AMPKα2.
Numerous epidemiological studies have demonstrated that PM2.5 exposure is strongly tied to cardiovascular diseases, including arrhythmias, ischemic heart disease, heart failure, and cardiac death [4,10,46]. In this regard, the finding that metformin reduced cardiac dysfunction in PM2.5-exposed mice is of considerable interest. Metformin has been shown to be effective in improving cardiac function in diabetic patients and experimental animal models, including rapid ventricular pacing-, TAC-, or doxorubicin-induced heart failure [25,35,47,48]. Mechanistically, the cardioprotective effect of metformin is associated with AMPK-mediated enhancement of the myocardial metabolic energy status, reducing oxidative stress and inhibiting cardiomyocyte apoptosis in failing hearts [[47], [48], [49], [50]]. The finding that metformin treatment decreases myocardial 3′-NT, 4-HNE and superoxide levels in PM2.5-exposed mice suggested that metformin attenuates PM2.5-induced cardiotoxicity by decreasing oxidative stress. Moreover, metformin also attenuates PM2.5-induced cardiac dysfunction and myocardial oxidative stress in AMPKα2−/− mice. These data were consistent with those of a previous study showing that metformin attenuates TAC-induced hypertrophy, LV dysfunction and myocardial oxidative stress in AMPKα2−/− mice [25]. Therefore, metformin may exert cardiac protective effects through an AMPKα2-independent pathway.
It is noticeable that PM2.5 exposure had no obvious effect on pulmonary PRDX3 and SOD2 expressions, but significant decreased myocardial PRDX3 and SOD2 expressions. Furthermore, there were also significant differences between pulmonary and myocardial PRDX3 and SOD2 expressions in the PM2.5-exposed AMPKα2−/− mice after metformin treatment. We speculated that these discrepancies were relative to with PM2.5 exposure mode, sensitivity to oxidative stress, and activity of redox-related transcription factors or kinase. However, more careful studies are needed to address this speculation.
Growing evidence has indicated that metformin exerts biological and physiological functions through both AMPK-dependent and AMPK-independent molecular pathways [51]. In the liver, metformin inhibits hepatic gluconeogenesis in an AMPK-independent manner [52,53]. Metformin also alleviates unilateral ureteral obstruction-induced renal fibrosis through both AMPKα2-dependent and AMPKα2-independent pathways [54]. Here, we demonstrated that metformin could attenuate PM2.5-induced inflammation and oxidative stress in an AMPKα2-independent manner, and the underlying mechanism is related to the regulation of mitochondrial antioxidant enzymes. There is evidence that some redox-related transcription factors or kinases, including signal transducer and activator of transcription 3 (STAT3) [55], hypoxia-induced factor 1α (HIF-1α) [56], mTOR [57,58] and SIRT1 [59], are regulated by metformin through an AMPKα-independent pathway. However, the extent to which these actions of metformin regulate mitochondrial antioxidant enzymes and attenuate PM2.5-induced lung injury and cardiac dysfunction independent of AMPKα2 is not clear.
In summary, our study indicates that metformin, in dependent of AMPKα2, protects against PM2.5 exposure-induced lung injury and cardiac dysfunction by maintaining the expression of mitochondrial antioxidant enzymes, thereby decreasing oxidative stress and inflammation. These results suggest that metformin administration is a potential strategy to treat air pollution-associated diseases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by grants from National Natural Science Foundation of China (91743104 and 91643206), University of Chinese Academy of Sciences and the Open Research Fund Program of Key Laboratory of Cosmetic (Beijing Technology and Business University), China National Light Industry (KLC-2019-YB2). We would like to thank Fang Li and Dandan Sun from University of Chinese Academy of Sciences for their kindly help in instrument operation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101345.
Contributor Information
Wenjun Ding, Email: dingwj@ucas.ac.cn.
Zhongbing Lu, Email: luzhongbing@ucas.ac.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hopke P.K., Cohen D.D., Begum B.A., Biswas S.K., Ni B., Pandit G.G., Santoso M., Chung Y.S., Rahman S.A., Hamzah M.S., Davy P., Markwitz A., Waheed S., Siddique N., Santos F.L., Pabroa P.C., Seneviratne M.C., Wimolwattanapun W., Bunprapob S., Vuong T.B., Duy Hien P., Markowicz A. Urban air quality in the Asian region. Sci. Total Environ. 2008;404(1):103–112. doi: 10.1016/j.scitotenv.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Deng X., Zhang F., Rui W., Long F., Wang L., Feng Z., Chen D., Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. In Vitro : An International Journal Published in Association with BIBRA. 2013;27(6):1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Tian L., Zhang W., Lin Z.Q., Zhang H.S., Xi Z.G., Chen J.H., Wang W. Impact of traffic emissions on local air quality and the potential toxicity of traffic-related particulates in Beijing, China. Biomed. Environ. Sci. : BES (Biomed. Environ. Sci.) 2012;25(6):663–671. doi: 10.3967/0895-3988.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Li R., Kou X., Geng H., Xie J., Yang Z., Zhang Y., Cai Z., Dong C. Effect of ambient PM(2.5) on lung mitochondrial damage and fusion/fission gene expression in rats. Chem. Res. Toxicol. 2015;28(3):408–418. doi: 10.1021/tx5003723. [DOI] [PubMed] [Google Scholar]
- 5.Kirrane E.F., Luben T.J., Benson A., Owens E.O., Sacks J.D., Dutton S.J., Madden M., Nichols J.L. A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ. Int. 2019;127:305–316. doi: 10.1016/j.envint.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N., Hao M., Phalen R.F., Hinds W.C., Nel A.E. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 2003;109(3):250–265. doi: 10.1016/j.clim.2003.08.006. (Orlando, Fla.) [DOI] [PubMed] [Google Scholar]
- 7.Hertz-Picciotto I., Baker R.J., Yap P.S., Dostal M., Joad J.P., Lipsett M., Greenfield T., Herr C.E., Benes I., Shumway R.H., Pinkerton K.E., Sram R. Early childhood lower respiratory illness and air pollution. Environ. Health Perspect. 2007;115(10):1510–1518. doi: 10.1289/ehp.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sint T., Donohue J.F., Ghio A.J. Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhal. Toxicol. 2008;20(1):25–29. doi: 10.1080/08958370701758759. [DOI] [PubMed] [Google Scholar]
- 9.McGuinn L.A., Ward-Caviness C.K., Neas L.M., Schneider A., Diaz-Sanchez D., Cascio W.E., Kraus W.E., Hauser E., Dowdy E., Haynes C., Chudnovsky A., Koutrakis P., Devlin R.B. Association between satellite-based estimates of long-term PM2.5 exposure and coronary artery disease. Environ. Res. 2016;145:9–17. doi: 10.1016/j.envres.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M., Wu Y., Tian Y.H., Cao Y.Y., Song J., Huang Z., Wang X.W., Hu Y.H. Association between PM2.5 and daily hospital admissions for heart failure: a time-series analysis in Beijing. Int. J. Environ. Res. Public Health. 2018;15(10) doi: 10.3390/ijerph15102217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbas I., Badran G., Verdin A., Ledoux F., Roumie M., Lo Guidice J.M., Courcot D., Garcon G. In vitro evaluation of organic extractable matter from ambient PM2.5 using human bronchial epithelial BEAS-2B cells: cytotoxicity, oxidative stress, pro-inflammatory response, genotoxicity, and cell cycle deregulation. Environ. Res. 2019;171:510–522. doi: 10.1016/j.envres.2019.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Nyhan M.M., Wilker E.H., Vieira C.L.Z., Lin H., Schwartz J.D., Gold D.R., Coull B.A., Aba A.M., Benjamin E.J., Vasan R.S., Koutrakis P., Mittleman M.A. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: the Framingham Heart Study. Environ. Int. 2018;121(Pt 2):1210–1216. doi: 10.1016/j.envint.2018.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carling D., Mayer F.V., Sanders M.J., Gamblin S.J. AMP-activated protein kinase: nature's energy sensor. Nat. Chem. Biol. 2011;7(8):512–518. doi: 10.1038/nchembio.610. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Lin Z., Huang H., He H., Yang L., Chen T., Yang T., Ren N., Jiang Y., Xu W., Kamp D.W., Liu T., Liu G. AMPK is required for PM2.5-induced autophagy in human lung epithelial A549 cells. Int. J. Clin. Exp. Med. 2015;8(1):58–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Dornhof R., Maschowski C., Osipova A., Giere R., Seidl M., Merfort I., Humar M. Stress fibers, autophagy and necrosis by persistent exposure to PM2.5 from biomass combustion. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Shen X., Tian G., Shi X., Huang W., Wu Y., Sun L., Peng C., Liu S., Huang Y., Chen X., Zhang F., Chen Y., Ding W., Lu Z. AMPKalpha2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radical Biol. Med. 2018;121:202–214. doi: 10.1016/j.freeradbiomed.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Pan K., Jiang S., Du X., Zeng X., Zhang J., Song L., Zhou J., Kan H., Sun Q., Xie Y., Zhao J. AMPK activation attenuates inflammatory response to reduce ambient PM2.5-induced metabolic disorders in healthy and diabetic mice. Ecotoxicol. Environ. Saf. 2019;179:290–300. doi: 10.1016/j.ecoenv.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Wu K., Tian R., Huang J., Yang Y., Dai J., Jiang R., Zhang L. Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem. Biol. Interact. 2018;291:1–6. doi: 10.1016/j.cbi.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X.Y., Li Y.Y., Huang C., Li J., Yao H.W. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget. 2017;8(14):22513–22523. doi: 10.18632/oncotarget.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C.S., Bang B.R., Kwon H.S., Moon K.A., Kim T.B., Lee K.Y., Moon H.B., Cho Y.S. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol. 2012;84(12):1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Wang G., Song Y., Feng W., Liu L., Zhu Y., Xie X., Pan Y., Ke R., Li S., Li F., Yang L., Li M. Activation of AMPK attenuates LPS-induced acute lung injury by upregulation of PGC1alpha and SOD1. Exp. Ther. Med. 2016;12(3):1551–1555. doi: 10.3892/etm.2016.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajiwara C., Kusaka Y., Kimura S., Yamaguchi T., Nanjo Y., Ishii Y., Udono H., Standiford T.J., Tateda K. Metformin mediates protection against Legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species. J. Immunol. 2018;200(2):623–631. doi: 10.4049/jimmunol.1700474. [DOI] [PubMed] [Google Scholar]
- 23.Haberzettl P., McCracken J.P., Bhatnagar A., Conklin D.J. Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2016;310(11):H1423–H1438. doi: 10.1152/ajpheart.00369.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soberanes S., Misharin A.V., Jairaman A., Morales-Nebreda L., McQuattie-Pimentel A.C., Cho T., Hamanaka R.B., Meliton A.Y., Reyfman P.A., Walter J.M., Chen C.I., Chi M., Chiu S., Gonzalez-Gonzalez F.J., Antalek M., Abdala-Valencia H., Chiarella S.E., Sun K.A., Woods P.S., Ghio A.J., Jain M., Perlman H., Ridge K.M., Morimoto R.I., Sznajder J.I., Balch W.E., Bhorade S.M., Bharat A., Prakriya M., Chandel N.S., Mutlu G.M., Budinger G.R.S. Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis. Cell Metabol. 2019;29(2):335–347. doi: 10.1016/j.cmet.2018.09.019. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Lu Z., Fassett J., Zhang P., Hu X., Liu X., Kwak D., Li J., Zhu G., Tao Y., Hou M., Wang H., Guo H., Viollet B., McFalls E.O., Bache R.J., Chen Y. Metformin protects against systolic overload-induced heart failure independent of AMP-activated protein kinase alpha2. Hypertension. 2014;63(4):723–728. doi: 10.1161/HYPERTENSIONAHA.113.02619. (Dallas, Tex. : 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffat J., Grueneberg D.A., Yang X., Kim S.Y., Kloepfer A.M., Hinkle G., Piqani B., Eisenhaure T.M., Luo B., Grenier J.K., Carpenter A.E., Foo S.Y., Stewart S.A., Stockwell B.R., Hacohen N., Hahn W.C., Lander E.S., Sabatini D.M., Root D.E. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124(6):1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Viollet B., Andreelli F., Jorgensen S.B., Perrin C., Geloen A., Flamez D., Mu J., Lenzner C., Baud O., Bennoun M., Gomas E., Nicolas G., Wojtaszewski J.F., Kahn A., Carling D., Schuit F.C., Birnbaum M.J., Richter E.A., Burcelin R., Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Investig. 2003;111(1):91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Lei T., Yuan J., Wu Y., Shen X., Gao J., Feng W., Lu Z. GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol. 2018;17:25–34. doi: 10.1016/j.redox.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell D.S.H., Goncalves E. Heart failure in the patient with diabetes: epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes. Metab. 2019;21(6):1277–1290. doi: 10.1111/dom.13652. [DOI] [PubMed] [Google Scholar]
- 30.Masoudi F.A., Wang Y., Inzucchi S.E., Setaro J.F., Havranek E.P., Foody J.M., Krumholz H.M. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA. 2003;290(1):81–85. doi: 10.1001/jama.290.1.81. [DOI] [PubMed] [Google Scholar]
- 31.Hundal R.S., Inzucchi S.E. Metformin: new understandings, new uses. Drugs. 2003;63(18):1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 32.Valencia W.M., Palacio A., Tamariz L., Florez H. Metformin and ageing: improving ageing outcomes beyond glycaemic control. Diabetologia. 2017;60(9):1630–1638. doi: 10.1007/s00125-017-4349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales D.R., Morris A.D. Metformin in cancer treatment and prevention. Annu. Rev. Med. 2015;66:17–29. doi: 10.1146/annurev-med-062613-093128. [DOI] [PubMed] [Google Scholar]
- 34.Vaez H., Najafi M., Toutounchi N.S., Barar J., Barzegari A., Garjani A. Metformin alleviates lipopolysaccharide-induced acute lung injury through suppressing toll-like receptor 4 signaling. Iran. J. Allergy, Asthma Immunol. 2016;15(6):498–507. [PubMed] [Google Scholar]
- 35.Xiao H., Ma X., Feng W., Fu Y., Lu Z., Xu M., Shen Q., Zhu Y., Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc. Res. 2010;87(3):504–513. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 36.Feng S., Gao D., Liao F., Zhou F., Wang X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016;128:67–74. doi: 10.1016/j.ecoenv.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Shen X., Liu J., Wu C., Gao J., Zhang Z., Zhang F., Ding W., Lu Z. The effect of exposure time and concentration of airborne PM2.5 on lung injury in mice: a transcriptome analysis. Redox Biol. 2019;26:101264. doi: 10.1016/j.redox.2019.101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue W., Tong L., Liu X., Weng X., Chen X., Wang D., Dudley S.C., Weir E.K., Ding W., Lu Z., Xu Y., Chen Y. Short term Pm2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy. Redox Biol. 2019;22:101161. doi: 10.1016/j.redox.2019.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., Kwak H.J., Cha J.Y., Jeong Y.S., Rhee S.D., Kim K.R., Cheon H.G. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J. Biol. Chem. 2014;289(33):23246–23255. doi: 10.1074/jbc.M114.577908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian R., Li R., Liu Y., Liu J., Pan T., Zhang R., Liu B., Chen E., Tang Y., Qu H. Metformin ameliorates endotoxemia-induced endothelial pro-inflammatory responses via AMPK-dependent mediation of HDAC5 and KLF2. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2019;1865(6):1701–1712. doi: 10.1016/j.bbadis.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Briede J.J., De Kok T.M., Hogervorst J.G., Moonen E.J., Op Den Camp C.L., Kleinjanst J.C. Development and application of an electron spin resonance spectrometry method for the determination of oxygen free radical formation by particulate matter. Environ. Sci. Technol. 2005;39(21):8420–8426. doi: 10.1021/es0485311. [DOI] [PubMed] [Google Scholar]
- 42.Riva D.R., Magalhaes C.B., Lopes A.A., Lancas T., Mauad T., Malm O., Valenca S.S., Saldiva P.H., Faffe D.S., Zin W.A. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 2011;23(5):257–267. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Xiao B., Han F., Shi Y. Metformin alleviated the neuronal oxidative stress in Hippocampus of rats under single prolonged stress. J. Mol. Neurosci. : MN. 2017;63(1):28–35. doi: 10.1007/s12031-017-0953-6. [DOI] [PubMed] [Google Scholar]
- 44.Cahova M., Palenickova E., Dankova H., Sticova E., Burian M., Drahota Z., Cervinkova Z., Kucera O., Gladkova C., Stopka P., Krizova J., Papackova Z., Oliyarnyk O., Kazdova L. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309(2):G100–G111. doi: 10.1152/ajpgi.00329.2014. [DOI] [PubMed] [Google Scholar]
- 45.Prasad S., Sajja R.K., Kaisar M.A., Park J.H., Villalba H., Liles T., Abbruscato T., Cucullo L. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pope C.A., 3rd, Turner M.C., Burnett R.T., Jerrett M., Gapstur S.M., Diver W.R., Krewski D., Brook R.D. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res. 2015;116(1):108–115. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki H., Asanuma H., Fujita M., Takahama H., Wakeno M., Ito S., Ogai A., Asakura M., Kim J., Minamino T., Takashima S., Sanada S., Sugimachi M., Komamura K., Mochizuki N., Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119(19):2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 48.Asensio-Lopez M.C., Lax A., Pascual-Figal D.A., Valdes M., Sanchez-Mas J. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radical Biol. Med. 2011;51(10):1861–1871. doi: 10.1016/j.freeradbiomed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Gundewar S., Calvert J.W., Jha S., Toedt-Pingel I., Ji S.Y., Nunez D., Ramachandran A., Anaya-Cisneros M., Tian R., Lefer D.J. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 2009;104(3):403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun D., Yang F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem. Biophys. Res. Commun. 2017;486(2):329–335. doi: 10.1016/j.bbrc.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 51.Ikhlas S., Ahmad M. Metformin: insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 2017;185:53–62. doi: 10.1016/j.lfs.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 52.Foretz M., Hebrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephenne X., Foretz M., Taleux N., van der Zon G.C., Sokal E., Hue L., Viollet B., Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54(12):3101–3110. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng Y., Wang S., Zhang Y., Xiao H. Metformin attenuates renal fibrosis in both AMPKalpha2-dependent and independent manners. Clin. Exp. Pharmacol. Physiol. 2017;44(6):648–655. doi: 10.1111/1440-1681.12748. [DOI] [PubMed] [Google Scholar]
- 55.Lin C.C., Yeh H.H., Huang W.L., Yan J.J., Lai W.W., Su W.P., Chen H.H., Su W.C. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am. J. Respir. Cell Mol. Biol. 2013;49(2):241–250. doi: 10.1165/rcmb.2012-0244OC. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X., Chen J., Yi G., Deng M., Liu H., Liang M., Shi B., Fu X., Chen Y., Chen L., He Z., Wang J., Liu J. Metformin suppresses hypoxia-induced stabilization of HIF-1alpha through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7(1):873–884. doi: 10.18632/oncotarget.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalender A., Selvaraj A., Kim S.Y., Gulati P., Brule S., Viollet B., Kemp B.E., Bardeesy N., Dennis P., Schlager J.J., Marette A., Kozma S.C., Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metabol. 2010;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben Sahra I., Regazzetti C., Robert G., Laurent K., Le Marchand-Brustel Y., Auberger P., Tanti J.F., Giorgetti-Peraldi S., Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71(13):4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 59.Song Y.M., Lee Y.H., Kim J.W., Ham D.S., Kang E.S., Cha B.S., Lee H.C., Lee B.W. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.