Abstract
The occurrence of conflicting reports on the improvement of male reproductive function among humans fed diet containing the seed of Mucuna pruriens (MP) have raised a major concern in Nigeria. We assessed the effect of MP seed powder on the reproductive function in 32 adult male albino rats randomized to 4 groups of 8 rats each for 8 weeks. Group 1 (control) was given standard rat chow while groups 2, 3 and 4 were fed diets containing MP seed powder at 0.75 g, 1.5 g and 2.25 g respectively. Serum level of follicle stimulating hormone, testosterone, luteinizing hormone, oxidative stress markers in the testis, epididymal sperm quality and cytoarchitectural structure of the testis were monitored using standard methods. Significant improvements (p < 0.05) in the serum levels of all the hormones, testicular antioxidant defence, epididymal sperm quality without testicular degenerative changes were observed in group 2 compared to other groups. Oxidative stress, severe degenerative architectural lesions in the testis as well as significant reductions (p < 0.05) in epididymal sperm count, percentage motility and significant (p < 0.05) increased in abnormal sperm cells were observed in group 4. MP seed enhances reproductive function in male rats only at a dose level of 0.75g. A dose value higher than 0.75 g may be toxic to the male reproductive system.
Keywords: Biochemistry, Plant biology, Aphrodisiac, Sperm quality, Mucuna pruriens, Reproductive hormones, Fertility, Oxidative stress
Biochemistry; Plant biology; Aphrodisiac; Sperm quality; Mucuna pruriens; Reproductive hormones; Fertility; Oxidative stress
1. Introduction
Male reproductive dysfunction, poor sexual performances and loss of libido are common public health disorders as well as a major health challenge among humans in many part of the world (Akinola et al., 2010) and this has been a major concern over the years. Exposures to environmental pollutants, consumption of conventional drug, among other factors, have been documented to induce these reproductive disorders (Olayemi, 2010). Management or treatment of these reproductive dysfunctions in many developed nations involves the use of conventional drugs, which are expensive and may not be accessible and available to the poor in most developing nations. The high cost of these drugs has therefore, compelled humans in developing nations such as Nigeria to source for alternative drugs that are cheap, readily available and effective. One dominant alternative medicine for enhancing reproductive functions as well as treating other ailments in Nigeria is the use of medicinal plants by the herbal practitioners.
The use of herbal remedies in Nigeria is on the increase and several indigenous plants have been used in herbal formulations to cure diseases, sicknesses and heal injuries (Sofowora, 1993; Agboola, 1998). One such major medicinal plant used by the herbal practitioners to remediate ailments in Nigeria is Mucuna pruriens, widely known as velvet bean and commonly referred to as ‘werepe’ among the Yoruba tribe in Nigeria. M. pruriens, which belongs to the family Fabaceae, is a tropical legume and native toIndia, the Caribbean and Africa. The plant is indigenous to and commonly found in South Western Nigeria. The plant has been screened for its phytochemical constituents and reported to be rich in alkaloids, tannins anthraquinones, saponins, flavonoids and cardiac glycosides (Vadivel and Janardhanan, 2000; Nebedum et al., 2010; Agbafor and Nwachukwu, 2011; Nwaoguikpe et al., 2011) making the various parts of the plant, including leaves, roots and seeds, to possess some medicinal properties (Agbafor and Nwachukwu, 2011; Nwaoguikpe et al., 2011).
The root and leave extracts of the plant have been used in the treatment of snake bite and cancer (Alo et al., 2012). The seed of the plant has been reported to improve reproductive performances in male animal and humans due to its active compound L-3,4dihydroxyphenylalanine (L-DOPA) (Ahmad et al., 2008; Shukla et al., 2009; Mutwedu et al., 2019). In addition, antioxidant, anti-inflammatory, neuroprotective, antidiabetic, antiepileptic, antibacterial and cardioprotective properties of the plant have been demonstrated (Misra and Wagner, 2004; Majekodunmi et al., 2011; Uma and Gurumoorthi, 2013; Obogwu et al., 2014). Of major interest is the reported use of the seed in the treatment of fertility related issue and improvement of fertility status among humans around the world.
In Nigeria, both the traditional herbal practitioners and the non-practitioners are claiming that the consumption of the seed of M. pruriens can boost the sexual behavior in male subject. Although this claim has not been scientifically validated in Nigeria, but the increasing rate at which the seed is now being used in indigenous herbal formulations and consumed without measures necessitates the need to scientifically prove its credibility. Moreover, while most people are claiming the positive impact of the seed on their reproductive functions, some blatantly opposed this claim having observed its negative effects on their sexual performances and reproductive functions. This conflicting opinion on the aphrodisiac property of the seed in Nigeria has greatly attracted public attention and is now a major concern among the populace. It is also important to note that most reproductive studies conducted on the seed of M. pruriens in the male reproductive organ in other countries only focused on the semen quality (Ahmad et al., 2008; Gupta et al., 2011; Mahajan et al., 2011; Suresh et al., 2013), however, testing the reproductive function-enhancing property of a medicinal agent involves the assessment of reproductive hormones, testicular cytoarchitecture, sperm morphology and related biochemical profile in the male subject. Although Mutwedu et al. (2019) recently demonstrated positive effect of dietary meal supplemented with the seed of M. pruriens on sexual behavior, semen characteristics, and biochemical parameters in rabbit bucks (Oryctolagus cuniculus), but additional knowledge of this is required in Nigeria.
In order to scientifically confirm the reproductive function-enhancing potential of the seed of M. pruriens in Nigeria and suggest a preliminary regulatory dose limit, we designed this study to evaluate the level of some reproductive hormones, semen quality (morphology, count, motility), oxidative stress parameters in the testes and testicular cytoarchitecture in male albino rats fed diets containing graded dose of M. pruriens seed powder.
2. Materials and methods
2.1. Seed collection and preparation
The seeds of M. pruriens var. cochichennensis were obtained from a botanical garden in Egbe community, Kogi state, Nigeria and properly de-hulled and identified at the Department of Plant Science, Olabisi Onabanjo University. The de-hulled seeds (Fig. 1) were air dried and ground into powder with a Moltinex electric blender and later screened for its phytochemical and proximate compositions.
Fig. 1.
De-hulled seeds of Mucuna pruriens var. cochichennensis.
2.2. Experimental animal
Thirty-two (32) adult male albino rats (115 ± 5 g) were used for this study. Rat's acclimatization was done under a standard laboratory condition (25 ± 5 °C; 65 ± 5% Relative Humidity) for a week before the commencement of actual study. The rats were individually housed in wooden cages with a dimension of 45 cm × 30 cm × 40 cm. Each cell of the wooden cages has strong iron base in square shape, with a flat metallic container placed beneath each cell to collect the droppings of each rat. The rats were fed with standard laboratory rat chow and clean drinking water ad libitum during this acclimatization stage. We conducted the experimental protocol according to the regulations of the local ethics committee in animal care unit of our university (OOUACU/RAT/2018/0013) and ethical guidelines of animal experimentation (regulation CEE 86/609) were followed.
2.3. Experimental design
The rats were randomized into four experimental groups (groups 1, 2, 3 and 4) with eight rats per group. Individual rat in group 1 was fed standard laboratory rat chow (control) at 12 % of their body weight while rats in groups 2, 3 and 4 were individually fed the rat chow containing 0.75g (equivalent to I seed) 1.5 g (equivalent to 2 seeds) and 2.25 g (equivalent to 3 seeds) of MP seed powder respectively at 12 % of their body weight (Vanderlip, 2001; Taylor, 2002). The quantity of food given was measured and readjusted every 5 days in accordance with body weight increase. However, clean drinking water was supplied to all the rats ad libitum and the whole experimental exercise lasted for 8 weeks. The rats were well nourished and show no sign of being malnourished or mortality throughout the feeding regime. At the end of the feeding trial, the rats in each treatment group were sacrificed and blood sample, epididymal sperm cells and testis were collected for laboratory analysis.
2.4. Sample collections
Blood samples were collected between the hours of 8:00 a.m. and 10:00 a.m. into a plain sample tubes by retro orbital sinus with micro haematocrit tube. The blood samples were centrifuged at 2500 rpm for 10 min to obtain serum samples within an hour after the blood collection. The sera obtained were later stored at -20 °C till assayed for hormonal profile. The rats were sacrificed by cervical dislocation and the excised testes were rinsed in saline solution. Caudal epididymis (free of fats, vas deferens and other tissue) from each side of the testis of the rats were dissected out into 10 mL of 0.87% warmed normal saline and slightly teased to release the sperm cells (Wyrobek et al., 1984; Bakare et al., 2005; Hassan and Barakat, 2008; Owagboriaye et al., 2017). The sperm cells were dropped on grease-free clean slides to determine their movement and swimming ability (motility) using microscope Parts of the excised testes were used for antioxidant assay while the remaining parts were subjected to histopathological examination.
2.5. Phytochemical screening and proximate analysis
The seed was screened for its alkaloids, anthra-quinone glycosides, cardiac glycosides, flavonoids, saponins, phenols and tannins contents (Table 1) using the standard protocols described by Harborne (1973); Sofowora (1993); Trease and Evans (1989). We adopted the standard method of the Association of Official Analytical Chemists (AOAC, 2005) to determine the proximate compositions of the seed for moisture, ash and nitrogen free extract (NFE) contents. Crude protein, fibre and fat contents of the seed (Table 2) were determined according to the method of Pearson (1976).
Table 1.
Phytochemical compositions of Mucuna pruriens seed powder.
| Secondary metabolites | Result |
|---|---|
| Alkaloid | ++ |
| Anthraquinone | +++ |
| Cardiac glycoside | + |
| Saponin | +++ |
| Tannins | +++ |
| Phylobatanins | + |
| Flavonoid | +++ |
| Phenols | - |
| Steroids | - |
| Terpenoids | - |
Key: - Absent, + Trace, ++ Moderate, +++ Abundant.
Table 2.
Result on proximate analysis of Mucuna pruriens seed powder.
| Nutritional composition | Percentage (%) |
|---|---|
| Crude protein | 28.62% |
| Crude fibre | 5.16% |
| Crude fat | 1.87% |
| Total ash | 4.23% |
| Moisture | 11.18% |
| NFE | 60.13% |
NFE- Nitrogen Free Extract.
2.6. Hormonal assay
Serum samples obtained were analysed with commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kit for testosterone, follicle stimulating hormone (FSH) (Enzo life sciences, PA) and luteinizing hormone (LH) (Eagle Biosciences Inc., Nashua). The sensitivity of testosterone and FSH assay was 0.09 ng/mL and 0.0017 ng/mL respectively and detection range was 0.01–15 ng/mL and 0.02–19 ng/mL, respectively. The limit of detection of LH assay was 0.04 ng/mL and detection range of 0.06–16 was observed. The protocols and procedures used for the assay were as described by the manufacturer.
2.7. Assessment of oxidative stress indices in the testis of the rats
The concentration of reduced glutathione (GSH) was determined using the method of Ellman (1959). GSH concentration expressed as Unit/g tissue. Lipid peroxidation (MDA) was estimated as thiobarbituric acid reactive substance (TBARS) according to the method of Okhawa et al. (1979). Catalase (CAT) activity was evaluated according to the method described by Sinha (1972). The enzymatic activity was measured in mmol/min/mg protein. Meanwhile, the activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) were determined using the Cayman assay kit as described by the manufacturer.
2.8. Sperm quality assessment
The sperm quality of the rats was examined by counting the sperm cells, determining percentage sperm motility and carrying out the morphological assessment of the sperm. The movement and swimming ability of sperm (motility) was determined using microscope and the sperm cells were counted using haemocytometer (Hassan and Barakat, 2008).
The percentage motility of the sperm cells was determined using the expression:
Sperm morphology assay was according to Bakare et al. (2005). Epididymal sperm cells were suspended in normal saline and 1% Eosin Y stain. The smear was prepared and the slides were allowed to air-dried and coded for subsequent microscopic examination at ×1000 magnification.1000 sperm cells were assessed for morphological abnormalities for each rat.
2.9. Histopathological examination of the testis
The histological examination of rat's testis was according to our previous study (Owagboriaye et al., 2017). The testicular tissue of each rat was fixed in Bouin's fluid for 48 h and routinely processed for paraffin embedding. The embedded tissues were subjected to serial sectioning of 4μm thickness using a Rotary Microtome and later, processed in alcohol-xylene series and were stained with hematoxylin and eosin (H & E). The prepared slides were examined at ×400 magnifications.
2.10. Statistical analysis
All the data were presented as the Mean + Standard Deviation (SD). Statistical analyses for all measurements were performed using Statistical package for Social Sciences (SPSS) version 20.0 (IBM Corp., 2011). The mean, standard error of the mean, and analysis of variance were conducted. Post hoc test was performed using the Student- Newman- Keuls. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Serum level of reproductive hormones in experimental rat
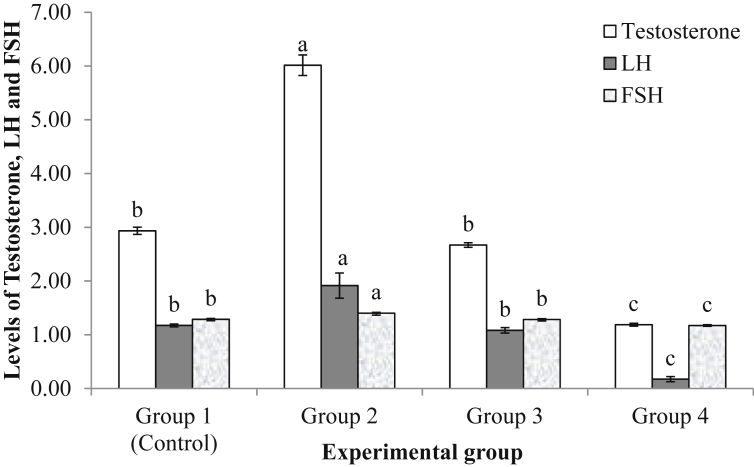
Testosterone, luteinizing hormone and follicle stimulating hormone levels in the male experimental rats daily fed M. pruriens seed dietary meal were observed to significantly reduce (p < 0.05) with increase in the number of M. pruriens seed fed (Fig. 2). Similarly, the level of these hormones in the experimental rats followed similar trend. They were significantly higher in the rats in group 2 which received 0.75g dietary seed powder (1 seed equivalent) of M. pruriens daily. There was however no significant difference (p > 0.05) in the level of these hormones recorded in rats in group 3 compared to control group. Rats in group 4 had significantly lower levels of the reproductive hormones.
Fig. 2.
Level of testosterone (ng/ml), luteinizing hormone (mlu/ml) and follicle stimulating hormone (mlu/ml) in male rats fed Mucuna pruriens seed dietary meal for 8 weeks. abcdMean having similar alphabets are not significantly different at p < 0.05; M. p = Mucuna pruriens. FSH = follicle stimulating hormone, LH = luteinizing hormone. Group 1 = rats fed diet without Mp (control); Group 2 = rats fed diet containing 0.75g Mp; Group 3 = rats fed diet containing 1.5g Mp; Group 4 = rats fed diet containing 2.25g Mp.
3.2. Oxidative stress markers in the testis of rats fed graded quantity of MucunaPruriens seed meal diet
The levels of some antioxidant enzymes in the testis of the experimental rats are shown in Table 3. Activities of CAT and GPx were significantly higher (p < 0.05) in the rats in group 3 compared to control group. There were no significant different (p > 0.05) in the enzyme activities in group 2 and control group. Rats in group 4 however had the lowest activities of CAT and GPx. On the other hand, activity of SOD and concentration of GSH were not significantly different between rats in group 2 and 3 compared to control. Activity of SOD and GSH concentration were however significantly lower in the rats in group 4. Lipid peroxidation was significantly higher (p < 0.05) in the testis of rats in group 4. There was however no significant difference (p > 0.05) in the level of lipid peroxidation observed in the testis of the rats between group 2 and 3 compared to control.
Table 3.
Level of antioxidant parameters in the testis of rats fed graded quantity of Mucuna pruriens seed meal diet for 8 weeks.
| Experimental group | CAT (U/mg protein) | SOD (U/mg protein) | GPx (U/mg protein) | GSH (U/g tissue) | MDA (nmol/g tissue) |
|---|---|---|---|---|---|
| Group 1 | 12.15 ± 0.06b | 9.54 ± 0.04a | 15.20 ± 0.03b | 10.10 ± 0.11a | 17.34 ± 0.16b |
| Group 2 | 12.22 ± 0.04b | 9.60 ± 0.03a | 15.21 ± 0.03b | 10.24 ± 0.01a | 17.28 ± 0.12b |
| Group 3 | 13.95 ± 0.16a | 9.54 ± 0.02a | 17.16 ± 0.03a | 10.55 ± 0.11a | 17.39 ± 0.02b |
| Group 4 | 7.14 ± 0.03c | 4.29 ± 0.03b | 7.47 ± 0.05c | 5.78 ± 0.56b | 30.39 ± 1.08a |
abcdMeans (±Standard deviation) in the same column having similar superscripts are not significantly different at p < 0.05; M.p = Mucuna pruriens. Group 1 = rats fed diet without Mp (control); Group 2 = rats fed diet containing 0.75g Mp; Group 3 = rats fed diet containing 1.5g Mp; Group 4 = rats fed diet containing 2.25g Mp.
3.3. Sperm motility and sperm count
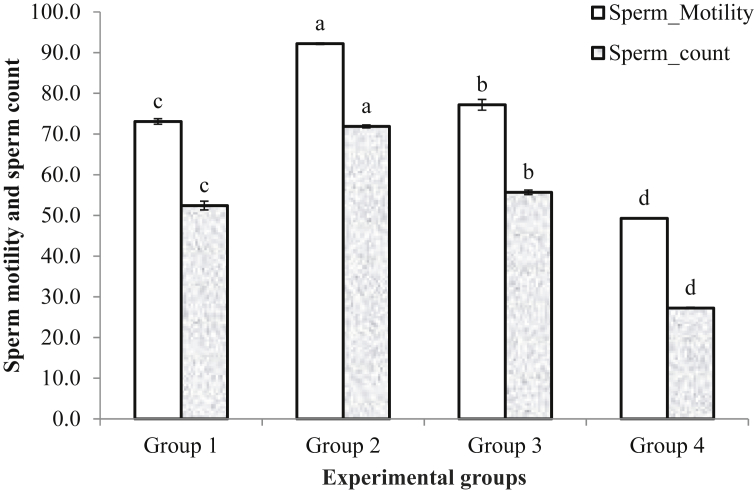
The sperm count and percentage motility in the experimental rats were significantly higher (p < 0.05) in the rats in group 2 compared to control (Fig. 3). This was followed by those in group 3 and the control group. Sperm motility and sperm count were however lowest in the rats in group 4.
Fig. 3.
Sperm motility (%) and sperm count (×106 cells/ml) of rats fed with Mucuna pruriens seed meal diet for 8 weeks. abcdMean having similar alphabets are not significantly different at p < 0.05 Group 1 = rats fed diet without Mp (control); Group 2 = rats fed diet containing 0.75g Mp; Group 3 = rats fed diet containing 1.5g Mp; Group 4 = rats fed diet containing 2.25g Mp.
3.4. Sperm morphological assessment
Sperm morphological parameters such as bent tail, sperms without head, sperms with damaged head, bent body, sperms without hook, banana shaped sperm, sperms without tail, total abnormality and percentage abnormality of the experimental rats are shown in Figs. 4, 5, 6, and 7 and Table 4. These sperm morphological parameters were significantly higher (p < 0.05) in the rats in group 4 compared to other experimental groups and the control. The numbers of sperms with bent tails were not significantly different (p > 0.05) between rats in group 2 and 3 compared to control group 1. In addition, sperms without head, damaged head, bent body, sperms without hook, banana shaped sperm and percentage sperm abnormality were significantly lower in group 2 and the control group. No sperm without tail was recorded in rats in group 2.
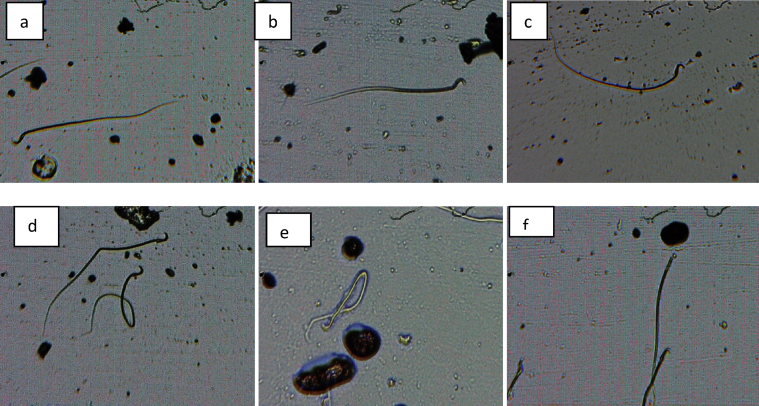
Fig. 4.
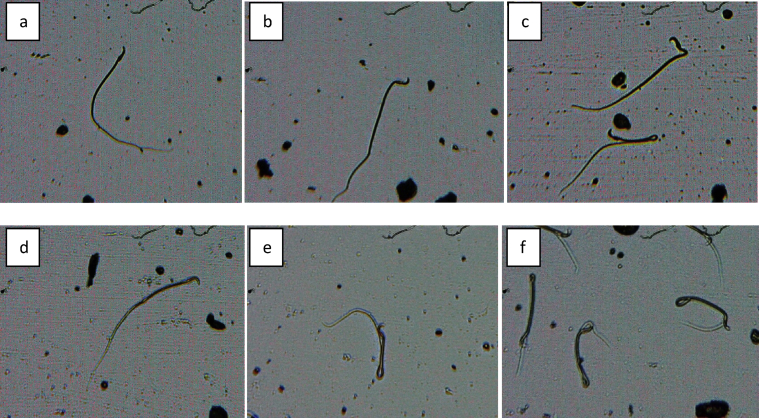
Abnormal sperm cells induced in rat control group (a) normal sperm cell (b) normal sperm cell (c)) normal sperm cell (d) banana shape (e) bent body (f) without head.
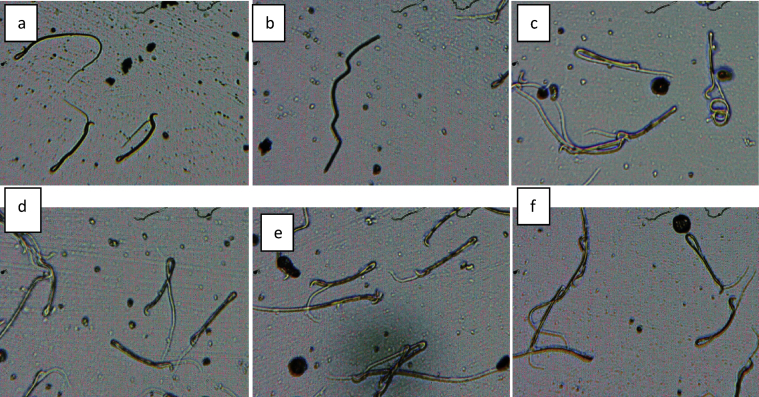
Fig. 5.
Abnormal sperm cells induced in rat in group 2 (a) normal sperm cell (b) normal sperm cell (c)) normal sperm cell (d) normal sperm cell (e) bent body (f) without head.
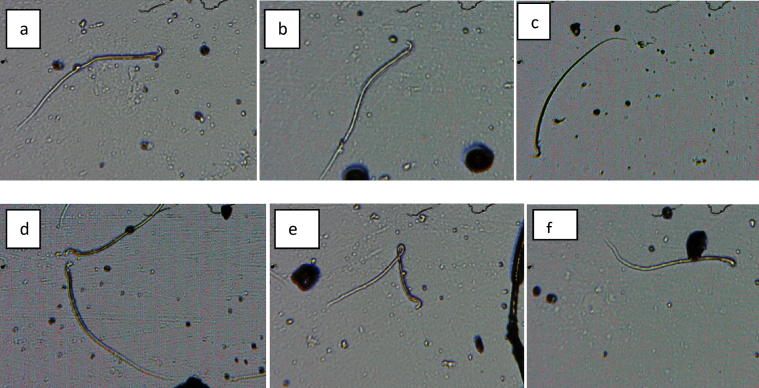
Fig. 6.
Abnormal sperm cells induced in rat in group 3 (a) bent body (b) normal sperm cell (c)) bent body and damaged head (d) normal sperm cell (e) bent body (f) banana and amorphous shape.
Fig. 7.
Abnormal sperm cells induced in rat in group 4 (a) bent tail and damaged head (b) without head and tail (c) bent tail, amorphous and banana shape (d) bent tail, amorphous and banana shape (e) bent tail and banana shape(f) bent tail and banana shape.
Table 4.
Sperm morphological assessment of rats fed with Mucuna pruriens seed meal for 8 weeks.
| Morphological parameter | Seeds of Mucuna pruriens |
|||
|---|---|---|---|---|
| Group 1 (n = 8) | Group 2 (n = 8) | Group 3 (n = 8) | Group 4 (n = 8) | |
| Bent tail | 13.0 ± 1.0b | 8.0 ± 0.5b | 10.0 ± 1.0b | 43.0 ± 1.0a |
| Without head | 1.0 ± 0.2c | 2.0 ± 0.4c | 7.0 ± 0.5b | 51.0 ± 1.0a |
| Damaged head | 3.0 ± 0.3c | 1.0 ± 0.1c | 5.0 ± 0.4b | 67.0 ± 1.0a |
| Bent body | 7.0 ± 0.3c | 8.0 ± 0.4c | 21.0 ± 1.0b | 38.0 ± 0.5a |
| Without hook | 3.0 ± 0.1c | 2.0 ± 0.4c | 9.0 ± 0.5b | 46.0 ± 1.0a |
| Banana shape | 5.0 ± 0.4c | 5.0 ± 0.6c | 11.0 ± 1.0b | 32.0 ± 2.0a |
| Without tail | 2.0 ± 0.1b | 0.0 ± 0.0c | 1.0 ± 0.2b | 34.0 ± 0.9a |
| TAS | 34.0 ± 1.0c | 26.0 ± 0.6d | 64.0 ± 1.0b | 311.0 ± 1.0a |
| % Abnormal | 3.4 ± 0.4c | 2.6 ± 0.3c | 6.4 ± 0.3b | 31.1 ± 0.1a |
abcdMeans (±Standard deviation) in the same row having similar superscripts are not significantly different at p < 0.05; TAS = Total abnormality; M.p = Mucuna pruriens; Group 1 = rats fed diet without Mp (control); Group 2 = rats fed diet containing 0.75g Mp; Group 3 = rats fed diet containing 1.5g Mp; Group 4 = rats fed diet containing 2.25g Mp.
3.5. Histopathological assessment of the testis
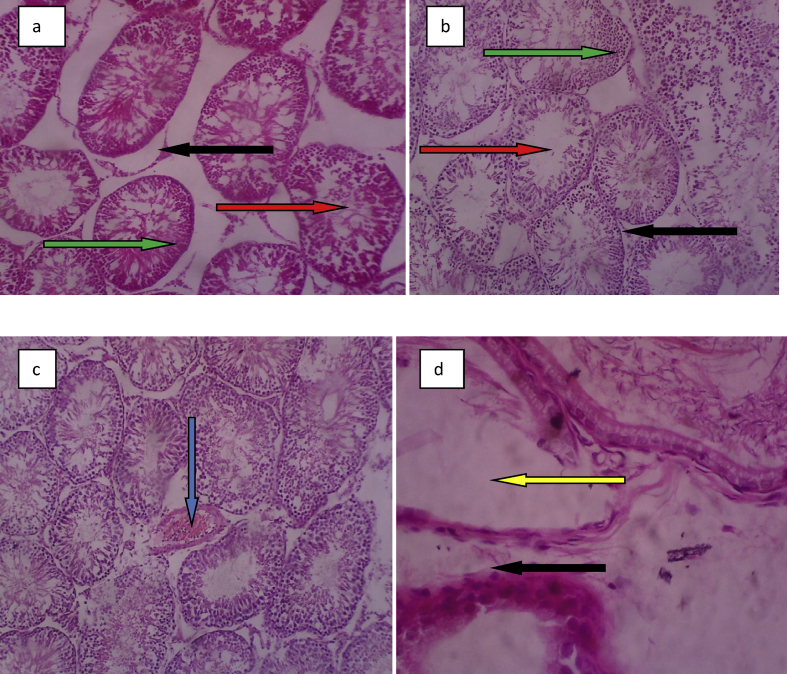
The photomicrographs of the histopathology of testis of rats fed Mucuna pruriens seeds dietary meal are shown in Fig. 8. Control group and group 2 revealed the normal cellular architectural structure of the tissues. Necrotic tissue was observed in testicular tissue of rats in group 3 while degenerative lesions such as depleted amount in the generation of sperm cells, interstitial cells and enlargement of seminiferous tubule were observed in the testis of rats in group 4.
Fig. 8.
Photomicrograph of testis tissue(a) control showing normal seminiferous tubule (red arrow), interstitial cells (black arrow), Spermatocytes (green arrow) b) group 2 showing normal seminiferous tubule (red arrow), Interstitial cells (black arrow) and spermatocytes (green arrow) (c) group 3 showing organized sperm cells but with necrotic tissue (blue arrow) (d) group 4 showing loss of interstitial cells (black arrow), loss of interstitial (black arrow) and spermatocytes (yellow arrow).
4. Discussion
The serum concentrations of testosterone, LH and FSH in rats in group 2 were observed to increase when compared to other experimental groups. These hormones have been reported to play a major role in the improvement of reproductive function in male reproductive system (Zitzmann, 2008; Nithya and Elango, 2015). Seed of M. pruriens is known to contain L-DOPA as its active substance which improves male reproductive function in animal (Misra and Wagner, 2007). In addition, studies have shown that L-DOPA stimulates the secretion of gonadotropin releasing hormone (GnRH), which in turn, stimulates the secretion of FSH and LH from the anterior portion of pituitary gland (Vermes et al., 1979; Singh et al., 2013).
Elevated serum levels of FSH and LH stimulate spermatogenesis processes through testosterone production. Therefore, it could be that concentration of L-DOPA in M. pruriens at 0.75 g (which is equivalent to 1 seed) is sufficient enough to stimulate the secretion of GnRH from the hypothalamus, thereby, resulted to higher serum levels of testosterone, LH and FSH as observed in this study. In addition, the higher levels of these reproductive hormones observed among rats in group 2 could also be attributed to the level of flavonoids, saponins, and tannins present in M. pruriens seeds at a dose level of 0.75 g (Misra et al., 2004; Mutwedu et al., 2019). These compounds have been documented to increase testosterone level through hypothalamo-pituitary-testicular axis stimulation in animals (Ahangarpour et al., 2013) and similar mechanism is reasonably suggested in this study. However, the L-DOPA and phenolic contents of the seed at 2.25 g (equivalent to 3 seeds) might have been too strong for, and as such, toxic to the proper functioning of the hypothalamic-pituitary axis. This may explain the observed reductions in the levels of these hormones in rats in group 4. Although the M. pruriens seed used in this study was not analysed for its L-DOPA contents, we suggest additional study looking into this area. Our finding partly agrees with Sahin et al. (2016) who observed improved secretion of testosterone level in rat treated with the seed of M. pruriens, but no changes in their serum levels of FSH and LH. Meanwhile, the seed was noted to increase the levels of testosterone and LH and decrease the level of FSH in infertile men (Ahmad et al., 2008; Shukla et al., 2008, 2009).
An imbalance between productions of free radicals or reactive oxygen species (ROS) is known to result into oxidative stress which lead to damage to lipids (lipid peroxidation), proteins, carbohydrates and nucleic acids and this has been identified as a major factor contributing to poor sexual performances among male humans (Agarwal and Said, 2005; Agarwal et al., 2008). The vital role of antioxidant defense, which focused on the protection of cells by scavenging the free radicals produced through oxidation in the body, has been reported (Pastore et al., 2003). The increase in the activities of antioxidant enzymes (CAT, SOD GPx) and GSH in the testes of rats in group 2 may indicate the antioxidant potential of M. pruriens seed to neutralize the toxic effect of ROS which are naturally formed from metabolic processes in animal cells. Flavonoids and tannins contents of the seed have been recognized as the major phenolic compound that act as primary antioxidant to scavenge free radicals or ROS (Potterat, 1997). In a similar trend, the antioxidant property of L-DOPA has been demonstrated (Gulcin, 2007). Hence, the antioxidant function of the seed in the testes of the rats observed in this study could be reasonably attributed to the identified phytochemical and L-DOPA contents of the seed at 0.75 g. However, this seed at higher quantity (2.25 g or 3 seeds) would have induced production of ROS by depleting the activities CAT, SOD GPx and GSH concentration in the testes of the rats, consequently resultedto the peroxidation of polyunsaturated fatty acids which led to the formation of MDA and lipid peroxidation.
The observed increase in the sperm count and percentage motility among rats in group 2 may directly results from the elevated levels of their reproductive hormones such as testosterone which promotes the process of spermatogenesis. A report by Mylchreest et al. (2002) has associated increased in epididymal sperm number to elevated level of serum level of testosterone in rats. In addition, the increase in sperm count observed in this group might be as a result of the increase in their testisticular antioxidant defense which could have suppressed oxidative stress, sperm cell apoptosis in the testis and enhanced epididymal sperm maturation (Ahmad et al., 2008; Shukla et al., 2008). In addition, the observed increase in sperm count and percentage motility in this group could be due to the level of protein content in M. pruriens seeds consumed by the rats as earlier documented by Suresh et al. (2009). Meanwhile, the impact of quality food with high protein content in enhancing semen quality was reported by Oyeyemi and Okediran (2007) and recently confirmed by Mutwedu et al. (2019). On the other hand, the seed at 2.25 g (3 seeds) might have induced sperm cell apoptosis in the testis of rats in group 4 due to a possible occurrence of oxidative stress as evidenced by the reductions in antioxidant defense parameters and induction of lipid peroxidation in their testis. Moreover, oxidative stress is known to affect sperm motility by causing an alteration in axoneme structure and consequently, leads to the sperm abnormality (Syntin and Robaire, 2001) as well as reduction in sperm motility (Giorgio et al., 2007). This may also explain the observed higher number of primary sperm abnormality (abnormal tail and head) and secondary abnormality (bent body, banana shape, bent tail, without hook) in group 4. In addition, an abnormal chromosome (Bruce et al., 1974), minor alterations in testicular DNA (Giri et al., 2002) or point mutation (Narayana et al., 2002) have been documented to induce abnormal sperm cells in animal. We can thus suggest here that during spermatogenesis, M. pruriens seed at dose level of 2.25 g could be interacting with the genetic material in the testes of the rats which can consequently lead to abnormal sperm cells. We also recommend additional studies testing the impact of consuming graded dose of M. pruriens seed powder on the genetic material of animal.
The observed degenerative lesions characterised by depletion in the amount of generation of sperm cells, interstitial cells and enlargement of seminiferous tubule in the testis of rats in group 4 are evidence of the toxicity of the seed at dose level of 2.25 g to the male reproductive system. Meanwhile, the interference of a toxic substance with spermatogenesis process has been reported to induce degenerative changes in the seminiferous tubule and interstitial cells in the testis of rats (Thakur et al., 2014). It is therefore possible for the seed at 2.25 g (3 seeds) to have directly interfered with the process of spermatogenesis in the testes of the rats in group 4 and this could probably be a pre-requisite for the observed abnormal sperm morphology produced by the damaged testes.
5. Conclusion
We have shown in this study that M. pruriens seed powder is a potent antioxidant assgent and also has the capacity to enhance reproductive function in male albino rat due to its higher phenolic content. The seed powder may improve male reproductive function by elevating the serum levels of testosterone, FSH and LH in animal. It is further revealed here that M. pruriens seed powder maintains and improves the semen quality and testicular cytoarchitectural integrity of the rats. This property of the seed was noted to be valid only at a dose level of 0.75 g which is equivalent to the weight of a single seed consumed. However, a dose value higher than 0.75 g, especially at 2.25 g which is equivalent to the weight of 3 seeds, appeared to be toxic to the male reproductive system through the induction of oxidative stress in the testis of the animal. Although several studies have reported the reproductive function-enhancing potential of M. pruriens seed in other countries, to the best of our knowledge, this is the first study to consider a graded dose (1 seed, 2 seeds and 3 seeds) of the seed in male subject in Nigeria. This finding is of great importance considering the rate at which M. pruriens seed is being used in herbal formulations and consumed without regulation and with a view of enhancing reproductive performance among humans in Nigeria. Considering the findings of this study, we recommend urgent need for additional study to evaluate the impact of M. pruriens seed on food palatability and the relative body weight of animals in order to buttress our understanding on the reproductive function assessment of the seed. In addition, there is need to provide an appropriate dose of M. pruriens seed per kilogram of human body weight in order to prevent a potential reproductive toxicity of the seed that may result through excessive consumptions.
Declarations
Author contribution statement
Ashidi, Joseph Senu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Owagboriaye, Folarin Ojo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yaya, Funmilola Balikis, Payne, Deborah Eyinjuoluwa: Performed the experiments.
Lawal, Olubukola Ireti: Analyzed and interpreted the data.
Owa, Stephen Olugbemiga: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Agarwal A., Makker K., Sharma R. Clinical relevance of oxidative stress inmale factor infertility: an update. Am. J. Reprod. Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Said T.M. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95:503–507. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- Agbafor K.N., Nwachukwu N. Phytochemical analysis and antioxidant property of leaf extracts of Vitex doniana and Mucuna pruriens. Biochem. Res. Int. 2011 doi: 10.1155/2011/459839. Article ID 459839, 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agboola A. Contraception. Textbook of obstetrics and gynaecology for medical students. Gynecology. 1998;1:189–190. [Google Scholar]
- Ahangarpour A., Oroojan A.A., Heydari H. Effect of hydroalcoholic extract of Dorema aucheri on serum levels of testosterone, FSH and sperm count in nicotinamide-STZ- induced diabetic rat models. Zanjan Univ. Med. Sci. J. 2013;21:22–31. [Google Scholar]
- Ahmad M.K., Mahdi A.A., Shukla K.K., Islam N., Jaiswar S.P., Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil. Steril. 2008;90:627–635. doi: 10.1016/j.fertnstert.2007.07.1314. [DOI] [PubMed] [Google Scholar]
- Akinola O.I., Fabanwo K.A., Akinoso O.A. Semen quality in male partness of infertile couple in Lagos state, Nigeria. Int. J. Trop. Med. 2010;5(2):37–39. [Google Scholar]
- Alo M.N., Okeh O.C., Anyim C., Orji J.O. The effects of ethanol extract of Mucuna pruriens leaves on aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase in albino rats. J. Nat. Prod. Plant Resour. 2012;2(4):465–470. [Google Scholar]
- AOAC . eighteenth ed. Association of Official Analytical, Chemists International; Maryland, USA: 2005. Official Methods of Analysis. [Google Scholar]
- Bakare A.A., Mosuro A.A., Osibanjo O. An in vivo evaluation of induction of abnormal sperm morphology in mice by land fill leachates. Mutat. Res. 2005;582:28–34. doi: 10.1016/j.mrgentox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bruce W.R., Furrer R., Wyrobek A.J. Abnormalities in the shape of murine sperm after acute testicular x-irradiation. Mutat. Res. 1974;23:381–386. doi: 10.1016/0027-5107(74)90112-2. [DOI] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Giorgio M., Trinei M., Migliaccio E., Pelicci P.G. Hydrogenperoxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- Giri S., Prasad S.B., Giri A., Sharma G.D. Genetoxic effect of malathion: an organophosphorus insecticide, using three mammalian bioassays in vivo. Mutat. Res. 2002;514:223–231. doi: 10.1016/s1383-5718(01)00341-2. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Comparison of In vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids. 2007;32:431–438. doi: 10.1007/s00726-006-0379-x. [DOI] [PubMed] [Google Scholar]
- Gupta A., Mahdi A.A., Ahmad M.K., Shukla K.K., Bansal N., Jaiswar S.P., Shankhwar S.N. A proton NMR study of the effect of Mucuna pruriens on seminal plasma metabolites of infertile males. J. Pharm. Biomed. Anal. 2011;55:1060–1066. doi: 10.1016/j.jpba.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Harborne J.B. Chapman and Hall publishers; London: 1973. Phytochemical Methods. A Guide to Modern Technique of Plant Analysis; pp. 51–59. [Google Scholar]
- Hassan A.M., Barakat A.H. Assessment of oxidative stress induced by nickel chloride and antioxidant effects of basil (ocimum basilicum L) and thyme (thymus vulgaris L) J. Genet. Eng. Biotech. 2008;6(2):29–38. [Google Scholar]
- IBM Corporation . IBM Corp; Armonk, NY: 2011. IBM SPSS Statistics for Windows, Version 20.0. [Google Scholar]
- Mahajan G.K., Mahajan A.Y., Mahajan R.T. Efficacy of aphrodisiac plants towards improvement in semen quality and motility in infertile males. J. Complement. Integr. Med. 2011;9 doi: 10.1515/1553-3840.1520. Article 6. [DOI] [PubMed] [Google Scholar]
- Majekodunmi S.O., Oyagbemi A.A., Umukoro S., Odeku O.A. Evaluation of the anti-diabetic properties of Mucuna pruriens seed extract. Asian Pac. J. Trop. Med. 2011;4:632–636. doi: 10.1016/S1995-7645(11)60161-2. [DOI] [PubMed] [Google Scholar]
- Misra L., Mishra H.O., Wagner H. IUPAC International Conference on Biodiversity and Natural Products: Chemistry and Medicinal Applications, 26–31 Jan. Delhi University; New Delhi, India: 2004. Biologically active principles from Mucuna pruriens seeds. [Google Scholar]
- Misra L., Wagner H. Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry. 2004;65:2565–2567. doi: 10.1016/j.phytochem.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Misra L., Wagner H. Extraction of bioactive principle from Mucuna pruriens. Indian J. Biochem. Biophys. 2007;44(1):56–60. [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Wallace D., Foster P. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats. Reprod. Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Mutwedu V.B., Ayagirwe R.B., Bacigale S.B., Mwema L.M., Butseme S., Kashosi T., Mitima B., Manyawu G.J., Nyongesa A.W. Effect of dietary inclusion of small quantities of Mucuna pruriens seed meal on sexual behavior, semen characteristics, and biochemical parameters in rabbit bucks (Oryctolagus cuniculus) Trop. Anim. Health Prod. 2019 doi: 10.1007/s11250-019-01808-2. [DOI] [PubMed] [Google Scholar]
- Narayana K., D’Souza U.J., SeetharamaRao K.P. Ribavirin-induced sperm shape abnormalities in Wister rat. Mutat. Res. 2002;513:193196. doi: 10.1016/s1383-5718(01)00308-4. [DOI] [PubMed] [Google Scholar]
- Nebedum J.O., Udeafor P.C., Okeke C.U. Comparative effects of ethanolic extracts of Ficus carica and Mucuna pruriens leaves on haematological parameters in albino rats. Biokemistri. 2010;22:77–84. [Google Scholar]
- Nithya R., Elango V. Pesticide effect in male hormones and antioxidant status in male albino rats. J. Acad. Ind. Res. 2015;4(4):140–143. [Google Scholar]
- Nwaoguikpe R.N., Braide W., Ujowundu C.O. The effects of processing on the proximate and phytochemical compositions of Mucuna pruriens seeds (velvet beans) Pak. J. Nutr. 2011;10:947–951. [Google Scholar]
- Obogwu M.B., Akindele A.J., Adeyemi O.O. Hepatoprotective and in vivo antioxidant activities of the hydroethanolic leaf extract of Mucuna pruriens (Fabaceae) in antitubercular drugs and alcohol models. Chin. J. Nat. Med. 2014;12:273–283. doi: 10.1016/S1875-5364(14)60054-6. [DOI] [PubMed] [Google Scholar]
- Okhawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Olayemi F.O. A review on some causes of male infertility. Afr. J. Biotechnol. 2010;9(20):2834–2842. [Google Scholar]
- Owagboriaye F.O., Dedeke G.A., Ademolu K.O., Olujimi O.O., Ashidi J.S., Aladesida A.A. Reproductive toxicity of Roundup herbicide exposure in male albino rat. Exp. Toxicol. Pathol. 2017;69:461–468. doi: 10.1016/j.etp.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Oyeyemi M.O., Okediran B.S. Testicular parameters and sperm morphology of chinchilla rabbits fed with different planes of soy meal. Int. J. Morphol. 2007;25(1):139–144. [Google Scholar]
- Pastore A., Piemonte F., Locatelli M., Russo A.L., Gaeta L.M., Tozzi G., Federici G. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin. Chem. 2003;47(8):1467–1469. [PubMed] [Google Scholar]
- Pearson V.A. seventh ed. Churchill Livingstone; London: 1976. The Chemical Analysis of Foods; pp. 3–4. [Google Scholar]
- Potterat O. Antioxidants and free radical scavengers of natural origin. Curr. Org. Chem. 1997;1:415–440. [Google Scholar]
- Sahin K., Orhan C., Akdemir F., Tuzcu M., Gencoglu H., Sahin N., Turk G., Yilmaz I., Ibrahim H., Juturu V. Comparative evaluation of the sexual functions and NF-κB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Complement Altern. Med. 2016;16:318. doi: 10.1186/s12906-016-1303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla K.K., Mahdi A.A., Ahmad M.K., Shankhwar S.N., Rajender S., Jaiswar S.P. Mucuna pruriens improves male fertility by its action on the hypothalamus pituitary-gonadal axis. Fertil. Steril. 2009;92:1934–1940. doi: 10.1016/j.fertnstert.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Shukla K.K., Mahdi A.A., Shankwar S.N., Ahmad M.K. Effect of Mucuna pruriens on hormonal status and semen quality in infertile males. Contraception. 2008;78:194. [Google Scholar]
- Singh A.P., Sarkar S., Tripathi M., Rajender S. Mucuna pruriens and its major constituent L-DOPA recover spermatogenic loss by combating ROS, loss of mitochondrial membrane potential and apoptosis. PLoS One. 2013;8(1):54655. doi: 10.1371/journal.pone.0054655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A.K. Colometric assay of catalase. Anal. Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Sofowora A. sixth ed. Chichester John Wiley and Sons; New York: 1993. Medicinal and Traditional Medicine in Africa; pp. 285–286. [Google Scholar]
- Suresh S., Prithiviraj E., Lakshmi N.V., Ganesh M.K., Ganesh L., Prakash S. Effect of Mucuna pruriens (Linn.) on mitochondrial dysfunction and DNA damage in epididymal sperm of streptozotocin induced diabetic rat. J. Ethnopharmacol. 2013;145:32–41. doi: 10.1016/j.jep.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Suresh S., Prithiviraj E., Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int. J. Androl. 2009;33:22–32. doi: 10.1111/j.1365-2605.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- Syntin P., Robaire B. Sperm structural and motility changesduring aging in the brown Norway rat. J. Androl. 2001;22:235–244. [PubMed] [Google Scholar]
- Taylor D. Harper Collins Limited; London, Great Britain: 2002. Small Pet Handbook. [Google Scholar]
- Thakur M., Gupta H., Singh D. Histopathological and ultra-structural effects of nanoparticles on rat testis following 90 days (Choronic study) of repeated oral administration. J. Nanobiotechnol. 2014;12:42. doi: 10.1186/s12951-014-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trease G., Evans W. thirteenth ed. Macmillian publishers; 1989. Pharmacognosy. Briallene Tindacallan. [Google Scholar]
- Uma S., Gurumoorthi P. Dietary antioxidant activities in different germplasms of Mucuna. J. Med. Food. 2013;16:618–624. doi: 10.1089/jmf.2012.2697. [DOI] [PubMed] [Google Scholar]
- Vanderlip S.L. Barron’s Educational Series. Inc.; New York, USA: 2001. Mice: A Complete Pet Owner’s Manual. [Google Scholar]
- Vadivel V., Janardhanan K. Nutritional and anti-nutritional composition of velvet bean: an under-utilized food legume in South India. Int. J. Food Sci. Nutr. 2000;51:279–287. doi: 10.1080/09637480050077167. [DOI] [PubMed] [Google Scholar]
- Vermes I., Toth E.K., Telegdy G. Effects of drugs on brain neurotransmitter and pituitary testicular function in male rats. Horm. Res. 1979;10:222–232. doi: 10.1159/000179004. [DOI] [PubMed] [Google Scholar]
- Wyrobek R.A., Watchmaker G., Gordon L. Handbook of Mutagenicity Test-Procedure. Elsevier Science; Amsterdam: 1984. Sperm morphology testing in mice; pp. 733–750. [Google Scholar]
- Zitzmann M. Effects of testosterone replacement and its pharmacogenetics on physical performance and metabolism. Asian J. Androl. 2008;10(3):364–372. doi: 10.1111/j.1745-7262.2008.00405.x. [DOI] [PubMed] [Google Scholar]