Abstract
Research focus recently shifted to mitochondrial dynamics and the role of fusion and fission in cardioprotection. The aim of this study was to evaluate (i) the function and dynamics of mitochondria isolated from hearts exposed to ischaemia/reperfusion (I/R) (ii) the effects of melatonin, a powerful cardioprotectant, on mitochondrial dynamics in I/R. Isolated perfused rat hearts were stabilized for 30 min, subjected to 20 min global ischaemia, followed by 30 min reperfusion. Tissue was collected, mitochondria isolated for measurement of mitochondrial oxidative function and lysates from mitochondrial and cytosolic fractions prepared for western blotting. Melatonin (0.3 or 50 μM) was administered for 10 min immediately before the onset of ischaemia and for 10 min at the onset of reperfusion. Infarct size was assessed after 35 min regional ischaemia/60 min reperfusion using triphenyltetrazolium staining. The results show that reperfusion significantly reduced mitochondrial QO2 (states 3 and 4), with minor effects by melatonin. Cytosolic Beclin 1 and the LC3 II/I ratio were reduced by ischaemia and increased by reperfusion. Both ischaemia and reperfusion reduced mitochondrial PINK1 and Parkin levels, while reperfusion increased p62. An alternative mitophagy pathway mediated by Rab9 is activated during myocardial ischaemia/reperfusion. Ischaemia reduced and reperfusion increased cytosolic ULK1 expression, associated with redistribution of Rab9 and Drp1 between the cytosol and mitochondria. Melatonin significantly reduced mitochondrial p62 expression upon reperfusion. Throughout the protocol, melatonin significantly (i) increased cytosolic total (t) and phospho (p) ULK1, and Rab9 levels (ii) increased the cytosolic and reduced the mitochondrial pDrp1 levels and p/t Drp1 ratio, suggesting inhibition of mitochondrial fission. Fusion was affected to a lesser extent. Cardioprotection by melatonin is associated with substantial effects on mitophagy, the significance thereof remains to be established.
Keywords: Cardiology, Musculoskeletal system, Physiology, Pharmacology, Laboratory medicine, Autophagy, Mitochondrial oxidative phosphorylation, Mitophagy, Mitochondrial fission, p62, ULK1, Drp-1, Cardioprotection, Melatonin
Cardiology; Musculoskeletal system; Physiology; Pharmacology; Laboratory medicine; Autophagy; Mitochondrial oxidative phosphorylation; Mitophagy; Mitochondrial fission; p62; ULK1; Drp-1; Cardioprotection.
1. Introduction
The mitochondrion has become a major focus of studies aimed at cardioprotection (for a recent review see ref [1]). Apart from its well-established role in myocardial energy production, it is also known that these organelles may represent a final common endpoint in several cardioprotective interventions. In this regard, research focus has recently shifted to another facet of mitochondrial behaviour, namely mitochondrial dynamics and the role of their fusion and fission in this regard.
In view of their importance in the regulation of cell fate, stringent quality control mechanisms are in place to ensure a healthy mitochondrial population. Removal of damaged mitochondria occurs via fission with the proteins dynamin-related protein (Drp-1) and mitochondrial fission protein 1 (Fis 1) being important role players. On the other hand, elongation of mitochondria by the process of fusion is controlled by the proteins mitofusin 1 and 2 (Mfn1, Mfn2) and optic atrophy 1 (Opa 1). Cardiac dysfunction in ischaemia/reperfusion (I/R) is associated with an imbalance between mitochondrial fission and fusion (for review see ref [2]). Numerous studies have shown that during reperfusion, mitochondria undergo fission and that there is a reduction in fusion [2]. Therefore, inhibition of excessive mitochondrial fission and increased incidence of mitochondrial fusion have been proposed as potential protective mechanisms against cardiac dysfunction after I/R injury. The process of degradation and recycling of damaged mitochondria is termed mitophagy, a type of autophagy triggered by reactive oxygen species (ROS), low mitochondrial membrane potential and opening of the mitochondrial permeability transition pore (MPTP) [3, 4], in which dysfunctional mitochondria are sequestered into autophagosomes and subsequently degraded in the lysosomes. One of the most well-defined mechanisms of mitophagy is the PINK1/Parkin mechanism [4, 5]. However, increasing evidence suggests an alternative non-conventional mitophagy pathway, which may play an important role in protecting the heart against ischaemic stress. A recent study provided compelling evidence for mitophagy mediated by a protein complex consisting of unc-51 like kinase 1 (ULK1), Rab9, receptor-interacting serine/threonine protein kinase 1 (Rip1) and Drp-1 and it was suggested that this alternative autophagy is the predominant form of mitophagy in cardiomyocytes during stress [6]. Their data also suggest that ULK1-Rab9-dependent mitophagy targets depolarized mitochondria using a mechanism distinct from that of PINK1-Parkin dependent mitophagy.
The harmful effects of reactive oxygen species (ROS) production on mitochondrial function and cell death during exposure of the heart to ischaemic stress/reperfusion as well as their effects on initiation of mitophagy, are well-established. In this regard, the cardioprotective effects of melatonin, have been shown by several groups [7, 8, 9]. Melatonin is known as a potent free radical scavenger and mitochondrial-targeted anti-oxidant, while it also functions as a signalling molecule to upregulate gene expression of anti-oxidant enzymes and a spectrum of stress responsive genes (for a review see ref [10]). It is believed that melatonin also exerts its beneficial effects by keeping the mitochondrial permeability transition pore (MPTP) in a closed confirmation [11, 12]. Recent evidence revealed an intricate relationship between melatonin and its actions and the mitochondrion per se: for example, measurement of the subcellular distribution of melatonin showed that the concentration of the indole in the mitochondria greatly exceeds that in the blood [10, 13]. Moreover, apart from passive diffusion across membranes, it has now been shown that the uptake of melatonin by the mitochondria is mediated by the oligopeptide transporters GLUT transporter/solute carrier family 2A (GLUT/SLC2A10) and PEPT1/2 (SLC15A1/2) [10,14]. This active transport of melatonin into the mitochondria is proposed to provide cellular protection [10].
A recent study in mouse brains [15] showed that melatonin can be synthesized in the mitochondrial matrix due to the presence of two key melatonin biosynthetic enzymes, namely arylalkylamine N-acetyltransferase (AANAT) and acetylserotonin O- methyltransferase (ASMT). Melatonin is bound by the high-affinity MT1 receptor located in the outer mitochondrial membrane. It was also demonstrated that melatonin activates the mitochondrial MT1/Gαi protein signal system, blocks adenylate cyclase activity and inhibits stress-induced release of cytochrome C [16]. The significance of the presence of melatonin within the mitochondrion and the ability of this organelle to take up or synthesize this hormone need to be further investigated. The protective effects of melatonin on mitochondria are multiple (for a summary see ref [10]), for example, it has been demonstrated several years ago that melatonin treatment of isolated hearts protects mitochondrial function against I/R injury [12]. However, the effects of this compound on mitochondrial dynamics under these conditions and their significance in cardioprotection are not known. The aims of this study were therefore to evaluate the effects of melatonin treatment of the isolated ischaemic/reperfused rat heart on the relationship between the mitochondrial oxidative phosphorylation process and autophagy/mitophagy as reflected by using appropriate markers for the PINK1/Parkin and non-conventional pathways. Two concentrations of melatonin were studied, namely a low concentration (0.3 μM) as was used by Lecour et al [17] and 50 μM as was routinely used by Lochner [8], Petrosillo and coworkers [12].
2. Results
2.1. Effect of melatonin on infarct size
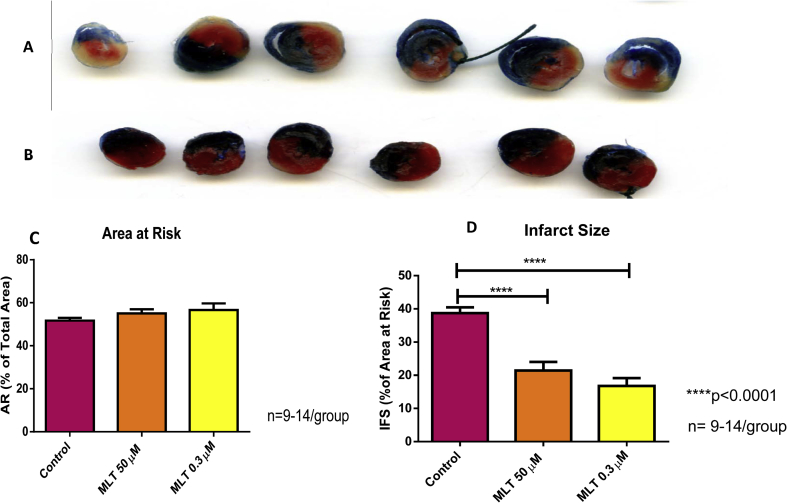
There were no significant differences between the areas at risk of the various groups (Fig. 1C: Control 51.69 ± 1.3; Mel 50 μM: 55.01 ± 1.97; Mel 0.3 μM: 56.56 ± 3.03). Melatonin at both 50 μM and 0.3 μM significantly (p < 0.0001) decreased the infarct size, compared to the control group (Fig. 1D). The reduction in infarct size induced by both concentrations of melatonin was associated with an improvement in functional performance during reperfusion as reflected by significant increases in aortic output, cardiac output and work performance (data not shown).
Fig. 1.
The effects of melatonin on infarct size. Melatonin (0.3 and 50 μM) administration was given for 10 min immediately before and for 10 min during reperfusion after 35min coronary artery ligation, followed by 60 min reperfusion. Tetraphenyltetrazolium staining was used to assess infarct size which was expressed as a % of the area at risk (A,B).
2.2. Effect of melatonin on mitochondrial oxidative phosphorylation function
In order to correlate the mitochondrial oxidative phosphorylation process with mitophagy and the effects of manipulation with melatonin on these parameters, mitochondria were isolated from hearts at different stages of the ischaemia/reperfusion protocol. This allowed evaluation of the effects of ischaemia per se and also those of reperfusion in the absence or presence of melatonin (0.3 μM or 50 μM). Two mitochondrial substrate protocols were used: in the carbohydrate protocol a substrate combination was used for electron flow through respiratory chain complexes I and II (glutamate plus malate); in the fatty acid protocol, respiration was measured with palmitoyl-L-carnitine plus malate. In the evaluation of the data, an ANOVA was initially performed on all parameters. In view of the effect of perfusion per se (as evidenced by comparison of values obtained from mitochondria prepared from hearts without perfusion (baseline) and those obtained after a stabilization perfusion period of 30 min), all values throughout the perfusion protocol were subsequently compared with values obtained after stabilization.
Apart from a reduction in the ADP/O ratio upon reperfusion of control hearts, the perfusion protocol had no significant effects on this parameter and similar values were obtained in mitochondria isolated from control perfused hearts after the stabilization period, ischaemia as well as after reperfusion. In addition, similar results were obtained regardless of the substrate present in the mitochondrial incubation medium (Supplementary Fig 1). Melatonin was without significant effect on this parameter.
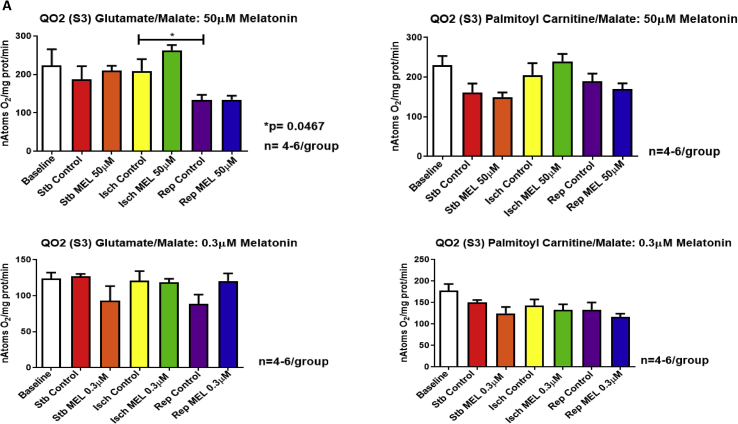
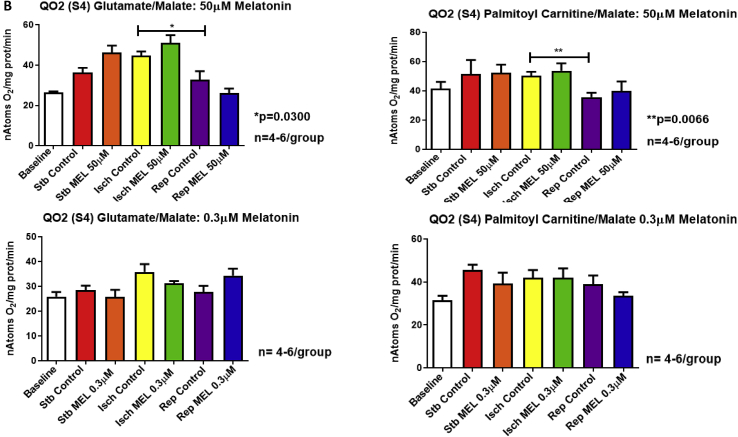
Exposure of the heart to 20 min global ischaemia was without effect on mitochondrial oxygen uptake (States 3 and 4), regardless of the substrate combination used. However, reperfusion caused a significant reduction in QO2 (states 3 and 4) with both substrates (Fig. 2A,B), as well as a reduction in the oxphos rate (ADP/O ratio X QO2 State 3) compared to ischaemia alone (substrates:glutamate/malate) (Supplementary fig. 2).
Fig. 2.
Mitochondrial oxidative phosphorylation function after exposure of the hearts to ischaemia and reperfusion: effects of melatonin. Melatonin (0.3 and 50 μM) was administered to isolated perfused hearts for 10 min before and for 10 min after ischaemia and mitochondria isolated for subsequent evaluation of mitochondrial oxidative phosphorylation function after (i) stabilization for 30 min (ii) stabilization followed by 20 min global ischaemia and (iii) stabilization, followed by 20 min global ischaemia and 30 min reperfusion. Respiratory activities were measured in the presence of glutamate (5mM) plus malate (2mM) (substrates for complexes I and II) or palmitoyl-L-carnitine (0.45mM) plus malate (2 mM) (mitochondrial fatty acid beta-oxidation substrate). Parameters evaluated were ADP/O ratio (Supplementary file Fig. 1); QO2 (State 3) (nAtoms oxygen uptake/mg protein/min in presence of ADP) (A); QO2 (State 4) (nAtoms oxygen uptake/mg protein/min after phosphorylation of added ADP) (B); oxidative phosphorylation rate (nmoles ATP produced/mg protein/min) (Supplementary file Fig. 2). Abbreviations: Stb: stabilization; Isch: 20 min global ischaemia; Rep: reperfusion after 20 min global ischaemia. n = 4–6 hearts/group.
Interestingly, melatonin had no significant effects on states 3 and 4 respiration throughout the perfusion protocol (Fig. 2A,B) with both substrate combinations, but at 0.3μM melatonin increased the oxphos rate with glutamate/malate as substrates (Supplementary Fig 2).
2.3. Evaluation of autophagy and mitophagy by western blot analysis
Using western blotting, the expression of the following mitochondrial proteins was evaluated: Parkin, PINK1, TOM70, p62/SQSTM1 (p62), Rab9, DRP-1 (phosphorylated and total), ULK1 (phosphorylated and total), mitofusin and Opa1. The expression of LC3, Beclin, PGC-1α, Sirt1, Drp-1 (phosphorylated and total), ULK1 (phosphorylated and total) and Rab9 was also studied in the cytosol. In addition to these markers two additional proteins, known to be associated with the effects of melatonin, were included in this series namely PGC-1 α and Sirt1 (see Table 1). In the initial studies the effects of two melatonin concentrations were evaluated, namely 50 and 0.3 μM, but no marked differences were observed between the effects of the high and low concentrations of melatonin. Therefore for the western blotting data, only the results obtained with the low concentration of melatonin are shown.
Table 1.
Mitochondrial and cytosolic proteins using western blotting.
| Cytosol | Mitochondria | |
|---|---|---|
| Autophagy | Beclin | |
| LC3 | ||
| Conventional | ||
| Pink/Parkin pathway | PINK | |
| Parkin | ||
| TOM70 | ||
| P62 | ||
| Alternative pathway | ULK1 | ULK1 |
| Rab9 | Rab9 | |
| Drp-1 | Drp-1 | |
| Mitochondrial biogenesis/fission | Sirt1 | |
| PGC1α | ||
| Fusion | Mfn2 | |
| Opa1 | ||
2.4. Autophagy (Fig. 3)
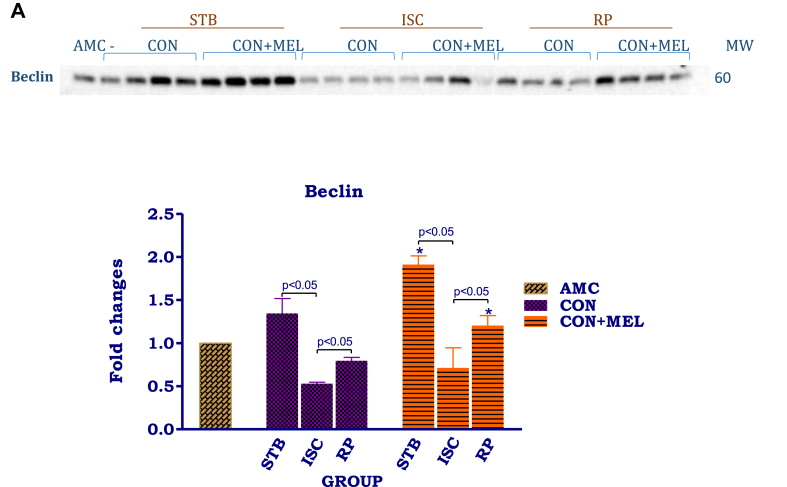
Fig. 3.
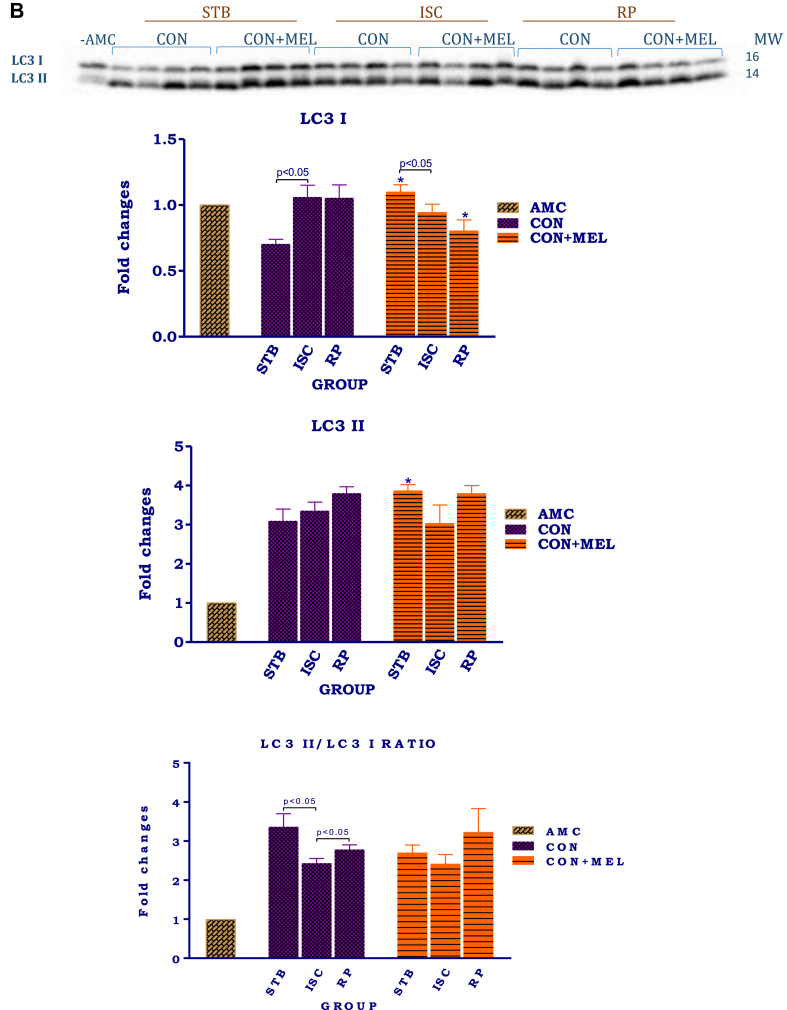
The effect of melatonin on autophagy in hearts exposed to ischaemia and reperfusion. Hearts were subjected to 20 min global ischaemia followed by 30 min reperfusion with and without melatonin (0.3 μM) (see Fig. 2) for subsequent preparation of cytosolic samples. Immunoblots and summary of the effects of melatonin on cytosolic Beclin 1 (A) and LC3 (B) expression in hearts after Stb, isch and rep. For full, non-adjusted images of gels see Supplementary file1 Fig. 3A, B. Abbreviations: Stb: stabilization; isch: ischaemia; rep: reperfusion; AMC: age-matched control; CON: control; CON + MEL: control + Melatonin. n = 4 hearts/group. LC3: microtubule-associated protein light chain. *p < 0.05 vs corresponding control group.
Cytosolic Beclin 1 and LC3 were used as makers of autophagy during the I/R protocol. Compared to stabilization, ischaemia lowered cytosolic Beclin 1 expression significantly (p < 0.05), while reperfusion was associated with an increase (p < 0.05) (Fig. 3A). The same response pattern was seen in hearts treated with melatonin, however, when compared with untreated controls, melatonin caused a significant increase in Beclin 1 expression after stabilization, as well as after reperfusion (p < 0.05). Interestingly, the cytosolic LC3 II/I ratio shows the same pattern in the control untreated hearts as Beclin 1 levels, namely a reduction during ischaemia and upregulation during reperfusion (Fig. 3B). Similar tendencies were observed in the melatonin treated hearts, but the values did not differ from those of untreated hearts.
2.5. Conventional PINK1/Parkin mitophagy (Fig. 4A-D)
Fig. 4.
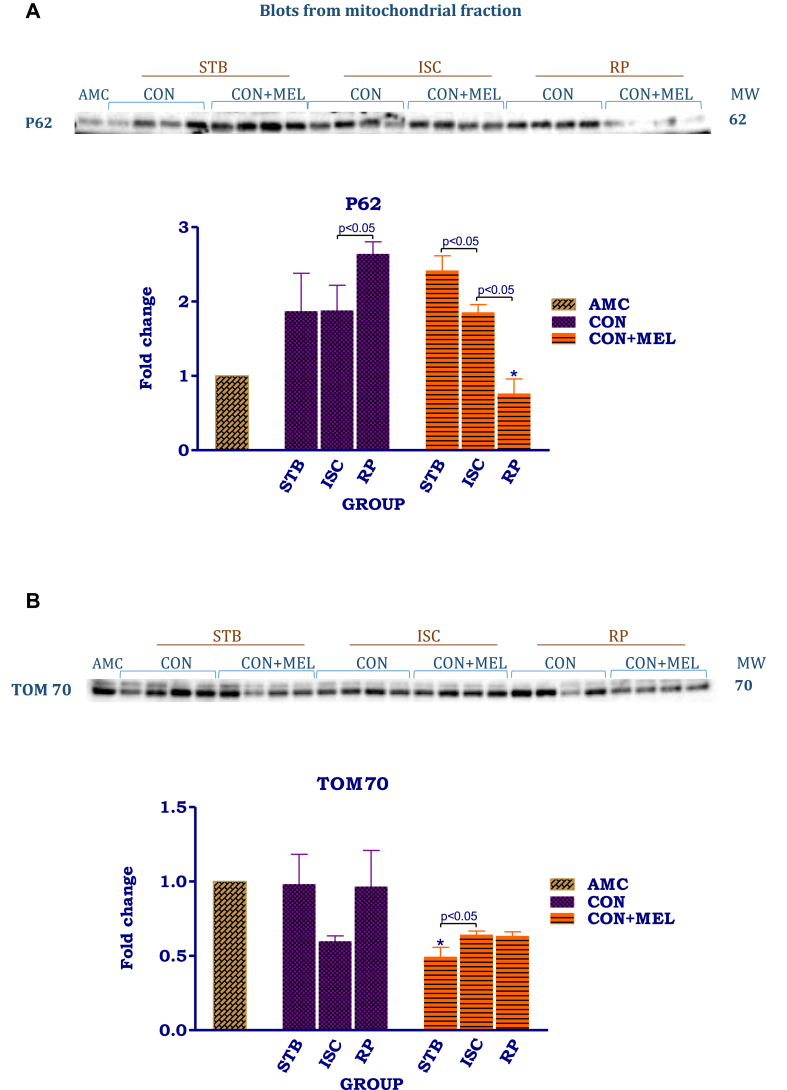
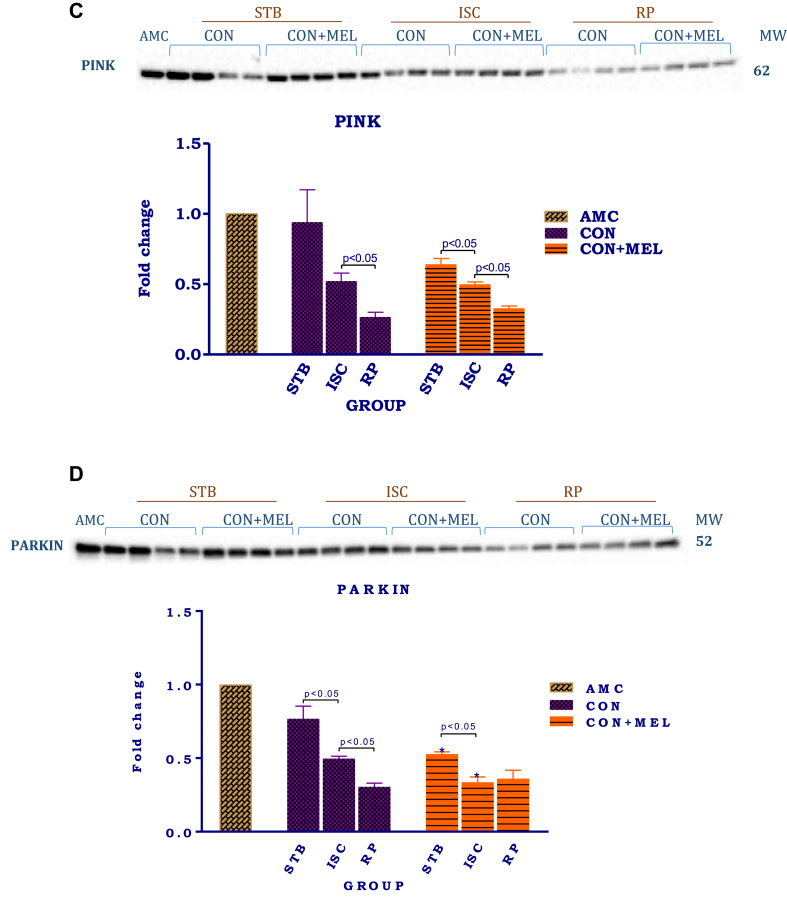
The effect of melatonin on indicators of mitochondrial mitophagy in hearts subjected to ischaemia and reperfusion. Hearts were subjected to 20 min global ischaemia followed by 30 min reperfusion with and without melatonin (0.3 μM) (Fig. 2) for subsequent preparation of mitochondria. Immunoblots and summary of the effects of 0.3 μM melatonin on p62 (A), TOM70 (B), PINK1 (C) and Parkin (D) expression in mitochondria isolated after Stb, isch and rep. For full non-adjusted images of gels see Supplementary file1 Figs 4A-D. Abbreviations: see Fig. 3. P62: p62/SQSTM1; TOM70: translocase of outer membrane 70; PINK1: PTEN-inducible kinase 1.
The most significant changes were observed in the levels of p62 of mitochondria from untreated control hearts, namely a significantly increased expression during reperfusion, compared to the values obtained after stabilization and ischaemia. The opposite pattern was seen with melatonin treatment, namely a significant reduction after ischaemia and a further reduction upon reperfusion (Arbitrary units (AU): Stb MEL 2.4 ± 0.2 vs Rep MEL 0.76 ± 0.19, p < 0.05) resulting in significantly lower levels at the end of reperfusion, compared to those of the untreated controls (AU: Rep Control 2.6 ± 0.16 vs Rep MEL 0.76 ± 0.19, p < 0.05).
The changes in TOM70 levels induced by exposure to I/R, were not significant due to rather large standard errors. Melatonin significantly lowered the TOM70 levels after stabilization (p < 0.05), when compared to its untreated counterparts. In contrast to the untreated controls, melatonin increased TOM70 expression after exposure to ischaemia, without having an effect after reperfusion.
Analysis of Parkin expression showed that ischaemia as well as reperfusion significantly lowered its expression when compared with the stabilization (AU: Stb Control-0.77 ± 0.09 vs. Isch Control 0.50 ± 0.02; p = 0.0090) or reperfusion (AU: Stb Control- 0.77 ± 0.09 vs. Rep Control-0.21 ± 0.02; p = 0.0001). Melatonin (0.3 μM) decreased Parkin expression during stabilisation (AU: Stb Control- 0.77 ± 0.09 vs. Stb MEL- 0.53 ± 0.014; p = 0.0262) as well as after ischaemia, while having no effect on its expression after reperfusion, compared to their untreated controls.
As was seen with Parkin, PINK1 expression was progressively lowered by I/R when compared with the stabilization controls (AU: Stb Control- 0.94 ± 0.23 vs. Rep-0.27 ± 0.03; p = 0.0023). Similar tendencies were observed in the presence of melatonin, namely a reduction in expression after exposure to ischaemia and a further significant reduction upon reperfusion. Melatonin, however, was without effect when compared with its untreated counterparts.
2.6. Alternative mitophagy pathways
In order to evaluate the role of an alternative autophagy pathway in our model of ischaemia/reperfusion and the effects of melatonin thereupon, the expression of ULK1, Rab9 and Drp-1 during an I/R protocol, was determined in both mitochondrial and cytosolic fractions.
ULK1 could not be detected in the mitochondrial fraction of hearts subjected to an I/R protocol (results not shown). However these interventions had a major effect on cytosolic ULK1 (see Fig. 5A). The expression of total (t) ULK1 was significantly decreased by exposure to both ischaemia (AU: Stb Control- 1.5 ± 0.11 vs. Isch Control- 0.17 ± 0.05; p < 0.05) and reperfusion (AU: Stb Control- 1.5 ± 0.11 vs. Rep Control- 0.53 ± 0.076; p = 0.0314) in untreated control groups in comparison to stabilisation. Its levels were significantly higher during reperfusion compared to ischaemia. In untreated controls, the expression of phospho (p)-ULK1 was significantly increased by ischaemia (AU: Stb Control- 0.21 ± 0.014 vs. Isch Control- 0.45 ± 0.073; p < 05) when compared to stabilisation. These were opposite to those observed for t ULK1.
Fig. 5.
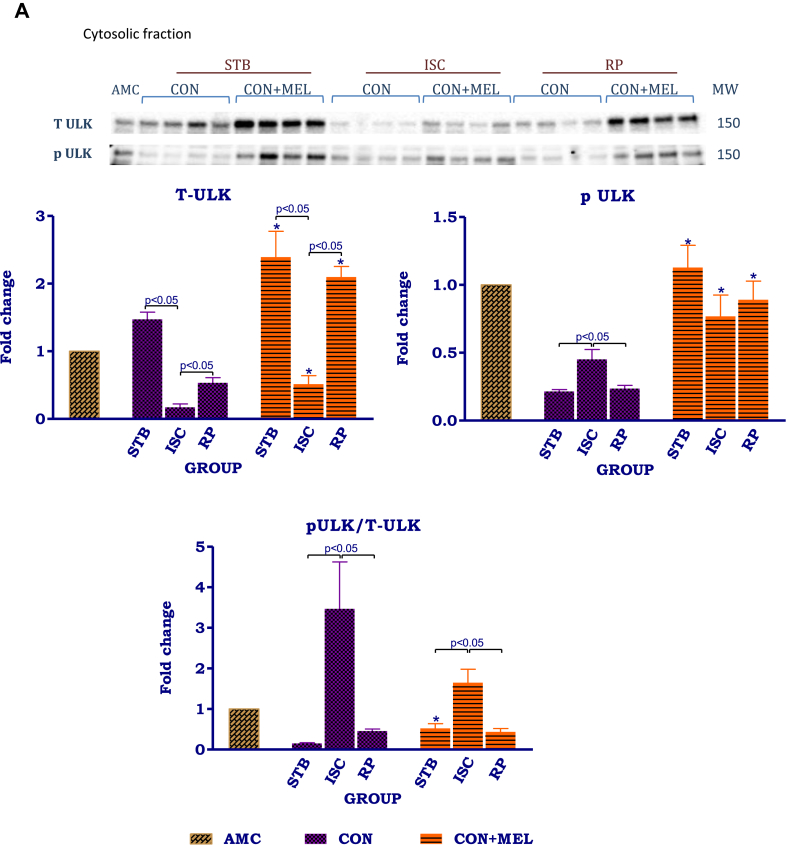
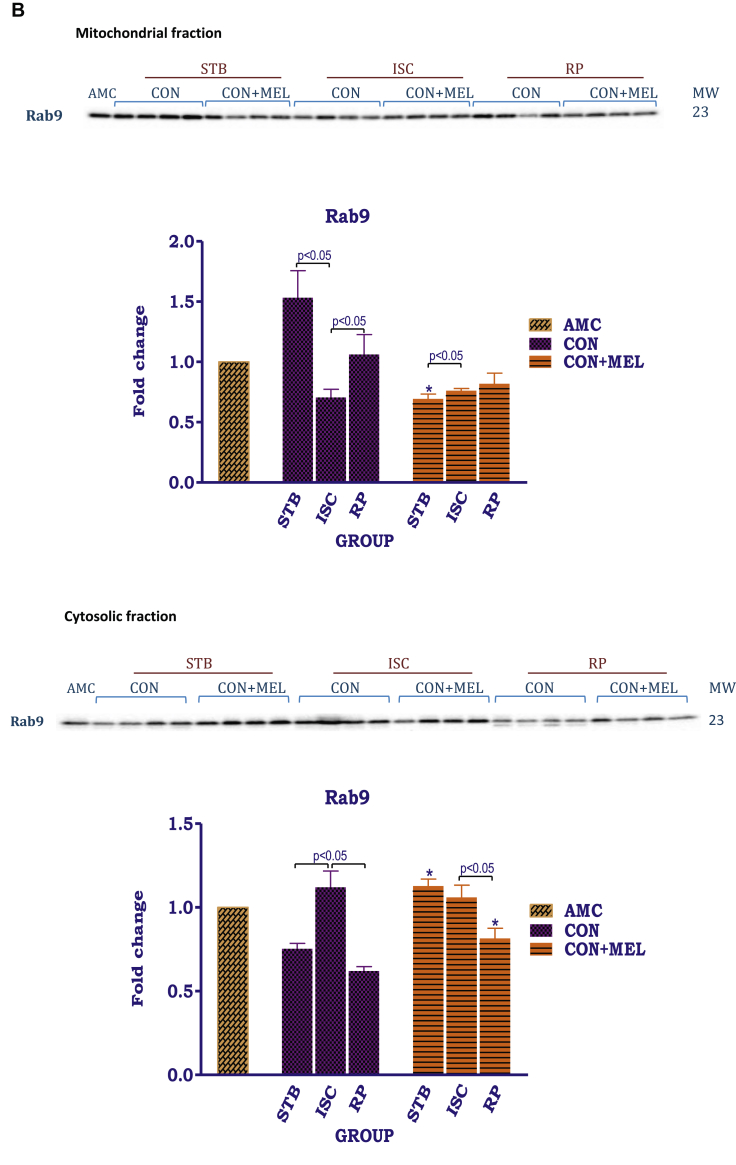
The effects of melatonin on mitochondrial and cytosolic ULK1 and Rab1. Hearts were subjected to 20 min global ischaemia and 30 min reperfusion with and without melatonin (0.3 μM) (Fig. 2) for subsequent preparation of cytosolic and mitochondrial fractions. Immunoblots and summary of the effects of melatonin on expression of myocardial cytosolic total ULK1 (tULK1), phospho ULK1 (pULK1) and phospho/total ULK1 (p/t ULK1) ratio (A); mitochondrial and cytosolic Rab9 (B) after Stb, isch and rep. For full non-adjusted images of gels see Supplementary file 1 Fig. 5A,B. Abbreviations: see Fig. 3. ULK1: unc-51 like kinase. *p < 0.05 vs corresponding control groups.
Melatonin administration during stabilisation had significant effects on t ULK1, p ULK1 as well as the p/t ULK1ratio throughout the protocol: for example t ULK1 was significantly increased during stabilization (AU: Stb Control- 1.5 ± 0.11 vs. Stb MEL- 2.4 ± 0.38; p = 0.0358), ischaemia (AU: Isch Control-0.17 ± 0.05 vs. Isch MEL- 0.51 ± 0.13; p = 0.0449) as well as during reperfusion (AU: Rep Control- 0.53 ± 0.076 vs. Rep MEL-2.1 ± 0.16; p = 0.002). Phosphorylated ULK1 levels during stabilisation (AU: Stb Control- 0.21 ± 0.014 vs. Stb MEL- 1.1 ± 0.16; p = 0.0003) as well as during reperfusion (AU: Rep Control- 0.24 ± 0.025 vs. Rep MEL- 0.89 ± 0.14; p = 0.0098) were also significantly increased by melatonin, compared to their untreated counterparts. Due to the reduction in t ULK1, the p/t ULK1 ratio was significantly increased during ischaemia in untreated controls; however despite the significant upregulation in both t and p ULK1 throughout the perfusion protocol by melatonin, it did not cause significant changes in the p/t ULK1 ratio.
In contrast to ULK1, Rab9 was present in both mitochondrial and cytosolic fractions (Fig. 5B). In the mitochondria, exposure to ischaemia caused a significant reduction in Rab9 expression (AU: Stb Control- 1.5 ± 0.23 vs. Isch Control- 0.71 ± 0.067, p < 0.05), when compared with its corresponding stabilisation control. This was followed by a significant increase during reperfusion. Compared to the untreated stabilisation group, mitochondrial Rab9 expression was significantly decreased by 0.3 μM melatonin (AU: Stb Control- 1.5 ± 0.23 vs. Stb MEL- 0.69 ± 0.040, p = 0.0023), but no further differences were observed between the control untreated and treated groups.
In the cytosol, the distribution pattern of Rab9 appeared opposite to that observed in the mitochondria: Rab9 expression was significantly increased in the untreated ischaemic group in comparison with the stabilisation control group (AU: Stb Control- 0.75 ± 0.03 vs. Isch Control-1.1 ± 0.01; p < 05). On the other hand, the control reperfusion group had lower Rab9 levels in comparison to the ischaemic control (AU: Isch Control- 1.1 ± 0.01 vs. Rep Control-0.62 ± 0.03; p < 0.05). In the melatonin treated groups, ischaemia and reperfusion decreased Rab9 expression in comparison to stabilisation (AU: Stb MEL- 1.1 ± 0.04 vs. Rep MEL- 0.82 ± 0.06; p = 0.0267). However, the stabilisation (AU: Stb Control-0.75 ± 0.03 vs. Stb MEL- 1.1 ± 0.04; p = 0.0052) and reperfusion melatonin groups (AU: Rep Control- 0.62 ± 0.02 vs. Rep MEL-0.82 ± 0.06; p = 0.0251) had increased Rab9 levels in comparison to their untreated counterparts.
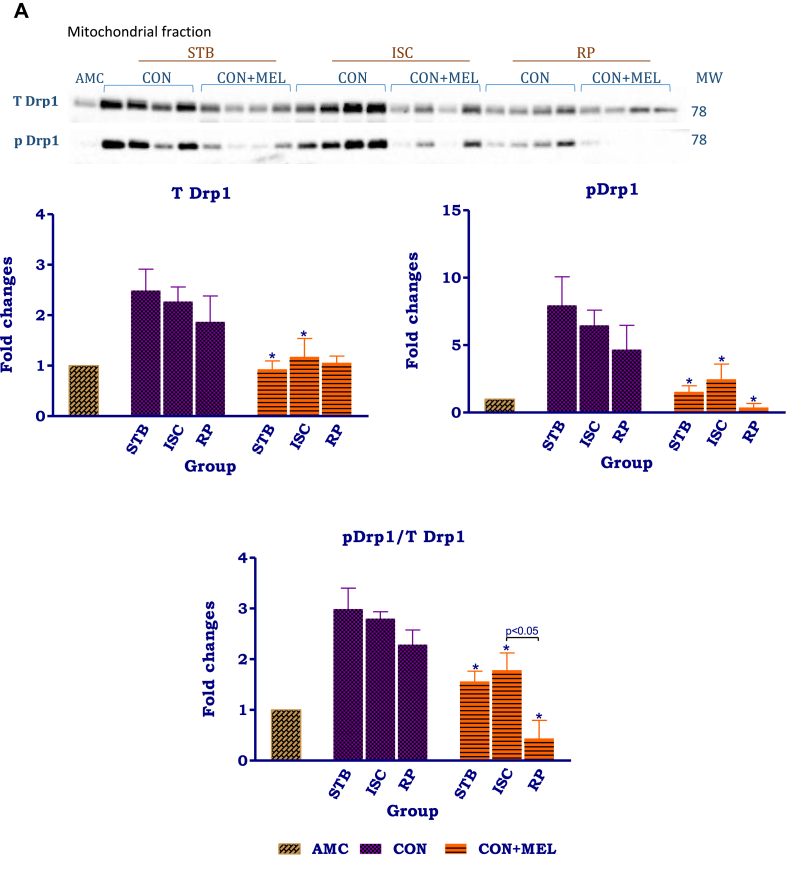
The distribution patterns of Drp-1 in the mitochondria and cytosol showed marked differences (Fig 6A,B). In the mitochondria isolated from untreated control hearts, the changes in expression of t and p Drp-1 as well as the p/t ratio during the perfusion protocol were insignificant. Melatonin treatment significantly reduced the expression of t and p Drp-1 at certain stages throughout the protocol: t Drp-1 levels were decreased in the stabilisation melatonin group compared to its untreated control (AU: Stb Control- 2.5 ± 0.42 vs. Stb MEL-0.93 ± 0.16, p = 0.0137); similarly p Drp-1 in the stabilisation (AU: Stb Control- 6.0 ± 2.1 vs. Stb MEL-1.5 ± 0.44; p = 0.0249) and ischaemic groups were significantly lower than their respective control counterparts. Melatonin significantly reduced the p/t Drp-1 ratio throughout the perfusion protocol when compared to the untreated controls.
Fig. 6.
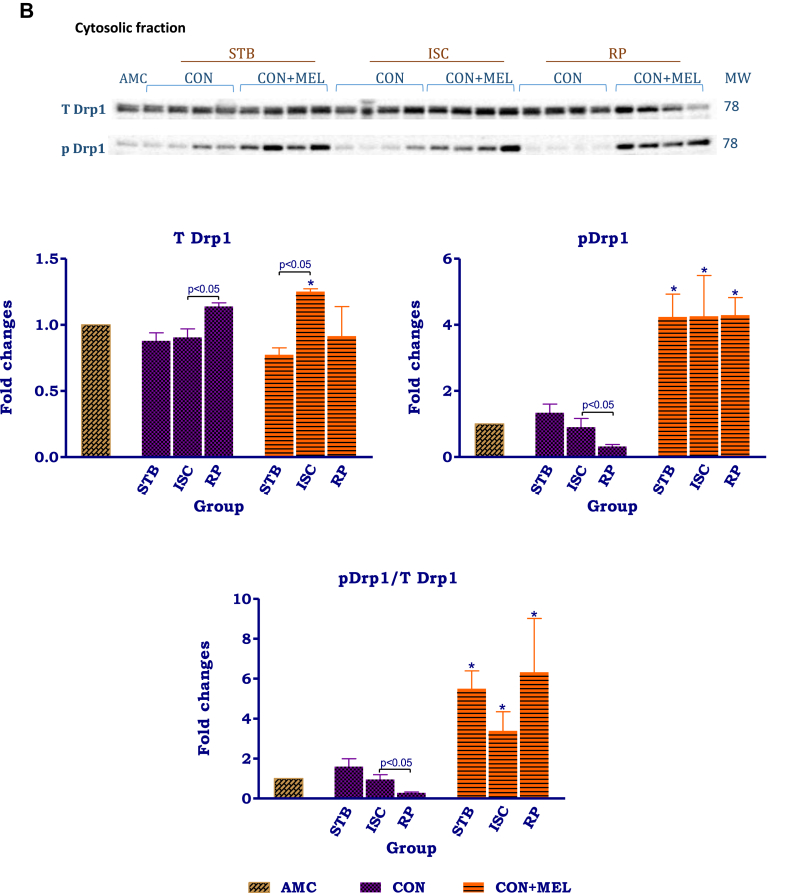
The effects of melatonin on mitochondrial and cytosolic Drp-1. Hearts were subjected to 20 min global ischaemia and 30 min reperfusion with and without melatonin (0.3 μM) (Fig. 2) for subsequent preparation of cytosolic and mitochondrial fractions. Immunoblots and summary of the effects of melatonin on expression of myocardial mitochondrial (A) and cytosolic (B) t Drp-1, p Drp-1 and p/t Drp-1 ratio after Stb, isch and rep. For full non-adjusted images of gels see Supplementary file1 Fig. 6A,B. Abbreviations: see Fig. 3. Drp-1: dynamin-related protein 1. *p < 0.05 vs corresponding control groups.
Different Drp-1 distribution patterns were observed in the cytosol: an increase in t Drp-1 was observed during reperfusion only, accompanied by a reduction in p Drp-1 as well as the p/t Drp-1 ratio particularly during reperfusion. Compared to their respective untreated control groups, melatonin significantly increased (>4x) p Drp-1 levels in the stabilisation (AU: Stb Control-0.9 ± 0.26 vs. Stb MEL- 4.2 ± 0.69; p = 0.0069), ischaemia (AU: Isch Control- 0.90 ± 0.26 vs. Isch MEL- 4.3 ± 1.20; p = 0.0373) and reperfusion (AU: Rep Control- 0.32 ± 0.06 vs. Rep MEL- 4.3 ± 0.53; p = 0.0003). Similarly, melatonin significantly increased the p/t Drp-1 ratio in the stabilisation (AU: Stb Control- 1.6 ± 0.39 vs. Stb MEL- 5.5 ± 0.89; p = 0.0069) and ischaemic (AU: Isch Control- 0.96 ± 0.23 vs. Isch MEL-3.4 ± 0.96; p = 0.0492) and reperfusion groups compared to their untreated controls.
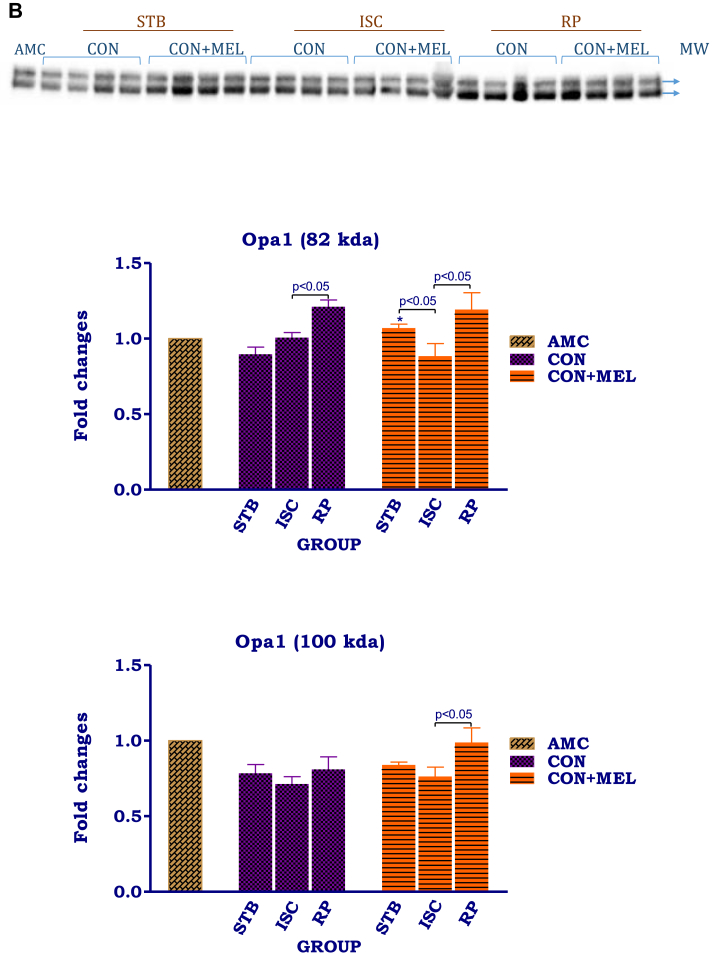
2.7. Mitochondrial fusion (Fig 7A, B)
Fig. 7.
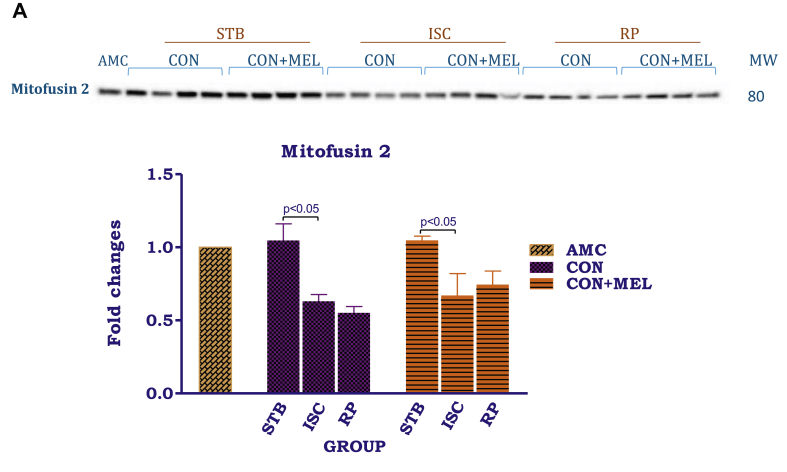
The effects of melatonin on mitochondrial mitofusin and Opa1. Hearts were subjected to 20 min global ischaemia and 30 min reperfusion with or without melatonin (0.3 μM) for subsequent preparation of mitochondria. Immunoblots and summary of the effects of melatonin on myocardial mitochondrial mitofusin (A) and Opa1 (B) expression after Stb, isch and rep. For full non-adjusted images of gels see Supplementary file 1 Fig. 7A,B. Abbreviations: see Fig. 3. Opa1: optic atrophy 1. *p < 0.05 vs corresponding control groups.
Ischaemia as well as reperfusion significantly reduced mitofusin 2 expression, when compared with values obtained after the stabilization phase. Interestingly, while Opa1 (100kDa) levels did not change throughout the protocol, the 82kDa isomer showed an increase particularly after reperfusion (p < 0.05). Melatonin treatment was without effect on mitofusin 2 as well as Opa1 levels and the values did not differ from their untreated controls.
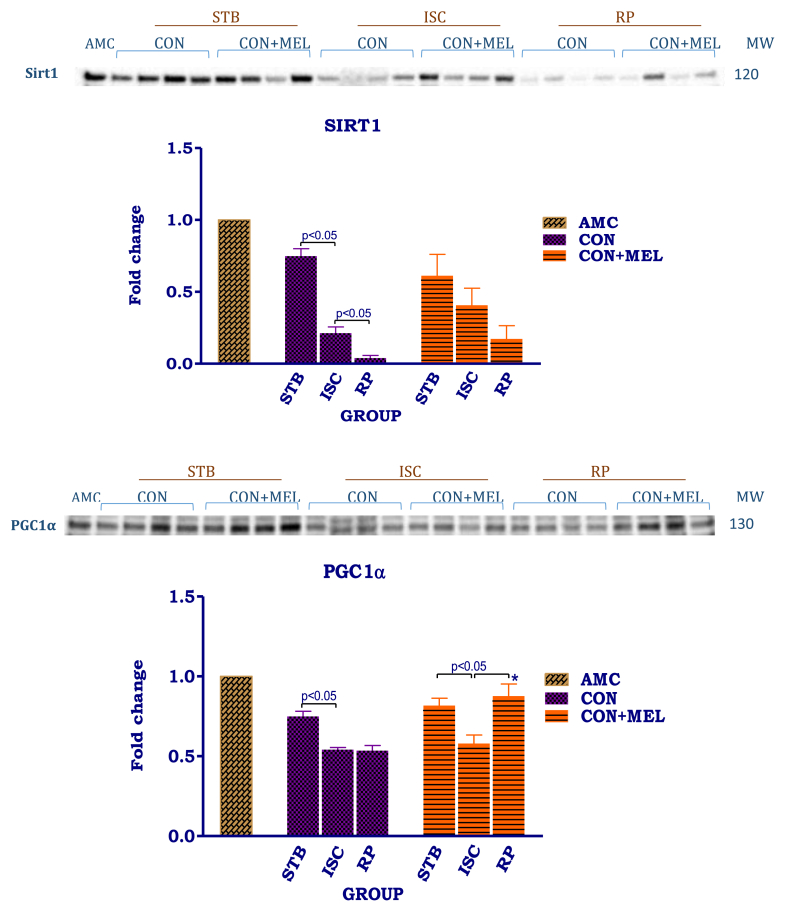
2.8. Sirt1 and PGC-1α (Fig. 8)
Fig. 8.
Effect of melatonin on cytosolic Sirt1 and PGC1α. Hearts were subjected to 20 min global ischaemia and 30 min reperfusion with and without melatonin (0.3 μM) for preparation of cytosolic fractions. Immunoblots and summary of the effects of melatonin on myocardial cytosolic expression of Sirt1 and PGC1α. For full non-adjusted images of gels see Supplementary file 1 Fig. 8. Abbreviations: see Fig. 3. Sirt1: silent information regulator; PGC1α: peroxisome-activated receptor gamma co-activator 1-alpha. *p < 0.05 vs corresponding control groups.
For assessment of the effects of melatonin on mitochondrial biogenesis, expression of cytosolic PGC-1α and Sirt1 was evaluated. The expression of both these proteins was lower after ischaemia. Ischaemia (AU: Stb Control- 0.75 ± 0.05 vs. Isch Control- 0.21 ± 0.04, p < 0.05) as well as reperfusion (AU: Stb Control-0.75 ± 0.05 vs. Rep Control- 0.039 ± 0.02 p = 0.0005) significantly reduced cytosolic Sirt1 expression. A similar stepwise depression in Sirt1 levels was seen with melatonin treatment, the changes being not significant.
Exposure to ischaemia alone or reperfusion significantly reduced the expression of cytosolic PGC-1α in untreated control groups. Melatonin caused a marked increase in PGC-1α expression after reperfusion (AU: Isch MEL 0.58 ± 0.05 vs Rep MEL 0.88 ± 0.08, p < 0.05), compared to the preceding ischaemia as well as the untreated reperfusion group (AU: Rep Control 0.54 ± 0.03, p < 0.001 vs Rep MEL 0.88 ± 0.08).
3. Discussion
For evaluation of the effects of I/R and melatonin treatment on mitochondrial function and mitophagy, use was made of the well-characterized in vitro model of the isolated perfused rat heart, a convenient and reproducible model to test mechanisms of myocardial injury and cardioprotection (for a review see ref 18). The in vitro approach was preferred since its throughput is higher than in vivo, it eliminates the confounding effects of interventions on systemic blood vessels and allows accurate manipulation of drug concentrations. Furthermore, although in vivo coronary artery ligation yields reliable infarct production and is closer to the clinical scenario, the surgery is technically more challenging.
The protective effects of melatonin on mitochondria are multiple and have recently been reviewed by Tan and coworkers (see reference 10). These include a reduction in mitochondrial oxidative stress, preservation of mitochondrial membrane potential and efficiency of ATP production, reduction in cytochrome C release and apoptosis and mitophagy, amongst others.
The present study described the effects of I/R on mitochondrial mitophagy focusing on the conventional as well as alternative pathways. Markers of alternative autophagy showed that I/R of the isolated perfused rat heart model not only had significant effects on the expression and distribution of these proteins within the cell, but that the beneficial effects of melatonin were also associated with major changes in this pathway.
3.1. Mitochondrial function
Exposure of the heart to 20 min global ischaemia per se was without effect on mitochondrial oxidative phosphorylation and significant changes were observed only after reperfusion (substrates: glutamate/malate) (Fig. 2). These observations differ from the well-established deleterious effects of I/R on mitochondrial function [19, 20] and are possibly due to the relatively short period of global ischaemia employed. For example, Petrosillo and coworkers [12] showed that exposure of the isolated rat heart to 30 min global ischaemia/15 min reperfusion caused significant changes in mitochondrial oxygen consumption, H2O2 production, lipid peroxidation, cardiolipin content and oxidation, and attributed attenuation of these changes by melatonin to the preservation of cardiolipin content and integrity.
Mitochondrial dysfunction is generally accepted as being a critical contributor to myocardial injury during I/R [19, 20, 21, 22] leading to decreased activity of complexes I [23, 24] and III, the latter being dominant in ROS generation [25, 26].
In view of the harmful effects of ROS on mitochondrial function [26, 27] and the fact that melatonin is a free radical scavenger [10, 28], it was surprising that its administration at both low and high concentrations had very little effect on mitochondrial function (Fig. 2), apart from an increase the oxphos rate during reperfusion (Supplementary Fig 2). Whether the latter contributed to cardioprotection, remains to be established. Failure to directly link the significant reduction in infarct size (Fig. 1) with changes in mitochondrial oxidative phosphorylation function may be due to differences in experimental protocol used (20 min global vs 35 min regional ischaemia).
3.2. Mitophagy: PINK1/Parkin pathway
The increase in cytosolic Beclin 1 expression and the LC3 II/I ratio of control hearts suggest upregulation of autophagy during reperfusion after 20 min global ischaemia (Fig. 3A, B). To further evaluate mitophagy, we used a snap-shot approach by preparing mitochondria after stabilization, after ischaemia and after reperfusion. Although this approach does not indicate flux, expression of p62 is regarded as a reliable indicator of autophagic flux [29] with a reduction in its levels indicating flux upregulation. Thus, we focused on the PINK1/Parkin pathway [4, 30] as indicators of mitophagy: steady state mitochondrial PINK1 as well as Parkin levels declined throughout ischaemia and reperfusion, a finding in contrast to expectations of PINK1 accumulation on the outer mitochondrial membrane and phosphorylation of proteins such as Mfn2, to trigger the mitophagic process [4, 30].
However the increase in mitochondrial p62 expression seen after reperfusion (Fig. 4) is suggestive of downregulation of mitophagy or decreased macroautophagy since cytosolic LC3 II/I can accumulate similarly to p62 if the autophagosomes are not cleared sufficiently. Upregulation of autophagy by myocardial I/R has been previously reported [31, 32], but as far as we are aware, the mitochondrial PINK1/Parkin pathway has not been evaluated simultaneously.
Although melatonin had few effects on the PINK1/Parkin pathway (Fig. 4), it significantly reduced mitochondrial p62 after reperfusion, suggesting upregulation of flux (possibly downstream of PINK1 and Parkin). The notion of melatonin-induced upregulation of autophagy is substantiated by the increased Beclin 1 levels and LC3 II/I ratio during reperfusion (the latter not being significant). Although melatonin has been reported to inhibit autophagy in tissues like the liver [33, 34], promotion of autophagy by this compound has been demonstrated by several others [35, 36, 37, 38] in heart muscle which could contribute to cardioprotection [39, 40, 41].
Expression of mitochondrial TOM70 was also evaluated as a possible indicator of mitophagy in myocardial ischaemia/reperfusion injury. TOM 70 is known to be essential for importing PINK1 into mitochondria [42] and to be critical for maintaining mitochondrial morphology and function [43]. For example Pei, and coworkers [43] showed that upregulation of TOM70 expression improved mitochondrial function and attenuated ischaemic injury. Although the changes were not significant, in the present study mitochondrial TOM70 expression was lowered by exposure of the heart to global ischaemia and increased during reperfusion, correlating with the well-known mitochondrial structural changes induced by ischaemia/reperfusion [44] (see Fig. 4B). Although TOM70 expression in tissue was suggested to be important in the beneficial effects of melatonin in a model of permanent coronary artery ligation [43], melatonin was without effect on its levels in mitochondria after I/R in global ischaemia (Fig. 4), the significance of which needs further investigation.
The relatively modest effects of melatonin on the PINK1/Parkin pathway in heart mitochondria are in contrast to the very marked effects of the compound on mitophagy and mitochondrial biogenesis in liver fibrosis [45]. This could be due to the relatively small changes in mitochondrial function induced by I/R in our model.
3.3. Alternative pathways
A role for an alternative pathway of mitophagy [6] in myocardial I/R was also considered. Using cytosolic ULK1 phosphorylation (Ser555) as indicator of activation, our data suggest up and downregulation of this pathway in ischaemia and reperfusion respectively (Fig. 5A). Ischaemia-induced AMPK activation [46] can account for the increased ULK1 phosphorylation during ischaemia, as reported by Tian and coworkers [47] while its reduction during reperfusion is probably due to reduced activation of AMPK during reperfusion [48]. Phosphorylation of ULK1 by AMPK regulates its translocation from the cytosol to mitochondria in Hela cells and MEFs [47]. However no ULK1 expression could be detected in mitochondria isolated from hearts during the perfusion protocol (data not shown). The reason for this is not clear and needs further investigation.
Interestingly, the cytosolic Rab9 expression followed a pattern opposite to that of total ULK1 expression in the cytosol, associated with exactly the opposite tendencies in mitochondrial Rab9 levels, suggestive of translocation of this protein from the mitochondria to the cytosol during ischaemia and vice versa during reperfusion. These observations suggest activation of an alternative mitophagy pathway during ischaemia/reperfusion, with significant movement of Rab9 between the mitochondria and cytosol, depending on the perfusion protocol.
Mitochondrial and cytosolic Drp-1 expression and phosphorylation were used to evaluate fission. Upon activation, cytosolic Drp-1 translocates to mitochondria where it dimerizes to initiate fission [49]. Dynamic changes in Drp-1 activity reflect rapid post-translational modification by phosphorylation and dephosphorylation, the best-characterized being phosphorylation at two sites: serine 616 phosphorylation causing activation and serine 637 phosphorylation causing inactivation [50, 51]. Phosphorylation of serine 637 was used as indicator of fission in this study.
Although the expression and phosphorylation of mitochondrial Drp-1 did not change significantly during the perfusion protocol, the significant reduction in cytosolic p and p/t Drp-1 suggested upregulation of fission in untreated ischaemic-reperfused hearts which was supported by the reduction in fusion (decreased Mfn2) (Fig. 6A,B; Fig. 7A). Although no measureable translocation of Drp-1 from the cytosol to the mitochondria was obtained in the present study, activation and translocation of Drp-1 from the cytosol to the mitochondria to initiate fission have been demonstrated in several experimental models of myocardial ischaemia/reperfusion, using in vivo and ex vivo hearts as well as cell models (see references 1,2 for reviews). For example Sharp and coworkers [49] showed that diminished phosphorylation of Drp-1 Serine637 was associated with translocation and mitochondrial fission using hearts subjected to 30 min global ischaemia/20 min reperfusion. However, most of these studies focused on the outcome of ischaemia/reperfusion or hypoxia/reoxygenation, rather than on the effects of ischaemia alone, as was also done in the present study.
The pattern of effects of I/R on mitochondrial Drp-1 (Fig. 6A) is similar to those observed for PINK1 and Parkin (Fig. 4). In COS cells loss of mitochondrial electron potential leads to recruitment of Drp-1 to mitochondria in the proximity of PINK1 and Parkin, suggesting that mitochondrial division occurs at sites where the PINK1/Parkin dependent mitochondrial clearance program is initiated [52]. Whether this also occurs in cardiomyocytes remains to be established.
The above changes in mitophagy may contribute to the reduced myocardial function during reperfusion: increased fission promotes I/R injury [49, 50], while pharmacological inhibition of Drp-1 by Mdivi is cardioprotective [1, 2, 49, 53, 54, 55, 56].
3.4. Melatonin and the alternative autophagy pathway
Melatonin had major effects on the alternative pathway during I/R, affecting the expression, phosphorylation and distribution of the proteins involved. Firstly, melatonin upregulated cytosolic tULK1 and pULK1, throughout the experimental protocol, probably due to AMPK activation, as demonstrated by Tian and coworkers [47]. Activation of AMPK by melatonin treatment has been demonstrated to occur in I/R hearts of diabetic rats [57]. Cytosolic Rab9 levels were also increased by melatonin, particularly during stabilization and reperfusion, albeit to a lesser extent.
Melatonin treatment profoundly affected intracellular distribution and phosphorylation of Drp-1 (Fig 6A,B), causing significant upregulation of cytosolic Drp-1 Ser637 phosphorylation and the p/tDrp-1 ratio throughout the perfusion protocol. Interestingly, this appears to be a rapid response, since it was observed after only 30 min stabilization perfusion, without any interventions. Since upregulation of Drp-1 Ser637 phosphorylation is indicative of a reduction in its activation and inhibition of translocation to the mitochondria, this indicates that melatonin administration causes a significant reduction in mitochondrial fission during ischaemia and reperfusion. These changes occur concomitantly with a reduction in mitochondrial tDrp-1 expression which may be due to decreased translocation from the cytosol to the mitochondria. However, despite the reduction in mitochondrial tDrp-1, lowering of its phosphorylation and the p/tDrp-1 ratio (particularly during reperfusion), indicates activation of residual mitochondrial Drp-1 by melatonin and thus activation of fission. An attempt was made to calculate whether the inactivation of cytosolic Drp-1 supercedes the increased Drp-1 activation in the mitochondria: the activation of the mitochondrial p/tDrp-1 ratio after exposure to ischaemia averaged 35%, compared to the 254% increase in cytosolic p/t ratio induced by melatonin (indicative of inactivation).
It is generally accepted that under most conditions, an elevated melatonin concentration results in decreased mitochondrial fission but elevated fusion [10]. Most of these results were obtained in neuronal tissue [58, 59, 60] or neuroblastoma SH-SY5Y cells [61, 62]. It has also been reported to affect mitochondrial fission/fusion dynamics in diabetic retinas [63].
As far as we are aware, this is the first report of the effects of melatonin on a number of proteins involved in alternative mitophagy in the I/R heart. Melatonin causes a substantial increase in cytosolic ULK and DRP-1 phosphorylation and their p/t ratios as well as Rab9 levels, while reducing the mitochondrial Rab9 expression and Drp-1 phosphorylation, particularly during reperfusion. Melatonin also appears to mediate rapid translocation of these proteins between the cytosol and mitochondria. The results suggest that the significant changes in phosphorylation of cytosolic Drp-1 induced by melatonin is indicative of inhibition of mitochondrial fission.
An inhibitory effect of melatonin on mitochondrial mitophagy was however not observed in carbon tetrachloride–induced liver fibrosis [45] and brain tissue-induced inflammation [64]. These observations suggest that melatonin effects on mitochondrial mitophagy may differ between tissues and the type of damage experienced.
3.5. Sirt1 and PGC-1α
A role for Sirt1 and PGC-1α in melatonin-induced effects on mitochondrial fission via Drp-1 was also investigated. Sirt1 physically interacts with and deacetylates PGC-1α, its major downstream target, promoting mitochondrial biogenesis. It has been shown that activation of AMPK by melatonin plays a role in this regard by initiation of mitochondrial biogenesis via SIRT1 dependent deacetylation of PGC-1α or upregulation of its expression [45].
Our results show the inhibitory effect of I/R on cytosolic Sirt1 levels (Fig. 8), confirming the in vivo findings of Yamamoto [65]. Melatonin significantly increased PGC-1α levels in the stabilization and reperfusion groups, indicating that this pathway may also contribute to its cardioprotective actions, via inhibition of Drp-1-mediated mitochondrial fission, confirming previous studies [45, 56]. Ding and coworkers [56] also suggested that melatonin attenuates the development of diabetes-induced cardiac dysfunction by preventing fission through this pathway.
3.6. Mitochondrial fusion
The mitochondrial proteins Mfn2 (outer membrane) and OPA 1 (inner membrane) were used as indicators of fusion [66, 67].
Our results show a reduction in mitochondrial Mfn2 levels by both ischaemia and reperfusion, while OPA1 shows an increase during reperfusion. The latter observation is in contrast to a previous report [68]. However, the role of myocardial fusion proteins in terms of susceptibility to I/R injury appears to be complex and conflicting results have been reported [10, 69]. Overexpression of Mfn2 [70] and Opa1 [71,72] was shown to be cardioprotective.
A recent study suggested that melatonin attenuates myocardial I/R injury via activation of the AMPK/Opa1 signalling pathway [73]. Little information is available regarding the effects of melatonin on mitochondrial Mfn2 in the heart. However, it has been shown to increase the expression of Mfn2 in the heart in post-myocardial infarction [74] and in renal convoluted tubules [75]. It is clear that the effects of melatonin on mitochondrial fusion in the myocardium need to be further investigated.
3.7. Limitations of the study
While appreciating the novelty of the discovery of melatonin effects on the alternative mitophagy pathway in myocardial stress, it is also clear that these beneficial effects need to further investigated and their significance in the outcome of I/R established. Firstly, despite the many advantages of the isolated perfused rat heart model [18], it may not fully represent the in vivo response, as will be the case when using coronary artery ligation in vivo. Secondly, the significant effects of melatonin on the expression and intracellular trafficking of components of the alternative mitophagy pathway, should be expanded by evaluation of RIP1, another role player in this pathway, by examining its phosphorylation by Ulk1 and its phosphorylation of S616 of Drp-1. Thirdly, the significance of the different role players, particularly Drp-1, in melatonin-induced cardioprotection, should be determined by use of appropriate inhibitors and activators. Using a combination of melatonin and an inhibitor of mitophagy in our well-characterized model of ischaemia/reperfusion should also shed light on the contribution of melatonin-induced inhibition of fission to its potent cardioprotective actions. It should also be kept in mind that all measurements made in this study represent a snapshot approach, without evaluation of flux, as advocated by Gottlieb and coworkers [29].
A study focusing on mitochondrial ultrastructure should also be useful in this regard. A recent study by Liu and coworkers [76] showed that intraperitoneal administration of melatonin before experimentation attenuated the ultrastructural changes characteristic of I/R.
4. Conclusions
The data obtained demonstrate for the first time that melatonin administration to the isolated perfused heart has, in addition to its other multiple actions, profound effects on the alternative mitophagy pathway, in addition to upregulation of autophagy during reperfusion. The results suggest that melatonin may, via its marked effects on cytosolic Drp-1 phosphorylation and activation, protect the myocardium in a manner similar to inhibitors of fission such as Mdivi [1, 2, 49, 53, 54, 55, 56], Thus we suggest that, in addition to its other cardioprotective actions, such as free radical scavenging [8] or its anti-adrenergic properties [77], melatonin also protects against I/R damage via inhibition of fission. Using a combination of melatonin and an inhibitor of mitophagy in our well-characterized model of ischaemia/reperfusion should also shed light on the contribution of melatonin-induced inhibition of fission to its potent cardioprotective actions.
5. Materials and methods
5.1. Experimental animals
Male Wistar rats (250–300g) were used for this study. All animals were housed in and obtained from the Stellenbosch University's Central Animal Research Facility located in the Faculty of Medicine and Health Sciences, Tygerberg. Animals were handled and cared for according to the accepted standards and use of animals in research and teaching as reflected in the South African National Standards document (SANS 10386:2008; available at www.sun.ac.za/research) of the South African Bureau of Standards. The animals were fed a standard rat chow diet and received access to food and water ad libitum. They were exposed to 12-hour dark/light cycles (with light from 6am-6pm) at a constant temperature of 20–21 °C.
Ethics approval was obtained from Research Ethics Committee: Animal Care and Use (REC: ACU) of Stellenbosch University (Faculty of Medicine and Health Science; Protocol #: SU-ACUM14-00039).
5.2. Isolated heart perfusions: infarct size
Rats were anaesthetised by intraperitoneal injection of sodium pentobarbitone (Bayer, Johannesburg, South Africa) (160 mg/kg). After rapid removal, the hearts were arrested in ice-cold modified Krebs-Henseleit buffer (KHB; substrate glucose 10mM) and subsequently mounted onto the aortic cannula and perfused with KHB as described before [77].
For determination of the effects of melatonin on infarct size, preference was given to exposure of the hearts to regional ischaemia. Hearts were stabilized for a period of 30 min (10 min retrograde perfusion at 100 cm H2O, 10 min working heart perfusion (preload 15 cm H2O; afterload 100 cm H2O), followed by 10 min retrograde perfusion), then subjected to 35 min of regional ischaemia by ligation of the left anterior descending coronary artery, followed by 60 min of reperfusion. Melatonin (0.3 or 50 μM) was administered retrogradely for 10 min immediately before and after regional ischaemia. Myocardial temperature was carefully controlled at 36.5 °C throughout the experimental protocol and monitored by inserting a temperature probe into the coronary sinus. Myocardial functional performance was measured during perfusion in the working mode at two time points namely prior to the induction of ischaemia as well as during reperfusion. Work performance was measured as described previously [78].
Melatonin (0.3 or 50 μM) was administered retrogradely for 10 min immediately before and for 10 min after regional ischaemia. Melatonin was dissolved in absolute ethanol (6mg/250 μl absolute ethanol) and the correct volume required added to 500 ml KHB buffer. A similar concentration of ethanol (without melatonin) added to the perfusate was without effect on all parameters measured (results not shown).
Infarct size was determined as previously described, using triphenyltetrazolium staining [78, 79]. The left coronary artery was ligated as high up as possible to ensure a reproducible area at risk which is prerequisite in studies on cardioprotection to allow comparison of infarct size between groups. The left ventricle area at risk, the infarcted and viable areas were drawn using computerized planimetry (UTHCSA Image Tool programme, University of Texas.
5.3. Isolated heart perfusions: mitochondrial studies
Mitochondria were isolated from hearts at different stages of the I/R protocol, i.e. at the end of 30 min of stabilization before onset of ischaemia (Stb), at the end of 20 min global ischaemia (Isch), as well as after 30 min reperfusion (10 min retrograde perfusion, 20 min working heart) (Rep).This allowed evaluation of the effects of ischaemia per se and also those of reperfusion in the absence or presence of melatonin (0.3 or 50 μM). For control purposes, a mitochondrial preparation isolated from a 5 min retrogradely perfused heart was included each day to serve as baseline.
Separate series of hearts were perfused for subsequent preparation of cytosolic samples at the same time points as listed above. These hearts were freeze-clamped and stored in liquid nitrogen until analysed.
5.4. Mitochondrial isolation procedure
Hearts allocated for mitochondrial isolation were placed in ice-cold isolation buffer (KE buffer: 0.18M KCL, 0.01M ethylenediaminotetraacetic acid (EDTA), pH 7.4, adjusted with 2M Tris) and mitochondria were isolated by differential centrifugation as described by Sordahl et al [80]. The mitochondrial pellet was divided in two: one half was dispersed in KE medium for immediate measurement of mitochondrial function, while the other half was dissolved in lysis buffer (see below) and stored at -80 °C for subsequent western blot analysis. Protein determination for mitochondrial functional studies was done by the technique of Lowry and coworkers [81].
Mitochondrial oxidative phosphorylation (oxphos) was measured polarographically at 27 °C using an oxygraph (Hansatech Instruments, Bannan UK) and Clark electrode. The mitochondrial incubation medium contained (in mM): sucrose 0.25, Tris-HCl 10, pH 7.4, K2HPO4 8.5, and glutamate 5/malate 2 (substrates for Complex I) or palmitoyl-L-carnitine 0.45/malate 2 (fatty acid beta-oxidation substrate) (pH 7.4). ADP (250–350 nmoles) was added to initiate State 3 respiration. Parameters investigated were the ADP/O ratio, State 3 respiration (mitochondrial respiration in the presence of ADP), State 4 respiration (mitochondrial respiration in absence of ADP). Mitochondrial respiratory rates (states 3 and 4) were expressed as nAtoms oxygen uptake/mg protein/min. The amount of ADP added to the incubation system was obtained spectrophotometrically (molar extinction coefficient of ADP: 15.4 at 259nm). The oxidative phosphorylation rates (nmoles ATP produced/mg prot/min) were calculated as follows: QO2 (state 3) x ADP/O ratio.
5.5. Western blot technique
Western blotting was performed on cytosolic as well as mitochondrial samples. The proteins studied as well as the relevant cell fractions are summarized in Table 1.
For cytosolic samples, heart tissue was homogenized with a Polytron homogenizer in 800 μL lysis buffer and the cytosolic fraction prepared as described by Fan et al [82].
An aliquot of the mitochondrial pellet was homogenized in 200 μL of lysis buffer using a Bullet BlenderTM (Next Advance Inc USA) at 4 °C for 5 min with a scoop of 0.15 mm zirconium oxide beads equivalent to the pellet size. Samples were then microfuged at 15 000 g for 10 min to obtain the supernatant, the protein content of which was determined using the Bradford method [83]. The following primary antibodies (obtained from Cell Signaling, Danvers, MA, USA) were used: anti-PINK1 (cat no 6946S), anti-p62 (cat no 7695), anti-Rab9 (cat no 5133), anti-beclin 1 (cat no 3495), anti-Opa1(cat no 804715), anti-total (cat no 8054) and anti-phosphorylated ULK1 (S555) (cat no 5869), anti-total (cat no 5391) and anti-p-Drp-1 (S637) (cat no 6319), Sirt1 (cat no 9475), and PGC1-α (cat no 2178). Antibodies for TOM 70 were obtained from Santa Cruz (Dallas, Tex, USA, cat no sc-390545). Parkin (cat no ab77924), LC3 A/B (cat no ab 128025) and mitofusin2 (cat no ab104274) from were purchased from Abcam (Cambridge, UK). All antibodies were monoclonal except for p62, Parkin and p-Drp-1 which were polyclonal. Dilution for all primary antibodies was 1:1000. Conjugated horseradish peroxidase was used as secondary antibody, dilution 1:4000 (Cell Signaling cat no 7074).
Depending on the protein of interest, 30–60 μg were loaded and separated using twenty-six well CriterionTM 4–20% precast gradient gels (BioRad) and stain-free technology. With stain-free technology, the transferred proteins on the PVDF membrane were visualized in the ChemiDoc MP system (Bio-Rad Laboratories) to confirm equal loading. Furthermore, the intensity of bands detected by the ECL reaction, was normalized to the total proteins that were transferred in each lane, negating the use of a loading control. Four samples/group were included on the same gel plus a sample prepared from a heart of an unperfused age-matched control animal to act as standard for normalization of all data.
5.6. Statistical analysis
All data points were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, Inc.). For infarct size evaluation, 9–14 hearts/group were used. For mitochondrial function as well as western blotting, 5 hearts per group were prepared. In the case of western blotting, 4 samples/group were loaded onto the gels. A one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc correction test was used for the comparison of all the groups within the same perfusion condition/protocol. Unpaired Student's t-test was used where appropriate. A p-value of ≤0.05 was deemed statistically significant.
Declarations
Author contribution statement
Kopano Dube, Karthik Dhanabalan: Performed the experiments; Analyzed and interpreted the data.
Ruduwaan Salie: Performed the experiments.
Marguerite Blignaut: Analyzed and interpreted the data.
Amanda Lochner, Barbara Huisamen: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the South African National Research Foundation (NRF grant number 93579), the Harry Crossley Foundation and Universiteit Stellenbosch.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to thank the the University of Stellenbosch for funding of the postdoctoral fellowship of Karthik Dhanabalan.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Boengler K., Lochnit G., Schulz R. Mitochondria “THE” target of myocardial conditioning. Am. J. Physiol. Heart Circ. Physiol. 2018 doi: 10.1152/ajpheart.00124.2018. [DOI] [PubMed] [Google Scholar]
- 2.Maneechote C., Palee S., Chattipakorn S., Chattipakorn N. Roles of mitochondrial dynamics modulation in cardiac ischaemia/reperfusion injury. J. Cell Mol. Med. 2017;21:2643–2653. doi: 10.1111/jcmm.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreira R.S., Lee Y., Ghochani M., Gustafsson A.B., Gottlieb R.A. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;2106:462–472. doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anzell A.R., Maizy R., Przyklenk K., Sanderson T.H. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol. Neurobiol. 2018;55:2547–2564. doi: 10.1007/s12035-017-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kane L.A., Lazarou M., Fogel A.I. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito T., Nah J., Oka S. An alternative mitophagy mediated by Rab9 protects the heart against ischemia. J. Clin. Investig. 2019 doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiter R.J., Tan D.-X. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc. Res. 2003;58:10–19. doi: 10.1016/s0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- 8.Lochner A., Huisamen B., Nduhirabandi F. Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front. Biosci. (Elite Ed). 2013;5:305–315. doi: 10.2741/e617. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y., Sun Y., Yi W. A review of melatonin as a suitable antioxidant against myocardial ischemia- reperfusion injury and clinical heart diseases. J. Pineal Res. 2014;57:357–366. doi: 10.1111/jpi.12175. [DOI] [PubMed] [Google Scholar]
- 10.Tan D.-X., Manchester L.C., Qin L., Reiter R.J. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016;17:2124. doi: 10.3390/ijms17122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter R.J., Rosales-Corral S., Tan D.X. Melatonin as a mitochondria-targeted anti-oxidant: one of nature’s best ideas. Cell. Mol. Life Sci. 2017;74:3853–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrosillo G., Di Venosa N., Pistolese M., Casanova G. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. FASEB J. 2006;20:269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z., Ma C., Meng C.L. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 2012;53:129–137. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 14.Huo X., Wang C., Yu Z., Peng Y. Human transporters, PEPT1/2, facilitate melatonin transport into mitochondria of cancer cells: an implication of the therapeutic potential. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 15.Suofu Y., Jean-Alphonse F.G., Jia J. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome release. Proc. Natl. Acad. Sci. 2017 doi: 10.1073/pnas.1705768114. e7997-e8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardeland R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017;74:3883–3896. doi: 10.1007/s00018-017-2615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nduhirabandi F., Lamont K., Albertyn Z., Opie L.H., Lecour S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J. Pineal Res. 2016;60:39–44. doi: 10.1111/jpi.12286. [DOI] [PubMed] [Google Scholar]
- 18.Merry L.L., Bolli R., Canty J.M., Du X.-J. Guidelines for experimental models of myocardial ischaemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 2018;314:H812–H838. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesnefsky E.J., Tandler B., Ye J., Slabe T.J., Turkaly J., Hoppel C.L. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. Heart Circ. Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q., Younus M., Thompson J. Intermediary metabolism and fatty acid oxidation: novel targets of electron transport chain-driven injury during ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2018;314:H787–H795. doi: 10.1152/ajpheart.00531.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafsson A.B., Gottlieb R.A. Heart mitochondria: gates of life and death. Cardiovasc. Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 22.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Liu J., Ma A., Chen Y. Cardioprotective effect of berberine against myocardial ischemia/reperfusion injury via attenuating mitochondrial dysfunction and apoptosis. Int. J. Clin. Exp. Med. 2015;8:14513–14519. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J. Pharmacol. Exp. Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Vazquez E.J., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 26.Lesnefsky E.J., Gudz T.I., Migita C.T., Ikeda-Saito M., Hassan M.O., Turkaly P.J., Hoppel C.L. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 27.Han D., Atunes F., Canali R. Voltage-dependent anion channels control release of superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 28.Galano A., Reiter R.J. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J. Pineal Res. 2018;65 doi: 10.1111/jpi.12514. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb R.A., Andres A.M., Sin J., Taylor D.P. Untangling autophagy measurements: all fluxed up. Circ. Res. 2015;116:504–514. doi: 10.1161/CIRCRESAHA.116.303787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narenda D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez R.E., Kubli D.A., Gustafsson A.B. Autophagy and mitophagy in myocardium: therapeutic potential and concerns. Br. J. Pharmacol. 2014;171:1907–1916. doi: 10.1111/bph.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie H., Xu Q., Jia J. Hydrogen sulphide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagic flux. Biochem. Biophys. Res. Commun. 2015;458:632–638. doi: 10.1016/j.bbrc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 33.San-Miguel B., Crespo I., Sanchez D.I. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J. Pineal Res. 2015;59:151–162. doi: 10.1111/jpi.12247. [DOI] [PubMed] [Google Scholar]
- 34.De Luxan-Delgado B., Potes Y., Rubio-Gonzalez A. Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 2016;61:108–123. doi: 10.1111/jpi.12333. [DOI] [PubMed] [Google Scholar]
- 35.Coto-Montes A., Boga J.A., Rosales-Corral S., Fuentes-Broto L., Tan D.X., Reiter R.J. Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol. Cell. Endocrinol. 2012;361:12–23. doi: 10.1016/j.mce.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Boqa J.A., Caballero B., Potes Y. Therapeutic potential of melatonin related to its role as an autophagy regulator. A review. J. Pineal Res. 2018:e12534. doi: 10.1111/jpi.12534. [DOI] [PubMed] [Google Scholar]
- 37.Govender J., Loos B., Marais E., Engelbrecht A.M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: a review of the protective role of melatonin. J. Pineal Res. 2014;57:367–380. doi: 10.1111/jpi.12176. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M., Lin J., Wang S. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J. Pineal Res. 2017;63 doi: 10.1111/jpi.12418. [DOI] [PubMed] [Google Scholar]
- 39.Dosenko V.E., Nagibin V.S., Tumanovska L.V., Moibenko A.A. Protective effect of autophagy in anoxia-reoxygenation of isolated cardiomyocyte? Autophagy. 2006;2:305–306. doi: 10.4161/auto.2946. [DOI] [PubMed] [Google Scholar]
- 40.Gurusamy N., Lekli I., Gorbunov N.V. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J. Cell Mol. Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Przyklenk K., Dong Y., Undyala V.V., Whittaker P. Autophagy as therapeutic target for ischaemia/reperfusion? Concepts, controversies and challenges. Cardiovasc. Res. 2012;94:197–205. doi: 10.1093/cvr/cvr358. [DOI] [PubMed] [Google Scholar]
- 42.Kato H., Lu Q., Rapaport D., Koziak-Pavlovic V. TOM70 is essential for PINK1 import into mitochondria. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pei H.F., Hon J.N., Wei F.P. Melatonin attenuates postmyocardial infarction injury via increasing TOM70 expression. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12371. [DOI] [PubMed] [Google Scholar]
- 44.Edoute Y., van der Merwe E.L., Sanan D., Kotze J.C.N., Steinmann Lochner A. Normothermic ischemic cardiac arrest of isolated working heart. Effects of time and reperfusion on myocardial ultrastructure, mitochondrial oxidative function and mechanical recovery. Circ. Res. 1983;53:668–678. doi: 10.1161/01.res.53.5.663. [DOI] [PubMed] [Google Scholar]
- 45.Kang J.W., Hong J.M., Lee S.M. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J. Pineal Res. 2016;60:383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- 46.Opie L.H. fourth ed. Lippincott, Williams & Wilkins; 2003. Heart Physiology. From Cell to Circulation; pp. p338–339. [Google Scholar]
- Tian W., Li W., Chen Y. Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2015;589:1847–1854. doi: 10.1016/j.febslet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Huisamen B.H., Genade S., Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7-36) amide in the rat heart and their role in protection against ischaemia. Cardiovasc. J. Africa. 2008;19:77–83. [PMC free article] [PubMed] [Google Scholar]
- 49.Sharp W.W., Fang Y.H., Han M. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–328. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang C.-R., Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jahani-Asl A., Slack R.S. The phosphorylation state of Drp1 determines cell fate. EMBO Rep. 2007;8:912–913. doi: 10.1038/sj.embor.7401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buhlman L., Damiane M., Bertolin G. Functional interplay between Parkin and Drp1 in mitochondrial fission and clearance. Biochim. Biophys. Acta. 2014;1843:2012–2026. doi: 10.1016/j.bbamcr.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Ong S.B., Kalkhoran S.B., Cabrera-Fuentes H.A., Hausenloy D.J. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur. J. Pharmacol. 2015;763:104–114. doi: 10.1016/j.ejphar.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Disatnik M.-H., Ferreira J.C., Campos J.C. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J. Am. Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding M., Dong Q., Liu Z. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovasc. Diabetol. 2017;16:19. doi: 10.1186/s12933-017-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding M., Feng N., Tang D. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1a pathway. J. Pineal Res. 2018;65 doi: 10.1111/jpi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu L., Gong B., Duan W. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC1a-SIRT3 signaling. Sci. Rep. 2017;7:41337. doi: 10.1038/srep41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dragicevic N., Copes N., O’Neal-Moffit G. Melatonin restores mitochondrial function in Alzheimer’s mice: a mitochondrial protective role of melatonin membrane receptor signaling. J. Pineal Res. 2011;51:75–86. doi: 10.1111/j.1600-079X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 59.Chaung J.I., Pan I.L., Hsieh C.Y., Huang C.Y. Melatonin prevents the Drp 1-dependent mitochondrial fission and oxidative insult in cortical neurones after MPP treatment. J. Pineal Res. 2016;61:230–240. doi: 10.1111/jpi.12343. [DOI] [PubMed] [Google Scholar]
- 60.Xu S., Pi H., Zhang L., Li Y. Melatonin prevents abnormal mitochondrial dynamics resulting from neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J. Pineal Res. 2016;60:291–302. doi: 10.1111/jpi.12310. [DOI] [PubMed] [Google Scholar]
- 61.Parameyong A., Govitrapong P., Chetsawang B. Melatonin attenuates the mitochondrial translocation of mitochondrial fission proteins and Bax, cytosolic calcium overload and cell death in metamphetamine-induced toxicity in neuroblastoma SHSY5Y cells. Mitochondrion. 2015;24:1–8. doi: 10.1016/j.mito.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Suwanjang W., Abramov A.Y., Charnkaew K., Govitrapong P., Chetsawang B. Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SHSY5Y cells. Neurochem. Int. 2016;97:34–41. doi: 10.1016/j.neuint.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Chang J.Y., Fu F., Shi L., Ko M.L., Ko G.Y. Melatonin affects mitochondrial fission/fusion dynamics in the diabetic retina. J. Diabetes Res. 2019 doi: 10.1155/2019/8463125. Art ID 8463125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin C., Chao H., Li Z. Melatonin attenuates traumatic brain injury-induced inflammation: a possible role for mitophagy. J. Pineal Res. 2016;61:177. doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto T., Sadoshima J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc. Med. 2011;21:27–32. doi: 10.1016/j.tcm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorn G.W., II Mitochondrial dynamics in heart disease. Biochim. Biophys. Acta. 2013;1833:233–241. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santel A., Frank S., Gaume B., Herrler M., Youle R.J., Fuller M.T. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J. Cell Sci. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- 68.Ong S.B., Hall A.R., Hausenloy D.J. Mitochondrial dynamics in health and disease. Antioxidants Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ong S.B., Kalkhoran S.B., Hernandez-Resendiz S. Mitochondrial-shaping proteins in cardiac health and disease-the long and short of it. Cardiovasc. Drugs Ther. 2017;31:87–107. doi: 10.1007/s10557-016-6710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong S.B., Subrayan S., Lim S.Y., Yellon D.M., Davidson S.M., Hausenloy D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 71.Le Page S., Niro M., Fauconnier J. Increase in cardiac ischemia/reperfusion injuries in Opa+/- mouse model. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varanita T., Soriano M.E., Romanello V. The opa1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metabol. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Wang Y., Xu J. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 2019;66 doi: 10.1111/jpi.12542. [DOI] [PubMed] [Google Scholar]
- 74.Pei H., Du J., Song X. Melatonin prevents adverse myocardial infarction remodeling via Notch/Mfn2 pathway. Free Radic. Biol. Med. 2016;97:408–417. doi: 10.1016/j.freeradbiomed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Stachiotti A., Favero G., Giugno L. Mitochondrial and metabolic dysfunction in renal convoluted tubules of obese mice: protective role of melatonin. PLoS One. 2014;9:e111141. doi: 10.1371/journal.pone.0111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L.-F., Qin Q., Qian M., Shi Q.-C. Protective effects of melatonin on ischemic reperfusion induced myocardial damage and hemodynamic recovery in rats. Eur. Rev. Med. Pharmacol. Sci. 2014;18:3681–3686. [PubMed] [Google Scholar]
- 77.Genade S., Genis A., Ytrehus K., Huisamen B., Lochner A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J. Pineal Res. 2008;45:449–458. doi: 10.1111/j.1600-079X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 78.Lochner A., Genade S., Moolman J.A. Ischaemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res. Cardiol. 2003;98:337–346. doi: 10.1007/s00395-003-0427-6. [DOI] [PubMed] [Google Scholar]
- 79.Salie R., Moolman J.A., Lochner A. The role of β-adrenergic receptors in the cardioprotective effects of beta-preconditioning (βPC) Cardiovasc. Drugs Ther. 2011;25:31–46. doi: 10.1007/s10557-010-6275-3. [DOI] [PubMed] [Google Scholar]
- 80.Sordahl L.A., Besch H.R., Jr., Allen J.C., Crow C., Lindenmayer G.E., Schwartz A. Enzymatic aspects of the cardiac muscle cell: mitochondria, sarcoplasmic reticulum and noncovalent cation active transport systems. Methods Achiev. Exp. Pathol. 1971;5:287–346. [PubMed] [Google Scholar]
- 81.Lowry A.O., Rosenbrough N.J., Farr A.L., Randall R.J. Protein with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 82.Fan W.-J., Van Vuuren D., Genade S., Lochner A. Kinases and phosphatases in ischaemic preconditioning: a re-evaluation. Basic Res. Cardiol. 2010;105:495–522. doi: 10.1007/s00395-010-0086-3. [DOI] [PubMed] [Google Scholar]
- 83.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;71:258–264. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.