Abstract
Researches documenting comprehensively the prevalence of seed vivipary in relation to phenology, as well as its impact on production are scant. This article reports the results of investigations carried out during four cropping seasons to quantitatively document seed vivipary in the oleaginous bottle gourd (Lagenaria siceraria). Field experiments were conducted during the first and second cropping season of 2014 and 2015 at the experimental station of Nangui Abrogoua University (Abidjan, Côte d’Ivoire). The assessment of the prevalence of seed vivipary was carried out using 185 L. siceraria accessions collected in different ecological zones of Côte d'Ivoire. To examine the influence of fruit maturation time on seed vivipary, four accessions (two viviparous and two non-viviparous) were cropped and harvested at 30 and 50 days after fertilization (DAF), complete whiteness of plants (CPW) and after 60 days of storage of fruits harvested on plants completely withered (CPWS). Finally, a comparative analysis of seed yield and its main components was conducted using four accessions including two highly viviparous and two non-viviparous. The results on seed vivipary prevalence showed that the oleaginous form of L. siceraria is highly susceptible and allowed the classification of the 185 accessions analyzed into three groups: non-viviparous (2.16%), viviparous (89.19%) and highly viviparous accessions (8.65%). No precocious seed germination was observed in non-viviparous accessions during fruit maturation stage. The fruits of highly viviparous accessions harvested at 30 DAF showed no precocious seed germination while 3.35–17.89% of fruits bearing viviparous seed were observed at 50 DAF. Plants from highly viviparous fruits showed significantly low yields compared those from non-viviparous fruits. These results suggested that an efficient control of seed vivipary allowing a quantitative and qualitative improvement of yield in the oilseed bottle gourd can be ensured by the selection of vivipary-tolerant genotypes and appropriate planning of the harvest time.
Keywords: Agriculture, Plant biology, Ecology, Genetics, Agronomy, Horticulture, Lagenaria siceraria, Cucurbits, Harvest time, Vivipary, Seed maturation, Yield
1. Introduction
Food security in the world is increasingly threatened by the reduction of arable land, the increase of human population and climate change (Kang et al., 2009). In the rural zones and particularly in sub-Saharan Africa, crop productivity is reduced by the desertification, global warming, erratic rain and soil degradation (Ngaira, 2007). Ensuring food security and economic prosperity of people in developing countries is based not only on cropping systems adapted to environmental stress, but also on the integration and promotion of underutilized species, rustic and tolerant to climate change (Adams et al., 1998). Lagenaria siceraria, commonly known as bottle gourd, with its high morphological diversity, well adapted to various environmental conditions, represents an excellent plant model for climate smart cropping systems implementation (Chimonyo and Modi, 2013). The integration of this underutilized crop species into agricultural development policies in support of cash crops is now being promoted by scientists and decision makers (Roy and Chakrabarti, 2003). Indeed, this crop, with its nutritional and therapeutic potentials, offers opportunities for diversification of agriculture, food, and income for the rural populations.
Bottle gourd is reported as one of the first plant species to be domesticated for human use (Clarke et al., 2006; Schlumbaum and Vandorpe, 2012). The genus Lagenaria contains six species five of which are wild (Jeffrey, 1980): L. breviflora (Benth.) Roberty, L. abyssinica (Hook f.) Jeffrey, L. rufa (Gilg.) Jeffrey, L. sphaerica (Sond.) Naudin, and L. guineensis (G. Don.) Jeffrey; L. siceraria being the only one cultivated species. Lagenaria siceraria has pantropical distribution but Africa is considered as its ancestral home. Based on results from morphological traits analysis, two subspecies have been recognized in L. siceraria (Heiser, 1973): the African L. siceraria ssp. siceraria and the Asian L. siceraria ssp. asiatica. L. siceraria is monoecious (staminate and pistilate flowers are separated), thus bound to experiment insect-mediated cross pollination which promotes random mating. The flowers are white in colour and appear 30 days after sowing. The male flowers with long peduncles appear first, at least seven days before the female flowers (Zoro Bi et al., 2003). With a strongly male-biased floral ratio (20 male:1 female) and large fruit (1–2.5 kg in the oleaginous form), this annual vine also experiments significant costs associated with male flower and fruit production (Delesalle and Mooreside, 1995). From a study examining mechanisms of pollen transfer within the genus Lagenaria in both natural and cropped field environments, Morimoto et al. (2004) concluded that hawkmoths are probably the major pollinators of L. siceraria.
Several species of cucurbits produce oilseeds that are consumed in West Africa. The most widely cultivated are Citrullus mucosospermus (Fursa) Fursa, Cucumis melo ssp. meloides Naudin, Cucurbita moschata Duchesne, Cucumeropsis mannii Naudin and Lagenaria siceraria (Molina) Standley (Zoro Bi et al., 2005; Achigan-Dako et al., 2008). In sub-Saharan Africa, the oilseed form of bottle gourd is grown for its seeds which are consumed during the popular ceremonies (Zoro Bi et al., 2003). The richness of these seeds in lipids, proteins and vitamins makes them appropriate in the resolution of diet problems, especially in rural areas. The plant also represents a significant asset to increase the income of small holders, since the price of 1 kg of oleaginous bottle gourd's kernels is higher than this of several cash crops such as cocoa and coffee (Zoro Bi et al., 2005; PIC-2010, 2012). The macronutrient composition and nutritional value of L. siceraria have been determined by Achu et al. (2005) and Loukou et al. (2007, 2011). These studies revealed that kernels of bottle gourd are rich in minerals (calcium, potassium, phosphorus, iron, magnesium and zinc), proteins (36%) and fats (50%). In addition, fruits of L. siceraria have essential constituents for normal and good health of humans (Rahman, 2003). Lagenaria siceraria is also intercropped with other crops with the aim to control the weed. Although this crop has been subjected to several research programs to enhance the production in tropical zones (Mashilo et al., 2016; Janaranji et al., 2016; Anzara et al., 2015; Zoro Bi et al., 2003), its yield remains low. Indeed, as all cucurbit crop species, bottle gourd is vulnerable to attacks of various pathogens and pests (Sharma and Dhankar, 1990). The main insect pests feeding different organs of L. siceraria in tropical Africa belong to three families (Adja et al., 2014; Anzara et al., 2015): Chrysomelidae, Coccinellidae, and Meloidae. Podosphaera xanthii, Golovinomyces cucurbitacearum, and Golovinomyces orontii, Lasiodiplodia theobromae, Macrophomina phaseolina, Colletotrichum sp. and Fusarium sp. are reported as the major fungi pathogens in bottle gourde (Jahn et al., 2002; Sultana and Ghaffar, 2009; Saha et al., 2016). Several viruses including cucumber green mottle mosaic virus (CGMMV), Zucchini yellow mosaic potyvirus (ZYMV), and cucumber mosaic cucumovirus (CMV) are currently indicated as pathogens in L. siceraria (Hseu et al., 1987; Ullman et al., 1991; Ling and Levi, 2007; Li et al., 2016). Species of the genus Lagenaria are also subject Pseudomonas lachrymans causing bacterial (Pirone, 1978).
The low yield of bottle gourd is also due to a low exploration of some important yield influencing factors such as seed quality. Seed quality is one of the most important factors of crops production, often neglected by poor farmers (Chimonyo and Modi, 2013).
Vivipary in plant, also named pre-harvest sprouting (Fang and Chu, 2008), is a phenomenon characterized by lack of dormancy with subsequent germination of seeds within the fruit while still attached to the parent plant (Cota-Sánchez and Abreu, 2007). The germination of viviparous seeds usually occurs prior to the complete desiccation of the fruit while they are still on the parent plant. Generally, orthodox seeds do not germinate in the fruits, even at full maturity. Indeed, the orthodox seeds undergo a period of metabolic quiescence before germination (Matilla and Matilla-Vazquez, 2008). However, several studies have reported vivipary in orthodox seed of many cereal crops such as wheat, barley, maize and rice, and grain legumes such as soybean, chickpea, blackgram, greengram, tomato (Dos Santos and Yamaguchi, 1979; Ahmad et al., 2014) and papaya (Saran et al., 2014). Among the cultivated cucurbits, Sechium edule (chayote) is the unique species in which vivipary is systematically observed (Monnerville et al., 2001). Indeed in cultivated plants of this species, the seed germinates when the fruit is still on the plant, while in wild plants germination occurs only the fruit is detached (Saade, 1996). This phenomenon, which decrease the yield of crops and causes substantial financial losses for farmers (Gao et al., 2013) is often observed in the culture of Lagenaria siceraria. Genotypes with thin fruit rind and edible seed express more frequently vivipary, compared to calabash type with hard fruit rind, mainly used as tool or utensil due to the hardness of its fruit rind. However, quantitative data on yield losses due to vivipary and the factors influencing its appearance in this crop are not documented. In traditional bottle gourd cropping systems, the harvested fruit are often stored in the field for a long period. This cultural practice can cause vivipary in fruit. According to Demir and Ellis (1992) delaying harvest of tomato and pepper seeds over 80 days after anthesis lead to 2–5% viviparous seeds. The high temperatures and dampness during seed maturation have also been reported to increase the vivipary (Andreoli et al., 2006). In such context, accessing the appropriate harvest time is a prerequisite to reduce or avoid the occurrence of vivipary in the fruits. This paper was aimed at a comprehensible documentation of the prevalence and variation of seed vivipary in the oilseed L. siceraria, with respect to fruit maturity. Data obtained are useful to determine the appropriate harvest time. Based on the knowledge of the vivipary rate in the Cucurbitaceae family, we hypothesize that vivipary occurs at a low rate in L. siceraria but the prevalence increases with the harvest time and causes yield losses.
2. Materials and methods
2.1. Site description
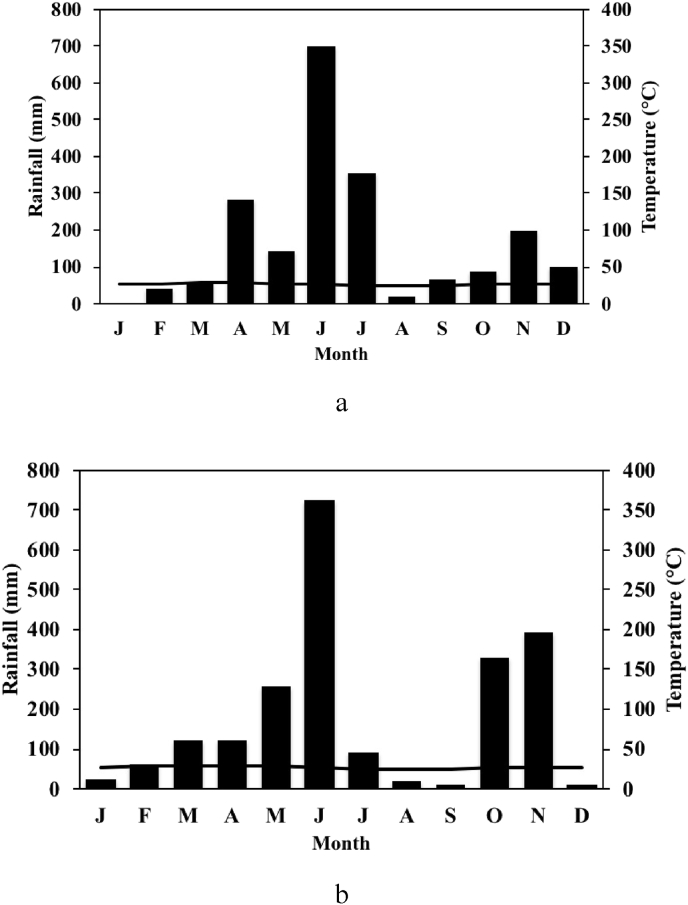
The study was conducted at the experimental station of the Nangui Abrogoua University in Abidjan (Côte d'Ivoire) for two consecutive cropping periods in 2014 and 2015. The screening of bottle gourd germplasm for vivipary was conducted in 2014. The experiments on the effect of harvest time on the vivipary prevalence and effect of vivipary on plant yield were carried out from March to June and from September to December 2015. The study site is located between 5°17′N and 5°31′N and 3°45′W and 4°22′W. The climate is a wet subequatorial, with the mean annual rainfall of 1350 mm, and a mean annual temperature of about 25 °C. The soil is ferrallitic, characterized by the forest land, and has a sand-clay texture and cluster structure (Yao-Kouamé and Kane, 2008). Meteorological data during 2014 and 2015 at the study site are shown in Fig. 1. The total rainfalls for the first and second cropping seasons in 2014 were 1179 mm and 454 mm respectively, while in 2015 the corresponding values were 1223 mm and 743 mm.
Fig. 1.
Ombrothermic diagrams for the experimental station of University Nangui Abrogoua (Abidjan, Côte d’Ivoire) in 2014 (a) and 2015 (b).
2.2. Plant material
The experimental materials for the study consisted of 200 accessions of Lagenaria siceraria from the collection of the Nangui Abrogoua University. The accessions are landraces collected from farmer's field, harvest or in market and conserved in the working gene bank of the Nangui Abrogoua University where samples are available for research purposes. Once seeds of fruits were collected, an accession alphanumeric code was provided and each accession was documented with local names, their meaning, origins, uses, and GPS coordinates. All collections, except those from markets, were made with the authorization of the field's owner, or a relative. Regenerations of accessions were not carried out before their use for vivipary screening. These accessions were representative of the varietal diversity and the five agro-ecological zones (South, East, North-east, North and Centre) in which they are regularly cultivated in Côte d’Ivoire. Based on results from the screening of 200 bottle gourd accessions for vivipary, two accessions strongly susceptible to vivipary and two non-viviparous accessions were selected and used to evaluate the effect fruit maturity on the prevalence of vivipary and the effect of vivipary on plant productivity.
2.3. Experimental design
The screening of bottle gourd germplasm for vivipary prevalence was carried out in a randomized layout implemented on a 9204 m2 (118 m × 76 m) plot. Each accession was planted in a row of five plants. Sowing spacing was 2 m between and within rows. Three seeds were sown per mound. Mounds were thinned to one seedling per mound at two weeks after sowing. Three hoe weeding was practiced to eradicate weeds. Poultry manure was applied at emergence to enhance plant production. Plots were treated with a broad spectrum cypermethrin based-insecticide at a dose of 0.8 l/ha. The fruits were harvested when the plants were completely withered.
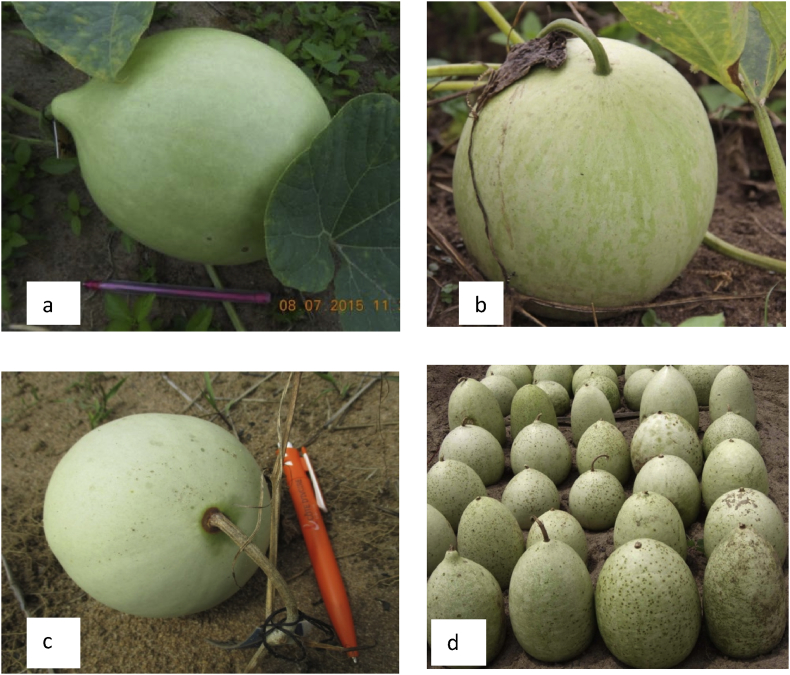
To evaluate the influence of fruit maturation stage on seed vivipary prevalence, plots (96 m × 48 m) were arranged in a complete randomized block design with factorial combination of four accessions and four stages of fruit maturation. The evolution of female flowers was monitored until the fertilization. A flower was considered to be fertilized when its petals were completely closed after blooming. The fertilized flowers were tagged and fruits were harvested at different stages of maturation, harvests being arranged according to the tagging day. Thus, for each accession, four groups of fruits were constituted (Fig. 2). The first group of 30 fruits was harvested at 30 days after fertilization (30 DAF). This stage corresponds to the time from which fruit growth stops. The second lot was harvested at 50 days after fertilization (50 DAF). At this stage fruit peduncle coloration changes from dark green to brown. It is presumed as the fruit maturity stage and the appropriate time for seed harvest (Oluoch and Welbaum, 1996). The third set of fruits was harvested when plants were completely withered (CPW). This is the usual stage of cucurbits harvest by rural farmers. The last set was fruits stored at the field conditions during 60 days after plant complete whiteness (CPWS). This practice is widely used by farmers. To get more precision concerning fruit appropriate harvesting time, in an additional trial using only the two viviparous accessions, fruits were harvested at 30 DAF, 35 DAF, 40 DAF, 45 DAF, 50 DAF, CPW. Four samples of 20 seeds from each accession and harvest time were submitted to germination tests to check their viability. Seed viability was evaluated using the germination percent (GP) in an on-farm trial using a completely randomized design with five replications (plots of 1 m × 0.5 m). Seeds were considered as germinated when the cotyledons appeared above the ground level. The seeds sown were surveyed daily for 14 days (ISTA, 1996).
Fig. 2.
Fruits of L. siceraria harvested at, a: 30 days after fertilization (DAF); b: 50 DAF; c: plant complete whiteness; d: 60 days after plant complete whiteness.
The experiments testing the effect of seed vivipary on plant productivity was arranged in a complete randomized blocks design with two replications implemented on a 1664 m2 (52 m × 32 m) plot. Each replication was represented by a row of 15 plants. The plots were pre-fertilized with poultry manure and regularly maintained by hoe weeding. The fruits were harvested at plant complete whiteness.
2.4. Crop traits
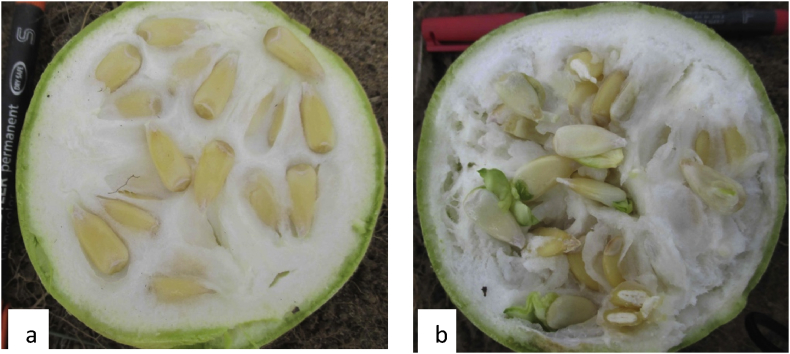
After harvest, the fruits were cut using a knife and the absence or presence of seed vivipary was observed (Fig. 3). The cut fruits were fermented for seven days. The prevalence of vivipary was evaluated on the basis of both viviparous seed containing fruits and viviparous seeds. To classify the accessions examined, three arbitral levels of vivipary were considered: (i) non-viviparous accessions bearing any viviparous seed; (ii) viviparous accessions characterized by 1–95% viviparous seeds and fruit bearing viviparous seeds; and (iii) highly viviparous accessions with 96–100% vivipary (seed and fruit). The two vivipary calculation criteria adopted in this study are of practical interest for both seed system and breeding purposes. For each sampled fruit, the seeds were extracted after fruit decomposition, washed, sun-dried and the number of non-viviparous seed as well as the total number were noted. Finally, the percentages of viviparous seed-bearing fruits (ratio between the number of fruits containing at least one viviparous seed and the total number of fruits) and seed (ratio between the number of viviparous seeds and the total number of seeds in a fruit) were calculated.
Fig. 3.
Transversal section of fruits harvested 50 days after fertilization from non-viviparous (a) and viviparous (b) accession of bottle gourd.
Four traits were recorded in trails examining the effect of fruit maturation time on vivipary prevalence: percent of viviparous seeds (VS), fruits bearing viviparous seeds (VF), immature seeds (IS), and seed germination (GP). For the experiment evaluating the effect of vivipary on plant productivity, height traits usually analyzed on L. siceraria as yield components (Yao et al., 2015) were scored. The measured traits were plant length (PL), number of branches (NB), number of fruits per plant (NF), fruit weight (FW), number of seeds per fruit (NS), 100-seed weight (100-SW), percent of viviparous seeds (VS), and seed yield per plant (SY). The measurement approaches of the selected traits followed Koffi et al. (2009).
2.5. Data statistical analysis
Mean values and standard deviations were calculated for each trait. The 185 accessions analyzed were classified into the three groups pre-defined on the basis of the calculated vivipary percent.
For the experiments testing the effect of fruit maturation on seed vivipary and the effect of seed vivipary on plant productivity, multivariate analysis of variance (MANOVA) appropriate for three-way fixed model was performed to check difference between the variable means for each factor tested. The General Linear Models (GLM) procedure of the SAS v.9.1 (SAS, 2004) was used to identify traits contributing to differences when MANOVA revealed significant difference for a factor. Least Significant Difference (LSD) multiple range-tests were used to identify differences among mean values.
3. Result
3.1. Screening of Lagenaria siceraria germplasm for vivipary
Plants bearing at least three matured fruits at complete whiteness were selected for data collection. On this basis, 185 accessions have been observed for vivipary prevalence. The selected plants produced 3–6 fruits, resulting in 7–30 fruits per accession. The distribution of the 185 accessions according to the three levels of vivipary prevalence defined is indicated in Table 1. Results related to seed vivipary indicated that 4 (2.16%) and 181 (97.84%) of the accessions analyzed were non-viviparous and viviparous, respectively. Calculation based on the fruits containing viviparous seeds showed that 16 (8.65%) accessions were highly viviparous.
Table 1.
Vivipary prevalence in 185 accessions of the bottle gourd Lagenaria siceraria.
| Calculation criterion | Accession number per vivipary level |
||
|---|---|---|---|
| 0% | 1–95% | 96–100% | |
| Viviparous seed | 4 | 181 | 0 |
| Fruit with viviparous seed | 4 | 165 | 16 |
3.2. Effect of fruits maturation on the prevalence of vivipary
MANOVA indicated that accession (F = 206.96; P < 0.001), fruit maturation time (F = 754.75; P < 0.001) and the interaction ‘accession × fruit maturation time’ (F = 37.41; P < 0.0001) had a significant effect on the four traits analyzed (SV, FV, IS, and GP). As interaction was statistically significant, data analysis and interpretation have been done with the interaction (Table 2). The seed vivipary in non-viviparous accessions (NI063 and NI189) did not vary during fruit maturation and on-field storage. However, 3.35 ± 6.02% (NI128) and 17.89 ± 12.77% (NI153) of vivipary seeds were noted in the two viviparous accessions at 50DAF. At 35DAF, vivipary seeds percent varied from 2.62 ± 5.41 to 6.77 ± 7.76 in viviparous accessions (Table 3). As expected, the percent of immature seeds decreased with fruit maturation and on-field storage but the extend did not vary significantly from 50DAF whatever the accessions considered. However, the percent of immature seeds were significantly higher in viviparous accessions (NI128 and NI153) whatever the fruit maturation stage. In these accessions, viviparous seeds appeared in all fruits at both CPW and CPWS stages. The germination percent evaluated after a 7-days observation period using non-viviparous seeds from the different accessions and fruit maturation stages varied from 57% to 93% (Table 2).
Table 2.
Interaction effect of bottle gourd accessions and fruit maturation stages on parameters related to vivipary.
| Traits1;2 | Vivipary level3 | 30DAF4 (%) | 50DAF (%) | CPW (%) | CPWS (%) |
|---|---|---|---|---|---|
| VS | NI063 | 0.00 ± 0.00aB | 0.00 ± 0.00cB | 0.00 ± 0.00cB | 0.67 ± 1.71cA |
| NI128 | 0.00 ± 0.00aD | 3.35 ± 6.02bC | 16.19 ± 11.71bB | 25.53 ± 11.83bA | |
| NI153 | 0.00 ± 0.00aD | 17.89 ± 12.77aC | 23.71 ± 9.83aB | 34.45 ± 10.87aA | |
| NI189 | 0.00 ± 0.00aA | 0.00 ± 0.00cA | 0.00 ± 0.00cA | 0.00 ± 0.00cA | |
| VF | NI063 | 0.00 ± 0.00aB | 0.00 ± 0.00cB | 0.00 ± 0.00c B | 18.33 ± 30.74bA |
| NI128 | 0.00 ± 0.00aD | 22.50 ± 39.14bC | 70.27 ± 39.89bB | 100.00 ± 0.00aA | |
| NI153 | 0.00 ± 0.00aC | 85.00 ± 32.42aB | 100.00 ± 0.00aA | 100.00 ± 0.00aA | |
| NI189 | 0.00 ± 0.00aA | 0.00 ± 0.00cA | 0.00 ± 0.00cA | 0.00 ± 0.00cA | |
| IS | NI063 | 70.50 ± 17.76bA | 13.82 ± 11.65bB | 8.09 ± 2.86bC | 7.97 ± 2.43bC |
| NI128 | 79.96 ± 16.36abA | 18.30 ± 10.85aB | 14.97 ± 6.64aC | 12.25 ± 3.36aC | |
| NI153 | 83.12 ± 18.73aA | 19.37 ± 9.44aB | 13.34 ± 5.29aC | 13.05 ± 4.54aC | |
| NI189 | 65.24 ± 10.52bA | 9.48 ± 4.29cB | 6.80 ± 1.86bC | 5.79 ± 2.15bC | |
| GP | NI063 | 57.00 ± 21.38aB | 90.00 ± 10.00aA | 90.00 ± 7.07aA | 92.00 ± 5.70abA |
| NI128 | 61.00 ± 21.33aB | 89.00 ± 8.21aA | 90.00 ± 7.90aA | 93.00 ± 5.70aA | |
| NI153 | 61.00 ± 20.43aB | 88.00 ± 9.74aA | 91.00 ± 7.41aA | 93.00 ± 6.70aA | |
| NI189 | 59.00 ± 22.19aB | 87.00 ± 10.36aA | 91.00 ± 6.51aA | 91.00 ± 7.41bA |
VS: percent of viviparous seeds; VF: fruits bearing viviparous seeds; IS: immature seed; GP seed germination percent.
For each trait, values followed by the different letter in a line (capital letters) and in a column (tiny letters) are significantly different (P < 0.05) using LSD.
NI063 and NI189 are non-viviparous accessions whereas NI128 and NI153 are highly viviparous.
DAF: days after fertilization; CPW: complete plant withered; CPWS: complete plant withered and stored at 60 days.
Table 3.
Prevalence of fruit and seed vivipary in two strongly viviparous accessions of Lagenaria siceraria at different maturation stages.
| Traits∗1,2 | Accession1 | 30DAF | 35DAF | 40DAF | 45DAF | 50DAF | CPW |
|---|---|---|---|---|---|---|---|
| VS | NI128 | 0.00 ± 0.00e A | 2.62 ± 5.41d B | 8.68 ± 8.10c B | 9.75 ± 6.60c B | 12.85 ± 5.75b B | 24.67 ± 10.32a B |
| NI153 | 0.00 ± 0.00e A | 6.77 ± 7.76d A | 16.00 ± 9.83c A | 18.99 ± 9.14c A | 22.48 ± 8.51b A | 37.04 ± 17.82a A | |
| VF | NI128 | 00.00 ± 0.00d A | 10.00 ± 28.03d B | 60.00 ± 33.80c B | 83.33 ± 24.39b B | 100.00 ± 0.00a A | 100.00 ± 0.00a A |
| NI153 | 00.00 ± 0.00d A | 26.66 ± 37.16c A | 83.33 ± 24.39b A | 100.00 ± 0.00a A | 100.00 ± 0.00a A | 100.00 ± 0.00a A | |
| IS | NI128 | 76.96 ± 9.83a B | 66.27 ± 14.50b A | 24.10 ± 9.76c A | 18.09 ± 6.84d B | 10.15 ± 3.79e A | 8.27 ± 3.04e B |
| NI153 | 84.30 ± 6.38a A | 56.75 ± 20.70b A | 25.75 ± 10.98c A | 24.17 ± 11.44c A | 13.76 ± 6.62d A | 13.33 ± 5.46d A |
Values followed by the different letter in a line (tiny letters) and in a column (capital letters) are significantly different (p < 0.05) using LSD.
VS: percent of viviparous seeds; VF: fruits bearing viviparous seeds; IS: immature seed.
DAF: days after fertilization; CPW: complete plant withered.
3.3. Effect of accession vivipary on plant yield parameters
MANOVA indicated significant effect of accessions (F = 48.35; P < 0.0001) on the yield and yield components analyzed. The effects of plant (F = 1.14; P = 0.21) and interaction ‘accession × plant’ (F = 0.97; P = 0.57) were not significant. The effect of accession vivipary on yield traits was significant (Table 4). Results showed that plants from non-viviparous accessions produced more seeds than those from viviparous accessions. The best values of the yield components analyzed were observed with non-viviparous accessions producing twice the seed yield of the viviparous accessions. Seed yields from the viviparous accessions were not significantly different whereas one of the non-viviparous accessions yielded statistically higher than the other (Table 4).
Table 4.
Yield parameters of viviparous and non-viviparous accessions of Lagenaria siceraria.
| Traits12 | Viviparous accessions |
Non-viviparous accession |
ANOVA result |
|||
|---|---|---|---|---|---|---|
| NI128 | NI153 | NI063 | NI189 | F | P | |
| PL (m) | 4.25 ± 1.07c | 3.41 ± 0.81d | 7.29 ± 1.10b | 9.15 ± 1.44a | 227.97 | <0.001 |
| NB | 13.07 ± 3.45c | 10.92 ± 3.30d | 15.80 ± 3.26b | 27.52 ± 4.21a | 189.28 | <0.001 |
| NF | 1.65 ± 0.66b | 1.50 ± 0.64b | 2.65 ± 0.66a | 2.90 ± 0.63a | 46.51 | <0.001 |
| FW (kg) | 0.94 ± 0.21c | 0.74 ± 0.20c | 1.19 ± 0.31b | 2.02 ± 0.57a | 181.33 | <0.001 |
| NS | 112.89 ± 33.76c | 112.31 ± 37.42c | 220.60 ± 33.09b | 274.87 ± 47.00a | 351.82 | <0.001 |
| 100-SW (g) | 13.48 ± 2.25c | 11.54 ± 2.17d | 17.25 ± 3.80b | 20.92 ± 4.90a | 71.63 | <0.001 |
| VS (%) | 35.88 ± 22.53b | 46.09 ± 21.87a | 0.00 ± 0.00c | 0.00 ± 0.00c | 249.20 | <0.001 |
| SY (kg/plant) | 0.054 ± 0.030c | 0.042 ± 0.025c | 0.218 ± 0.094b | 0.362 ± 0.016a | 71.82 | <0.001 |
PL: plant length; NB: number of branches; NF: number of fruit per plant; FW: fruit weight; NS: number of seed per fruit; 100-SW: 100-seed weight; VS: percentage of viviparous seed; SY: plant seed yield.
For each trait, values followed by the different letter in a line are significantly different (P < 0.05) using LSD.
4. Discussion
First hurdle to plant life cycle, seed germination is deterministic in crop establishment and yielding. Thus, crop husbandry and breeding technics that promote seed agronomic performances are of great interest for both breeders and farmers. One of factors affecting seed quality is seed vivipary (pre-harvest sprouting). The present investigation was the first comprehensible attempt to quantify seed viviparous germination with reference to common post-harvest practices in the oleaginous form of Lagenaria siceraria.
Results showed that seed vivipary is predominant (97.84%) in the oleaginous bottle gourd. In the Cucurbitaceae family, only chayote (Sechium edule) characterized by single-seeded fruit is reported to be true vivipay (Aung et al., 1990). However, vivipary is reported in the main cultivated species of Cucurbitaceae family such as melon (Ochi et al., 2013), fluted pumpkin (Ajayi et al., 2006), muskmelon (Welbaum, 1999), and watermelon (Kobayashi et al., 2010). The particularly high prevalence of viviparous accessions noted in the present study compared to this usually observed in the farmer's fields can be explained by the heavy rainfall that occurred during the experiments. It has been shown in rice (Baek and Chung, 2014), wheat (Ellis and Yadav, 2016) and coconut (Shareefa et al., 2014) that high amount of rainfall causes an increase in seed vivipary. Heavy rains that occurred during the present experiments were appropriate for a relevant on-farm selection of vivipary-tolerant genotypes. Thus, fully and moderately vivipary-tolerant genotypes identified in this study represent good materials for L. siceraria production in its different cropping areas, regardless of the amount of rainfall.
The second experiment examined the effect of fruit maturation time on seed vivipary in bottle gourd. Trials showed that seeds extracted from the viviparous accession fruits harvested 30DAF expressed no vivipary. From 35DAF, the vivipary rates observed were significantly high. The plant seed dormancy hormone (abscisic acid: ABA), antagonistic that triggers vivipary inducing hormone (gibberellic acid: GA), usually reaches its peak concentration at the early stage of fruit development i.e. at 4–6 DAF (Guinn, 1982; Fong et al., 1983). After this phase, a decrease in the concentration of ABA is usually noted, while that of GA increases (Nonogaki and Nonogaki, 2017). The results obtained in this study suggested that at 30DAF, the critical value of ABA content (Steinbach et al., 1997; Kucera et al., 2005) or the ABA/GA balance (White et al., 2000) were not reached, resulting in a low rate of seed vivipary. Thorough biochemical and genetic analyzes are needed to test this hypothesis. The results of such analyzes should offer interesting perspectives in terms of development of genetic markers that are needed for marker-assisted selection (Cao et al., 2016; Lin et al., 2016). The identification of the date at which seed vivipary rate is low, with a high percentage of mature seeds is of practical interest for the seed system development and production improvement in L. siceraria. It is worth noting that at 30DAF, plants of L. siceraria still bear a dense foliage. This result suggested a potential diversification in the used of L. siceraria, particularly in animal feeding and pharmacopeia. Such a dual-purpose cropping for both leaf and seed production can be highly profitable for rural farmers.
The study also showed that vivipary-tolerant genotypes were more productive than the viviparous. As demonstrated in the first part of this study, vivipary appears at early fruit development stage in L. siceraria (35DAF). It affects a high number of fruits with low quality seeds, therefore removed from the harvest. In addition, a high rate of unfilled seeds was observed in fruit from viviparous accessions. This resulted in low seed yield in viviparous genotypes. Vivipary can also negatively influence yield through its action on plant growth and development. It has been shown in cactus and tomato that vivipary significantly affects seedling establishment (Cota-Sánchez and Abreu, 2007; Cota-Sanchez, 2017), which can indirectly influence yield through slow-downing the phenology stages (Ellis, 1992).
Finally, it appeared from this study that seed vivipary is an important production factor in L. siceraria, similarly to the case of many industrial crops, including maize (Eyster, 1931; Tan et al., 1997; White et al., 2000), pepper (Marrush et al., 1998) and tomato (Cota-Sanchez, 2017; Wang et al., 2016). The trails carried out, which to our knowledge, are the first on L. siseraria showed that the vivipary can be controlled by genetic (selection) or agronomic (harvest planning and post-harvest treatment) approaches.
Declarations
Author contribution statement
Bi Irié A. Zoro: Conceived and designed the experiments.
Aya L.F. N’Gaza: Performed the experiments; Wrote the paper.
Kouadio I. Kouassi and Kouakou L. Kouakou: Analyzed and interpreted the data.
Kouamé K. Koffi: Contributed reagents, materials, analysis tools or data.
Jean-Pierre Baudoin: Analyzed and interpreted the data; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Achigan-Dako E.G., Fagbemissi R., Avohou H.T., Vodouhe R.S., Coulibaly O., Ahanchede A. Importance and practices of Egusi crops (Citrullus lanatus (Thunb.) Matsum. & Nakai, Cucumeropsis mannii Naudin and Lagenaria siceraria (Molina) Standl. cv.’Aklamkpa’) in sociolinguistic areas in Benin. Biotechnol. Agron. Soc. Environ. 2008;12:393–403. [Google Scholar]
- Achu M.B., Fokou E., Tchiégang C., Fotso M., Tchouanguep F.M. Nutritive value of some Cucurbitaceae oilseeds from different regions in Cameroon. Afr. J. Biotechnol. 2005;4:1329–1334. [Google Scholar]
- Adams R., Hurd B., Lenhart S., Leary N. Effects of global climate change on agriculture: an interpretative review. Clim. Res. 1998;11:19–30. [Google Scholar]
- Adja N.A., Danho M., Alabi T.A.F., Gnago A.J., Zimmer J.-Y., Francis F., Kouassi K.P., Baudoin J.-P., Zoro B.I.A. Entomofauna associated with African oleaginous cucurbits (Lagenaria siceraria Molina (Standl.1930) and Citrullus lanatus Thumb (Matsum & Nakai 1916)) and impact of pests on production. An. Soc. Entomol. Fr. 2014;50:301–310. [Google Scholar]
- Ahmad S., Khulbe R.K., Roy D. Evaluation of mungbean (Vigna radiata) germplasm for pre-harvest sprouting tolerance. Legume Res. 2014;37:259–263. [Google Scholar]
- Ajayi S.A., Berjak P., Kioko J.I., Dulloo M.E., Vodouhe R.S. Responses of fluted pumpkin (Telfairia occidentalis Hook. f.; Cucurbitaceae) seeds to desiccation, chilling and hydrated storage. South Afr. J. Bot. 2006;72:544–550. [Google Scholar]
- Andreoli C., Bassoi M.C., Brunetta D. Genetic control of seed dormancy and preharvest sprouting in wheat. Sci. Agric. 2006;63:564–566. [Google Scholar]
- Anzara G.K.G.R., Koffi K.K., Coulibaly S.S., Fouha B.N.D., Baudoin J.-P., Campa C., Zoro Bi I.A. Influence of herbivorous insects on the production of Lagenaria siceraria (Molina) Standley (Cucurbitaceae) Afr. J. Plant Sci. 2015;9:449–456. [Google Scholar]
- Aung L.H., Ball A., Kushad M. Developmental and nutritional aspects of chayote (Sechium edule, Cucurbitaceae) Econ. Bot. 1990;44:157–164. http://www.jstor.org/stable/4255225 [Google Scholar]
- Baek J.-S., Chung N.-J. Influence of rainfall during the ripening stage on pre-harvest sprouting, seed quality, and longevity of rice (Oryza sativa L.) Korean J. Crop Sci. 2014;59:406–412. [Google Scholar]
- Cao L., Hayashi K., Tokui M., Mori M., Miura H., Onishi K. Detection of QTLs for traits associated with pre-harvest sprouting resistance in bread wheat (Triticum aestivum L.) Breed Sci. 2016;66:260–270. doi: 10.1270/jsbbs.66.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimonyo V., Modi A. Seed performance of selected bottle gourd (Lagenaria siceraria (Molina) Standl.) Am. J. Exp. Agric. 2013;3:740–766. [Google Scholar]
- Clarke A.C., Burtenshaw M.K., McLenachan P.A., Erickson D.L., Penny D. Reconstructing the origins and dispersal of the Polynesian bottle gourd (Lagenaria siceraria) Mol. Biol. Evol. 2006;23:893–900. doi: 10.1093/molbev/msj092. [DOI] [PubMed] [Google Scholar]
- Cota-Sánchez J., Abreu D. Vivipary and offspring survival in the epiphytic cactus Epiphyllum phyllanthus (Cactaceae) J. Exp. Bot. 2007;58:3865–3873. doi: 10.1093/jxb/erm232. [DOI] [PubMed] [Google Scholar]
- Cota-Sanchez J.H. Precocious germination (vivipary) in tomato: a link to economic loss? Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017 [Google Scholar]
- Demir I., Ellis R.H. Changes in seed quality during development and maturation in tomato. Seed Sci. Res. 1992;2:81–87. [Google Scholar]
- Delesalle V.A., Mooreside P.D. Estimating the costs of allocation to male and female functions in a monoecious cucurbit, Lagenaria siceraria. Oecologia. 1995;102:9–16. doi: 10.1007/BF00333304. [DOI] [PubMed] [Google Scholar]
- Dos Santos D., Yamaguchi M. Seed-sprouting in tomato fruits. Sci. Hortic. 1979;11:131–139. [Google Scholar]
- Ellis R.H. Seed and seedling vigour in relation to crop growth and yield. Plant Growth Regul. 1992;11:249–255. [Google Scholar]
- Ellis R.H., Yadav G. Effect of simulated rainfall during wheat seed development and maturation on subsequent seed longevity is reversible. Seed Sci. Res. 2016;26:67–76. [Google Scholar]
- Eyster W.H. Vivipary in maize. Genetics. 1931;16:514–590. doi: 10.1093/genetics/16.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Chu C. Abscisic acid and the pre-harvest sprouting in cereals. Plant Signal. Behav. 2008;3:1046–1048. doi: 10.4161/psb.3.12.6606. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2634458/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong F., Smith D.J., Koehler D.E. Early events in maize seed development 1-methyl-3-phenyl-5-(3-[trifluoromethyl]phenyl)-4-(1 H)-pyridinone induction of vivipary. Plant Physiol. 1983;73:899–901. doi: 10.1104/pp.73.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Hu C.H., Li H.Z., Yao Y., Meng M., Dong J., Zhao W., Chen Q., Li X. Factors affecting pre-harvest sprouting resistance in wheat (Triticum aestivum): areview. J. Anim. Plant Sci. 2013;23:556–565. https://pdfs.semanticscholar.org/b97a/9b09b851b78d027f92f7cc297f3a6b6f5ff2.pdf [Google Scholar]
- Guinn G. Fruit age and changes in abscisic acid content, ethylene production, and abscission rate of cotton fruits. Plant Physiol. 1982;69:349–352. doi: 10.1104/pp.69.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser C.B. Tropical forest Ecosystems in Africa and South America: A Comparative Review. Smithsonian Institution Press; Washington DC (USA): 1973. Variation in the bottle gourd; pp. 121–128. [Google Scholar]
- Hseu S.H., Huang C.H., Chang C.A., Yang W.Z., Chang Y.M., Hsiao C.H. The occurrence of five viruses in six cucurbits in Taiwan. Plant Protec. Bull. Twn. 1987;29:233–244. [Google Scholar]
- ISTA . International Seed Testing Association (ISTA); 1996. Seed Science and Technology: Rules. Supplement. Zürich (Switzerland) [Google Scholar]
- Jahn M., Munger H.M., McCreight J.D. Breeding cucurbit crops for powdery mildew resistance. In: Bélanger R.R., Bushnell R.W., Dik A.J., Carver T.L.W., editors. The Powdery Mildews: A Comprehensive Treatise. American Phytopathological Society (APS Press); St. Paul (USA): 2002. pp. 239–248. [Google Scholar]
- Janaranji K.G., Kanthaswamy V., Kumar R.S. Heterosis combining ability and character association in bottle gourd for yield attributes. Int. J. Veg. Sci. 2016;22:490–515. [Google Scholar]
- Jeffrey C. A review of the Cucurbitaceae. Bot. J. Linn. Soc. 1980;81:233–247. [Google Scholar]
- Kang Y., Khan S., Ma X. Climate change impacts on crop yield, crop water productivity and food security. Rev. Prog. Nat. Sci. 2009;19:1665–1674. [Google Scholar]
- Kobayashi Y., Nabeta K., Matsuura H. Chemical inhibitors of viviparous germination in the fruit of watermelon. Plant Cell Physiol. 2010;51:1594–1598. doi: 10.1093/pcp/pcq103. [DOI] [PubMed] [Google Scholar]
- Koffi K.K., Anzara G.K., Malice M., Djè Y., Bertin P., Baudoin J.P., Zoro Bi I.A. Morphological and allozyme variation in a collection of Lagenaria siceraria (Molina) Standl. from Côte d’Ivoire. Biotechnol. Agron. Soc. Environ. 2009;13:257–270. http://www.pressesagro.be/base/text/v13n2/257.pdf [Google Scholar]
- Kucera B., Cohn M.A., Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005;15:281–307. [Google Scholar]
- Li J., Zheng H., Zhang C., Han K., Wang S., Peng J., Lu Y., Zhao J., Xu P., Wu X., Li G., Chen J., Yan F. Different virus-derived siRNAs profiles between leaves and fruits in cucumber green mottle mosaic virus-infected Lagenaria siceraria plants. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01797. 1797–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Zhang D., Liu S., Zhang G., Yu J., Fritz A.K., Bai G. Genome-wide association analysis on pre-harvest sprouting resistance and grain color in U.S. winter wheat. BMC Genomics. 2016;17:794–809. doi: 10.1186/s12864-016-3148-6. https://www.ncbi.nlm.nih.gov/pubmed/27729004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K.-S., Levi A. Sources of resistance to Zucchini yellow mosaic virus in Lagenaria siceraria germplasm. Hortscience. 2007;42:1124–1126. [Google Scholar]
- Loukou A.L., Gnakri D., Djè Y., Kippré A.V., Malice M., Baudoin J.P., Zoro Bi I.A. Macronutrient composition of three cucurbit species cultivated for seed consumption in Côte d’Ivoire. Afr. J. Biotechnol. 2007;6:529–533. [Google Scholar]
- Loukou A.L., Lognay G., Barthelemy J.-P., Maesen P., Baudoin J.P., Zoro Bi I.A. Effect of harvest time on seed oil and protein contents and compositions in the oleaginous gourd Lagenaria siceraria (Molina) Standl. J. Sci. Food Agric. 2011;91:2073–2080. doi: 10.1002/jsfa.4422. [DOI] [PubMed] [Google Scholar]
- Marrush M., Yamaguchi M., Saltveit M.E. Effect of potassium nutrition during bell pepper seed development on vivipary and endogenous levels of abscisic acid (ABA) J. Am. Soc. Hortic. Sci. 1998;123:925–930. [Google Scholar]
- Mashilo J., Shimelisa H., Odindoa A. Yield-based selection indices for drought tolerance evaluation in selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces. Soil Plant Sci. 2016;67:1–8. [Google Scholar]
- Matilla A., Matilla-Vazquez M. Involvement of ethylene in seed physiology. Plant Sci. 2008;175:87–97. [Google Scholar]
- Monnerville K., Boc Y., Jean-Charles O., Dornier M., Reynes M. Principales caractéristiques de Sechium edule Sw. Fruits. 2001;56:155–167. [Google Scholar]
- Morimoto Y., Gikungu M., Maundu P. Pollinators of the bottle gourd (Lagenaria siceraria) observed in Kenya. Int. J. Trop. Insect Sci. 2004;24:79–86. [Google Scholar]
- Ngaira J.K.W. Impact of climate change on agriculture in Africa by 2030. Sci. Res. Essays. 2007;2:238–243. http://www.academicjournals.org/SRE [Google Scholar]
- Nonogaki M., Nonogaki H. Prevention of preharvest sprouting through hormone engineering and germination recovery by chemical biology. Front. Plant Sci. 2017;8:1–7. doi: 10.3389/fpls.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi Y., Ito T., Hohjo M., Tsukagoshi S., Johkan M., Maruo T., Shinohara Y. Inhibition of viiparous sprouting on melon seeds using high level of potassium fertilization or abscisic acid application. J. Jpn. Soc. Hortic. Sci. 2013;82:227–233. www.jstage.jst.go.jp/browse/jjshs [Google Scholar]
- Oluoch M.O., Welbaum G.E. Effect of postharvest washing and post-storage priming on viability and vigor of six-year-old muskmelon (Cucumis melo L.) seeds from eight stages of development. Seed Sci. Technol. 1996;24:195–209. [Google Scholar]
- PIC-2010 . Université Nagui Abrogoua; Abidjan (Côte d’Ivoire): 2012. Valorisation et promotion de la production de deux cucurbites protéagineuses : Citrullus lanatus et Lagenaria siceraria (Rapport annuel de projet (mai 2010-juin 2011)) [Google Scholar]
- Pirone P.P. fifth ed. John Wiley & Sons; New Jersey (USA): 1978. Diseases and Pests of Ornamental Plants. [Google Scholar]
- Rahman A.S.H. Bottle gourd (Lagenaria siceraria)-A vegetable for good health. Nat. Prod. Rad. 2003;2:249–250. http://nopr.niscair.res.in/bitstream/123456789/12282/1/NPR%202%285%29%20249-256.pdf [Google Scholar]
- Roy S.K., Chakrabarti A.K. Encyclopedia of Food Sciences and Nutrition. second ed. Academic Press; Oxford: 2003. Vegetables of tropical climates: commercial and dietary importance; pp. 5956–5961. [Google Scholar]
- Saade R.L. International Plant Genetic Resources Institut (IPGRI); Rome (Italy): 1996. Chayote, Sechium edule (Jacq.) Sw., Promoting the conservation and use of underutilized and neglected crops. [Google Scholar]
- Saha Arnab, Das S., Chakraborty P., Saha B., Saha D., Saha A. Two new bottle gourd fruit rot causing pathogens from sub-Himalayan West Bengal. J. Agric. Technol. 2016;12:337–348. [Google Scholar]
- Saran P.L., Choudhary R., Solanki I.S., Kumar P.R. New fruit and seed disorders in papaya (Carica papaya L.) in India. Afr. J. Biotechnol. 2014;13:574–580. [Google Scholar]
- Schlumbaum A., Vandorpe P. A short history of Lagenaria siceraria (bottle gourd) in the Roman provinces: morphotypes and archaeogenetics. Veg. Hist. Archaeobotany. 2012;21:499–509. [Google Scholar]
- Shareefa M., Thomas R.J., Nampoothiri C.K., Karun A. Studies on vivipary in dwarf coconut cultivars. Indian J. Hortic. 2014;71:464–468. [Google Scholar]
- Sharma N.K., Dhankar B.S. Variability studies in bottle gourd (Lagenaria siceraria Standl.) Haryana J. Hortic. Sci. 1990;19:305–312. [Google Scholar]
- Steinbach H.S., Benech-Arnold R.L., Sánchez R.A. Hormonal regulation of dormancy in developing sorghum seeds. Plant Physiol. 1997;113:149–154. doi: 10.1104/pp.113.1.149. http://www.plantphysiol.org/content/plantphysiol/113/1/149.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N., Ghaffar A. Seed-borne fungi associated with bottle gourd [Lagenaria siceraria (Mol.) Standl] Pak. J. Bot. 2009;41:435–442. [Google Scholar]
- Tan B.C., Schwartz S.H., Zeevaart J.A.D., McCarty D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12235–12240. doi: 10.1073/pnas.94.22.12235. http://www.jstor.org/stable/43047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman D.E., Cho J., German L. Occurrence and distribution of cucurbit viruses in the Hawaiian Islands. Plant Dis. 1991;75:367–370. [Google Scholar]
- Wang X., Zhang L., Xu X., Qu W., Li Jingfu, Xu X., Wang A. Seed development and viviparous germination in one accession of a tomato rin mutant. Breed Sci. 2016;66:372–380. doi: 10.1270/jsbbs.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum G.E. Cucurbit seed development and production. HortTechnology. 1999;9:341–348. [Google Scholar]
- White C.N., Proebsting W.M., Hedden P., Rivin C.J. Gibberellins and seed development in maize. I. Evidence that gibberellin/abscisic acid balance governs germination versus maturation pathways1. Plant Physiol. 2000;122:1081–1088. doi: 10.1104/pp.122.4.1081. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC58942/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao-Kouamé A., Kane F. Biochemical characteristics of Lippia multifora (Verbenaceae) leaves with respect to fertilizer applied to the soil. J. Plant Sci. 2008;3:287–291. [Google Scholar]
- Yao K.A.G., Koffi K.K., Ondo-Azi S.A., Baudoin J.-P., Zoro B.I.A. Seed yield component identification and analysis for exploiting recombinative heterosis in bottle gourd. Int. J. Veg. Sci. 2015;21:441–453. [Google Scholar]
- Zoro Bi I.A., Koffi K.K., Djè Y. Caractérisation botanique et agronomique de trois espèces de cucurbites consommées en sauce en Afrique de l'Ouest: Citrullus sp.,Cucumeropsis mannii Naudin et Lagenaria siceraria (Molina) Standl. Biotechnol. Agron. Soc. Environ. 2003;7:189–199. http://www.pressesagro.be/base/text/v7n3-4/189.pdf [Google Scholar]
- Zoro Bi I.A., Koffi K.K., Djè Y., Malice M., Baudoin J.-P. Biodiversity of cucurbits consumed as sauce thickener in Côte d’Ivoire: a capital resource for the economic prosperity of rural women. In: Segers H., editor. Tropical Biodiversity: Science, Data, Conservation. Global Biodiversity Information Facility (GBIF); Brussels (Belgium): 2005. pp. 158–167. [Google Scholar]