Highlights
-
•
Guideline-based CRC screening is low among average- and elevated-risk individuals.
-
•
Cancer Risk Intake System (CRIS) generates tailored CRC screening recommendations.
-
•
44% of elevated- and 90% of average-risk patients received guideline-based orders.
-
•
Only about half of elevated-risk group underwent guideline-concordant colonoscopy.
-
•
Half of the average-risk group received guideline-concordant screening.
Abbreviations: CRIS, Cancer Risk Intake System; CRC, colorectal cancer
Keywords: Colorectal cancer screening, Tailored, Primary care
Abstract
Despite demonstrated primary and secondary prevention benefits, screening for colorectal cancer (CRC) is sub-optimal. We implemented the Cancer Risk Intake System (CRIS) among a convenience sample of patients presenting for primary care in Dallas County safety-net clinics. CRIS, which assesses individuals’ CRC risks and generates guideline-based screening recommendations for them and their providers, had been found in a randomized trial to facilitate risk-based screening, compared to usual care. Here, of 924 patients ages ≥50 who used CRIS, 699 were identified as needing screening, with 39.2% needing colonoscopy rather than FIT. However, following use of CRIS and patients’ and providers’ receipt of guideline-concordant recommendations, 20.9% elevated-risk patients received no screening orders, only 44.1% received guideline-concordant colonoscopy orders, and less than half of these (48.4%) completed colonoscopy. Guideline-concordant screening orders were more common for average-risk patients (62.5% received orders for FIT and 26.6% for colonoscopy). However, like their elevated-risk counterparts who received screening orders, more than half of average-risk patients in each order group (52.3% for FIT and 52.8% for colonoscopy) did not complete screening. We found no correlates for receiving screening orders, but higher comorbidity scores were associated with less screening completion among the average-risk group. We had hoped CRIS would facilitate risk-based screening but, although orders for and receipt of colonoscopy were more common for elevated- than average-risk patients, they were still suboptimal in this clinical setting with a “FIT-first” strategy. A stronger intervention may be necessary to increase guideline-concordant recommendations and screening among patients 50 and older.
1. Introduction
Despite demonstrated primary and secondary prevention benefits (Edwards et al., 2010, Yang et al., 2014, Siegel et al., 2017), screening for colorectal cancer (CRC) remains low (Meester et al., 2015). In 2015, 42% of adults ≥50 were non-adherent with recommendations for average-risk individuals (White et al., 2017). Fewer data exist for elevated-risk individuals whose screening recommendations differ from their average-risk counterparts. Depending on risk factors, some elevated-risk individuals only satisfy screening guidelines by undergoing colonoscopy (Bibbins-Domingo et al., 2016, Qaseem and Denberg, 2012, Rex et al., 2017). The challenge for identifying and delivering risk-based “precision” screening is that risk factors determining which modality and screening interval is appropriate are neither routinely captured nor summarized in electronic health records (EHRs).
To facilitate risk-based screening, we developed the Cancer Risk Intake System (CRIS) for use by primary care patients prior to visits. Described elsewhere (Skinner et al., 2015, Skinner et al., 2016); CRIS “assesses individual’s risks, uses algorithms to match them with national screening guidelines, and generates tailored guideline-concordant recommendations for patients and their providers” (Skinner et al., 2017). We previously reported results from a randomized controlled trial (RCT) showing patients who used CRIS were more likely to be screened than those who did not. In that trial, conducted among commercially insured, non-minority patients in an academic medical center, most patients who became adherent did so by undergoing colonoscopy – a test that meets guidelines for both elevated- and average-risk patients. (Skinner et al., 2015).
We hypothesized CRIS might be important for facilitating risk-based screening in safety-net clinics serving under-insured and racial/ethnic minority patients, where fecal immunochemical testing (FIT) is the preferred modality. For providers accustomed to recommending FIT for all patients, CRIS could help identify those at elevated risk who would only meet guidelines by completing colonoscopy.
We recruited a convenience sample of patients who used CRIS before appointments in two primary care safety-net clinics in Dallas, Texas. Both clinics employ a “FIT-first” strategy rather than routinely referring for screening colonoscopy. Here we report proportions receiving orders for guideline-concordant, non-guideline-concordant, or no screening, proportions completing guideline-concordant, non-concordant, or no screening, and clinical or demographic factors correlated with order receipt and screening completion.
2. Methods
From 2013 to 2016, bilingual staff recruited patients at community-based clinics of Parkland Health & Hospital System (Dallas-County’s safety net), after approval from the UT Southwestern IRB. Patients, who verbally consented and used CRIS prior to appointments, were ages 50–64 and spoke English or Spanish. (Results for patients under age 50 have previously been reported (Skinner et al., 2017). Patients with history of CRC or total colectomy were ineligible. Those lacking 6 months of follow-up were excluded from analyses. Screen-up-to-date was defined for average-risk patients as colonoscopy within 10 years, sigmoidoscopy within 5 years, or a stool test (FOBT/FIT) within one year. CRIS algorithms determined whether elevated-risk patients were up-to-date according to guidelines associated with their risk factors; for most, this meant colonoscopy within 10 years.
Described elsewhere (Skinner et al., 2016); CRIS employs complex algorithms based on US Multi-Society Task Force (USMTF) guidelines for average- and elevated-risk individuals (Winawer et al., 2003, Levin et al., 2008) and generates guideline-concordant screening recommendations. Patients and providers receive printouts of the CRIS-generated screening recommendation along with brief descriptions of risk factors warranting recommendations (personal history of polyps and inflammatory bowel disease and family history of CRC, polyps, familial adenomatous polyposis, or Lynch-Syndrome-associated cancers). Screening orders and test completion were retrieved from the EHR at 6 months post visit. Because patients only had insurance coverage to receive CRC testing within the Parkland system, it was extremely unlikely that patients underwent testing not ascertainable via the EHR.
Screening orders placed by healthcare providers and screening completion were defined as guideline-concordant, non-guideline-concordant, or none. Potential clinical or demographics factors correlated with order receipt and screening included age, sex, race/ethnicity, risk level, specific risk factors, clinic, provider type, insurance type, Charlson comorbidity score, and number of primary care visits within 12 months.
2.1. Analysis
We calculated proportions of guideline-concordant vs. non-concordant orders placed by providers and patients who underwent concordant vs. non-concordant screening. “Guideline-concordant” was FIT or colonoscopy if either was recommended by guidelines or colonoscopy if it was the only guideline-recommended test. Non-guideline concordant orders were for FIT when only colonoscopy met guidelines. To identify significant independent factors and correlates associated with guideline-concordant orders and screening in the two risk groups, we used multivariate generalized linear mixed effect models (GLMM) with backward selection and nested structure of patients within clinics. For multivariate analysis, we dichotomized outcomes in the elevated-risk group as concordant or not (non-concordant or no order). For screening receipt, the outcome was guideline-concordant screening receipt vs not (non-concordant or no screening). For average-risk participants, both outcomes were already dichotomized – guideline-concordant order and screening receipt vs. no order and screening. We used SAS Version 9.4 Proc GLIMMIX for multivariate analysis (Cary, NC).
3. Results
Of 924 patients ages ≥50 who used CRIS, 699 were identified as being in need of screening and had 6 months of follow-up through which we could access screening via EHRs. CRIS identified 211 of these (30.2%) with elevated risk and 488 (69.8%) as average-risk. Elevated-risk patients were primarily female, African American, and had >3 primary-care visits in the past year (Table 1). The most common single risk factors that put patients at elevated risk were family history of CRC (21.8%), of polyps (18%) and patient history of polyps (14.2%). Most common combination of risk factors was family history of CRC and polyps (19.4%).
Table 1.
Participant characteristics.
| Variable | Average risk (Colo or FIT) (N = 488) | Elevated risk (Colo Only) (N = 211) | All (N = 699) |
|---|---|---|---|
| Age, years | |||
| 50–54 | 44.67% | 44.08% | 44.49% |
| 55–59 | 33.40% | 31.28% | 32.76% |
| 60–64 | 21.93% | 24.64% | 22.75% |
| # of risk factors | 0.102 ± 0.304 | 1.431 ± 0.593 | 0.504 ± 0.737 |
| Charlson comorbidity | |||
| 0 | 47.13% | 41.71% | 45.49% |
| 1 | 32.58% | 39.34% | 34.62% |
| 2+ | 0.29% | 18.96% | 19.89% |
| Clinic | |||
| Bluitt-Flowers | 50.41% | 62.09% | 53.93% |
| deHaro-Saldivar | 49.59% | 37.91% | 46.07% |
| CRIS visit payor | |||
| State/Fed Gov’t coverage | 20.90% | 19.91% | 20.60% |
| Commercial | 2.87% | 0.95% | 2.29% |
| Charity | 71.93% | 75.36% | 72.96% |
| Self-pay | 4.30% | 3.79% | 4.15% |
| CRIS visit provider | |||
| Physician | 76.84% | 84.36% | 79.11% |
| Physician assistant | 3.69% | 5.69% | 4.29% |
| Nurse | 19.47% | 9.95% | 16.60% |
| # primary care visits | |||
| 0 | 25.20% | 15.64% | 22.32% |
| 1 | 30.53% | 28.44% | 29.90% |
| 2 | 25.20% | 24.17% | 24.89% |
| 3+ | 19.06% | 31.75% | 22.89% |
| Race/Ethnicity | |||
| White | 6.76% | 7.58% | 7.01% |
| Black | 49.59% | 63.98% | 53.93% |
| Hispanic | 42.83% | 27.96% | 38.34% |
| Other/Unknown | 0.82% | 0.47% | 0.72% |
| Sex | |||
| Female | 61.27% | 76.30% | 65.81% |
| Male | 38.73% | 23.70% | 34.19% |
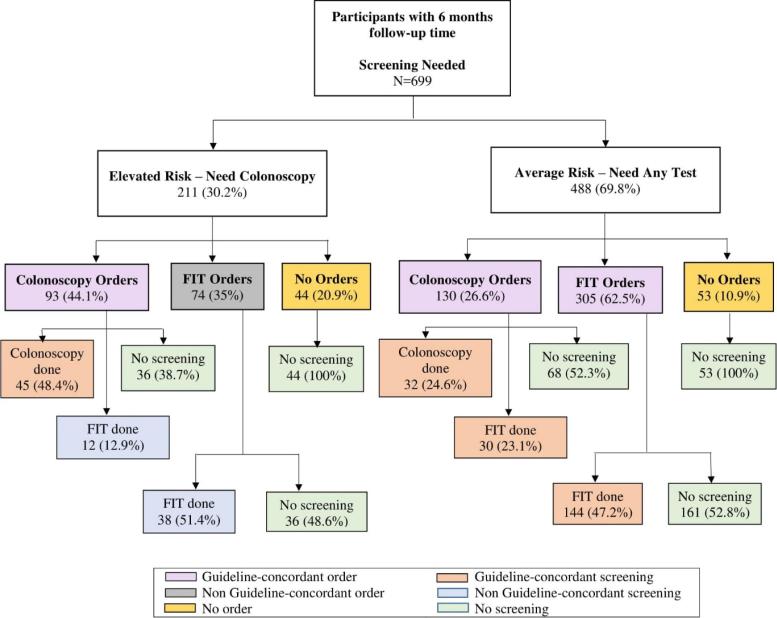
Fig. 1 depicts screening orders and screening completion, by 6 months, for elevated- (n = 211) and average-risk (n = 488) patients.
Fig. 1.
Proportion of type of CRC screening and screening orders placed.
Most elevated-risk patients received screening orders, but fewer than half (44.1%) were guideline-concordant (35% received FIT orders and 20.9% received no orders). Most average-risk patients received guideline-concordant orders (62.5% for FIT and 26.6% colonoscopy); 10.9% received no orders.
Most of the 93 elevated-risk patients who received colonoscopy orders underwent some type of screening by 6 months – 48.4% with colonoscopy and 12.9% via FIT. Just over half of elevated-risk patients with (non-concordant) orders for FIT had completed the test by 6 months and, predictably, none of those with no orders were screened.
Like the elevated-risk group, none of the 53 average-risk patients without orders underwent screening. Among average-risk patients with orders, just over half in colonoscopy and FIT order groups (52.3% and 52.8%, respectively) remained unscreened by 6 months. Of those receiving colonoscopy orders, nearly equal groups completed colonoscopy (24.6%) and FIT (23.1%).
Multivariate analysis to assess correlates associated with orders placed revealed no factors significantly associated with guideline-concordant orders in either risk group. Nor were any factors significantly associated with guideline-concordant screening completion among elevated-risk patients. However, among average-risk patients, Charlson score was associated with guideline-concordant screening. Patients with a comorbidity index ≥ 2 had a lower likelihood of being screened, compared to those with scores of 0 (adjusted odds ratio = 0.539, 95% CI 0.325–0.894, p = 0.017).
4. Discussion
CRIS, a touch-screen computer program used by patients to input information about screening and personal and family risk factors, classified most patients in need of screening as average-risk, therefore satisfying guidelines via any screening modality. About a third reported risk factors warranting screening via colonoscopy. Just under 90% of average-risk patients received orders for guideline-concordant testing (mostly for FIT) but fewer than half completed screening. Among those receiving colonoscopy orders, about half who were screened completed FIT instead. None who received FIT orders “crossed over” to colonoscopy. Neither orders for nor completion of guideline-concordant testing was as common for elevated- as average-risk patients. Just over 20% of elevated-risk patients received no screening orders. Among the roughly half who received colonoscopy orders, about half completed the procedure by six months, more than a third completed FIT instead, and the rest were not screened.
In a previous RCT among commercially insured and non-minority patients, CRIS showed increased participation in CRC screening, compared with usual care (Skinner et al., 2015). Here, we employed CRIS in routine care and identified test ordering and screening completion in safety-net clinics serving primarily minority patients.
It seems counterintuitive that higher-risk patients were less likely to receive any screening orders and that so many who did receive orders were for non-guideline-concordant FIT. Providers may have discounted patients’ risk level or they may have considered patients recently screened via FIT as being “up-to-date” even if, by guidelines, they needed colonoscopy. Simple identification of risk level and reminder of guidelines was not a powerful enough intervention to overcome providers’ standard practice of ordering FIT for all patients ≥50.
We had hoped to identify clinical or demographics factors correlated with receipt of both guideline-concordant orders and screening completion; the only significant finding was lower screening participation among average-risk patients with higher comorbidity scores. Prior studies have reported inconsistent results for CRC screening rates among patients with comorbidities (Gimeno Garca, 2012, Fleming et al., 2011, Sewitch et al., 2007, Fisher et al., 2007), but have not reported results by risk level. We are unsure why the significant association was only in the average-risk group. Lack of difference by insurance type is likely because all had a fixed co-pay of $5 for FIT and $25 for colonoscopy.
Despite sub-optimal outcomes for guideline-concordant test ordering and screening participation, we should note the large majority of CRIS users in need of screening did receive test orders and, within both average- and elevated-risk groups, about half completed some screening by 6 months. That a larger proportion of elevated- (48.4%) than average-risk patients (24.6%) received colonoscopy orders also suggest some effect of identifying patients’ risk levels and reminding them and their providers about screening guidelines.
Study limitations and strengths should be noted. First, CRIS uses algorithms verified to match reported risk factors with guideline-based recommendations, but the information is only as accurate as patients’ self-reports. For example, if someone classified by CRIS’ relatively low specificity as being at elevated risk was “reclassified” as average-risk through correction of a relative’s age at diagnosis during provider discussion, a physicians’ recommendation for FIT may have been appropriate. Indeed, a strength of using CRIS in the clinic setting is the opportunity for such clarification to occur during the visit.
The study is limited in the fact that, unlike the previous RCT among commercially insured patients (Skinner et al., 2015), it did not include a comparison group of patients who did not use CRIS. Nor do we have specific information about nonparticipants who visited the clinics during the same time period. However, we do know participants were similar to the overall screening-eligible Parkland primary-care clinic population in: age, Charlson comorbidity score, insurance type, race/ethnicity, and sex (Balasubramanian et al., 2017). A study strength is the fact that CRIS was used within the context of regular clinical care rather than facilitated by research assistants in a RCT. Finally, a larger sample size might have resulted in the observed trend for more colonoscopy among elevated-risk patients being a significant difference.
Although one might argue that introduction of CRIS could have resulted in confusion into our system’s “FIT-first” strategy, ultimately reducing rather than enhancing screening participation, data from a cohort of screen-eligible primary care patients in the system during the time period showed an opportunistic screening rate of 30% (Singal et al., 2016), which suggests that CRIS did not reduce screening uptake.
In this descriptive study, we found overall screening completion rates among CRIS users just slightly lower than screening completion in the previous RCT (Skinner et al., 2015), through which screening was mainly via colonoscopy irrespective of risk level whereas here, screening was usually via FIT, irrespective of risk level. A final strength is analysis of not only patient participation in screening but also whether and what tests were ordered by providers.
5. Conclusions
When used in routine practice in safety-net clinics, CRIS identified almost a third of patients as likely candidates for colonoscopy rather than FIT, due to previous screening history and personal and familial risks. Although orders for and receipt of colonoscopy were more common in this elevated-risk group than for patients with average risk, they were still suboptimal in this clinical setting with a “FIT-first” strategy. Previous studies have identified multiple barriers to CRC screening at the patient and provider levels (Guerra et al., 2007). A stronger and more precise intervention may be necessary to increase guideline-concordant recommendations and screening among patients 50 and older, perhaps especially among those with elevated risks.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
NCI RO1 CA12233, NCI RO1 CA1223301, NIH/NCI 5P30 CA142543 and NIH/NCATS UL1TR001105, NCI U54 CA163308.
References
- Balasubramanian B.A., Garcia M.P., Corley D.A., Doubeni C.A., Haas J.S., Kamineni A. Racial/ethnic differences in obesity and comorbidities between safety-net- and non safety-net integrated health systems. Medicine (United States) 2017;96:1–5. doi: 10.1097/MD.0000000000006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K., Grossman D.C., Curry S.J., Davidson K.W., Epling J.W., García F.A.R. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- Edwards B.K., Ward E., Kohler B.A., Eheman C., Zauber A.G., Anderson R.N. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.A., Galanko J., Dudley T.K., Shaheen N.J. Impact of comorbidity on colorectal cancer screening in the veterans healthcare system. Clin. Gastroenterol. Hepatol. 2007;5:991–996. doi: 10.1016/j.cgh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Fleming S.T., Schoenberg N.E., Tarasenko Y.N., Pearce K.A. Prevalence of colorectal cancer screening among a multimorbid rural appalachian population. South. Med. J. 2011;104:811–818. doi: 10.1097/SMJ.0b013e31823a8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno Garca A.Z. Factors influencing colorectal cancer screening participation. Gastroenterol. Res. Pract. 2012:1–8. doi: 10.1155/2012/483417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C.E., Schwartz J.S., Armstrong K., Brown J.S., Halbert C.H., Shea J.A. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J. Gen. Intern. Med. 2007;22:1681–1688. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B., Lieberman D.A., McFarland B., Smith R.A., Brooks D., Andrews K.S. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- Meester R.G.S., Doubeni C.A., Zauber A.G., Goede S.L., Levin T.R., Corley D.A. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281–2285. doi: 10.1002/cncr.29336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A., Denberg T.D., Hopkins R.H., Jr, Humphrey L.L., Levine J. Screening for colorectal cancer : a guidance statement from the American College of Physicians. Ann. Intern. Med. 2012;156:378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- Rex D.K., Boland C.R., Dominitz J.A., Giardiello F.M., Johnson D.A., Kaltenbach T. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest. Endosc. 2017;86:18–33. doi: 10.1016/j.gie.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Sewitch Maida J., Fournier Caroline, Dawes Martin, Yaffe Mark, Snell Linda, Roper Mark. Do physician recommendations for colorectal cancer screening differ by patient age ? Can. J. Gastroenterol. 2007;21:435–438. doi: 10.1155/2007/938978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- Singal A.G., Gupta S., Tiro J.A., Skinner C.S., McCallister K., Sanders J.M. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer. 2016;122:456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner C.S., Halm E.A., Bishop W.P., Ahn C., Gupta S., Farrell D. Impact of risk assessment and tailored versus non-tailored risk information on colorectal cancer testing in primary care: a randomized controlled trial. Cancer Epidemiol. Biomarkers Prev. 2015;24:1523–1530. doi: 10.1158/1055-9965.EPI-15-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner C.S., Gupta S., Bishop W.P., Ahn C., Tiro J.A., Halm E.A. Tailored information increases patient/physician discussion of colon cancer risk and testing: the Cancer Risk Intake System trial. Prev. Med. Rep. 2016;4:6–10. doi: 10.1016/j.pmedr.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner C.S., Ahn C., Halm E.A., Bishop W.P., McCallister K., Sanders J.M. Recommendation of colorectal cancer testing among primary care patients younger than 50 with elevated risk. Prev. Med. (Baltim.) 2017;102:20–23. doi: 10.1016/j.ypmed.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A., Thompson T.D., White M.C., Sabatino S.A., de Moor J., Doria-Rose P.V. Cancer screening test use — United States, 2015. MMWR Morbidity and Mortality Weekly Rep. 2017;66:201–206. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer S., Fletcher R., Rex D., Bond J., Burt R., Ferrucci J. Colorectal cancer screening and surveillance: clinical guidelines and rationale - update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- Yang D.X., Gross C.P., Soulos P.R., Yu J.B. Estimating the magnitude of colorectal cancers prevented during the era of screening 1976 to 2009. Cancer. 2014;120:2893–2901. doi: 10.1002/cncr.28794. [DOI] [PubMed] [Google Scholar]