Abstract
Epidemiological evidence shows that physical activity lowers the risk of developing breast cancer and decreases the risk of disease recurrence [1,2]. The main hypothesis on the positive effects of exercise-oncology has focused on lowering the basal systemic levels of cancer risk factors with exercise training. Recently, the effects of cancer progression control by components released after acute exercise bouts have gained attention [3,4]. However, the evaluation of the antiproliferative potential of a single exercise bout needs technical improvement.
Here, we present data of a pilot study showing how to evaluate the anti-cancer potential of single exercise bouts with an in vitro three-dimensional cell growth assay, using a triple-negative breast cancer cell line cultured with exercise-conditioned serum.
Keywords: Exercise, Triple negative breast cancer, Three-dimensional in vitro culture, Cell proliferation
Specifications Table
| Subject | Sport Sciences, Therapy and Medicine |
| Specific subject area | Evaluation of the effects of exercise using cancer cell growth assays |
| Type of data | Graph Figure |
| How data were acquired | MTS assay and soft-agar assay. Statistical analysis has been performed with Prism5 software, using 1-way ANOVA followed by Bonferroni-corrected multiple comparisons. |
| Data format | Raw and analyzed |
| Experimental Factors | Cells were cultured in standard conditions. During the experiments, the standard culture medium was replaced by Dulbecco's Modified Eagle's Medium without red phenol, with 0.8/1.2 mg/mL of glucose, supplemented with 5% of human pre- or post-exercise serum. Cells were cultured in an anchorage-dependent manner or in anchorage-independent conditions (soft agar assay) in a 0.3% soft-agar layer. |
| Experimental features | The cell viability was evaluated by the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) after 72h of incubation with pre- or post-exercise sera. The cancer progression control potential was evaluated in anchorage-independent culture conditions, counting the colonies composed by more than 20 cells, formed in each well after 18 days of cell incubation with pre- or post-exercise sera. |
| Data source location | Institution: University of Urbino Carlo Bo – Department of Biomolecular Sciences, Hygiene Unit and Division of Exercise and Health Sciences City/Town/Region: Urbino Country: Italy |
| Data accessibility | All data are presented within this article |
|
1. Data
This Data in Brief presents the optimization of methods for the evaluation of triple negative breast cancer (TNBC) [1,2] cell MDA-MB-231 responses induced by acute exercise-conditioned sera [3,4], considering also the capacity of exercise-conditioned sera to modulate three-dimensional (3D) anchorage-independent cancer cell growth. The time schedule of the aerobic exercise session performed by the three subjects is presented in Fig. 1.
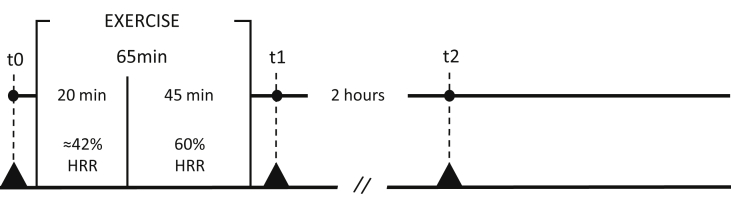
Fig. 1.
Details of the acute exercise interventions. The time schedule of pre- (t0) and post-exercise (t1; t2) sampling is shown in the timeline. Blood samples for in vitro assays were drawn according to protocol exercise at t0, t1 and t2.//, rest period; HRR, heart rate reserve.
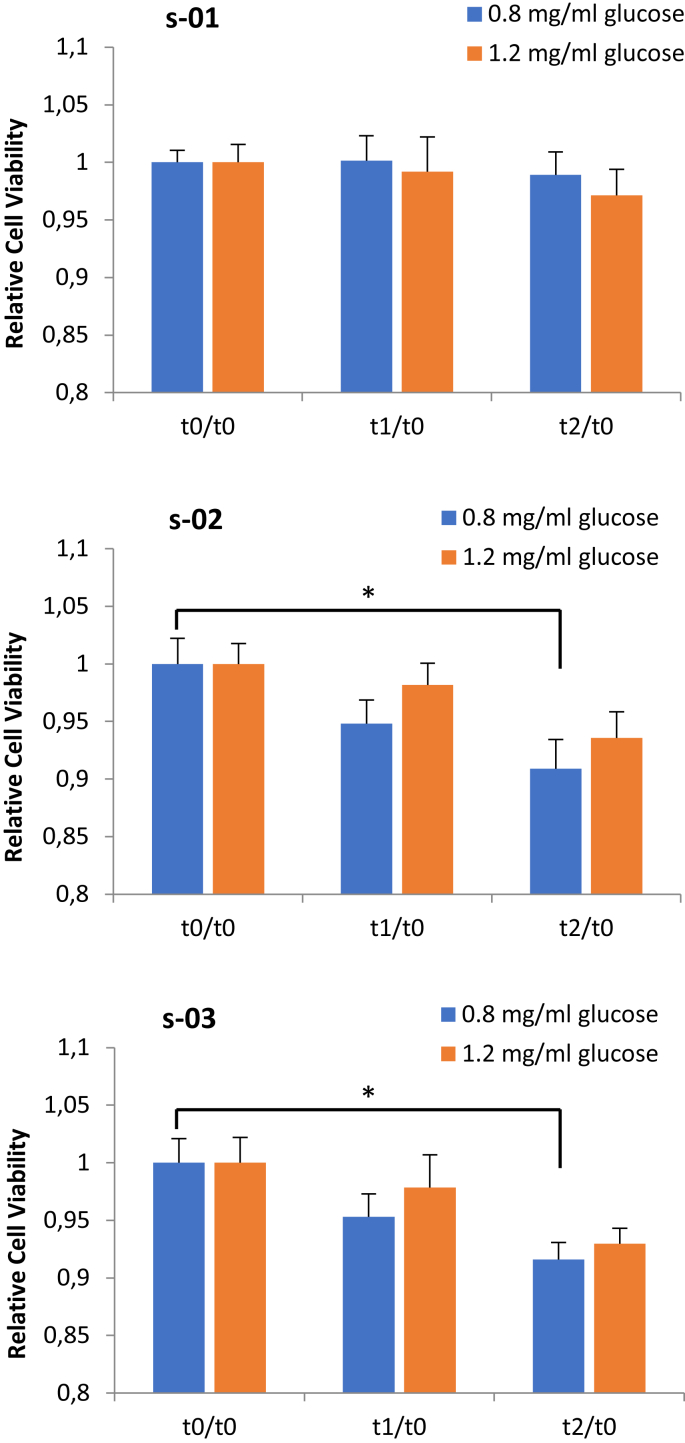
The effects of the sera sampled pre- or post-exercise on the capacity of TNBC cell to proliferate were monitored by exposing MDA-MB-231 cells to 5% of human sera for 72 hours, after which the cell viability was evaluated by MTS assay (Fig. 2). Data of supplementation of the culture medium with a physiological or hyperglycemic concentration of glucose (0.80 mg/mL or 1.20 mg/mL) showed a lower ability of the exercise-conditioned sera (t1 and t2) to induce TNBC cell proliferation than the sera collected pre-exercise (at rest sera, t0). Interestingly, the inhibition obtained was higher with a physiological concentration of glucose (0.80 mg/mL), leading to a statistically significant reduction in the ability of cells to proliferate in two out of three subjects considered (s-02 and s-03). In particular, in these two subjects, t1 and t2 sera led to a reduction of 5% and 9%, respectively (n.s. and p < 0.05, respectively). Analyzing the highest concentration of glucose tested (1.2 mg/mL), t1 and t2 sera led to a reduction of cell proliferation of only 2% and 6% (n.s.), considering the sera of subjects s-02 and s-03. Raw data are presented as supplementary file “RAW DATA FIG.2”.
Fig. 2.
MTS assay. Evaluation of cell viability after 72 hours of incubation with 5% of pre- or post-exercise sera and with culture medium supplemented with 0.8 or 1.2 mg/mL of glucose. t0: pre-exercise serum; t1: immediately after exercise serum; t3: 2 hours post-exercise serum. s-01, subject 01; s-02, subject 02; s-03, subject 03. Data are expressed as mean ± SEM of five experiments; *p < 0.05.
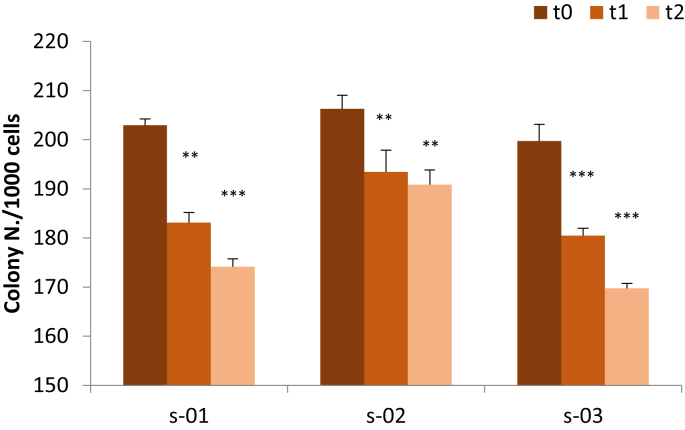
The effects of exercise-conditioned sera on the tumorigenic potential of MDA-MB-231 cells was monitored performing the anchorage-independent three-dimensional growth assay (soft-agar), which is considered one of the most reliable tests to assess the malignant transformation process in vitro [5]. In this technique, TNBC cells were dispersed in the central layer, composed by 0.3% agar and exposed to 0.80 mg/mL of glucose and 5% of pre-exercise (t0) or post-exercise sera (t1 or t2). Data of 3D anchorage-independent cancer cell growth are presented in Fig. 3, expressed as the total number of colonies formed by more than 20 cells, counted in each well after 18 days of incubation. Data show that all exercise-conditioned sera (t1 and t2 sera) reduced in a statistically significant manner the ability of TNBC cells to form colonies in soft agar, in comparison to pre-exercise sera (t0) (p < 0.01; p < 0.001). In particular, exercise-conditioned sera of the subject s-01 induced a reduction of colony number of 10% and 14% (t1 and t2, respectively); analyzing the subject s-02, the post-exercise sera induced reduction was of 6% and 8% (t1 and t2, respectively) and evaluating the subject s-03, the reduction induced by t1 and t2 sera was of 10% and 15%, respectively. Raw data are presented as supplementary file “RAW DATA FIG.3”.
Fig. 3.
Soft-agar assay. Evaluation of MDA-MB-231 colony formation after 18 days of incubation with 5% of pre- or post-exercise human sera. s-01, subject 01; s-02, subject 02; s-03, subject 03. Data are expressed as mean ± SEM of four experiments; **p < 0.01, ***p < 0.001, respect to t0.
2. Experimental design, materials, and methods
2.1. Subjects
Three healthy and sedentary pre-menopausal women were included in the study. Their median (range) age, height and weight were 43.3 ± 9.8 yrs, 164.4 ± 4.7 cm and 60.2 ± 5.8 kg, respectively. The study was carried out according to the Helsinki Declaration for research with human volunteers and all signed an informed consent form to participate.
2.2. Acute exercise session
On the experimental day, participants performed a single bout of exercise. In particular, the participants performed 65 min of moderate to baseline vigorous intensity aerobic exercise on a treadmill. In the first 20 minutes, subjects ran at a heart rate reserve (HRR; ≈42%) corresponding to 50% of their own estimated VO2max, then exercise intensity was increased to 65% of VO2max (i.e., 60% HRR) and maintained for 45 min [6].
2.3. Blood samples, cell line and cell cultures
Blood samples were collected in venous blood collection tubes (BD Vacutainer, 10mL, no additives) just before exercise, immediately after and 2 hours post-exercise (Fig. 1). Serum was obtained centrifuging blood samples at 1×103 x g for 15 minutes at 4 °C, after an incubation of 15–30 minutes at room temperature. Sera were aliquoted and stored at −80 °C; before the experiments, sera were heat-inactivated at 56 °C for 30 minutes, centrifuged at 12.000 rpm at 4 °C for 10 minutes and transferred to new sterile tubes.
TNBC cell line MDA-MB-231 was purchased from the American Type Cell Culture Collection (ATCC, Rockville, MD, USA) and cultured in Dulbecco's Modified Eagle Medium (DMEM) high glucose, supplemented with 10% of heat-inactivated Fetal Bovine Serum (FBS), 2 mM glutamine, 0.1 g/L streptomycin, 100 units/ml penicillin, 1 mM Na-pyruvate and 1 × MEM Non-essential Amino Acid Solution. During the experiments, DMEM high-glucose was replaced by DMEM without red phenol (DMEM-RPF), supplemented with 5% of heat-inactivated human sera (HS) pre- or post-exercise, 2 mM glutamine, 0.1 g/L streptomycin, 100 units/ml penicillin, 1 mM Na-pyruvate, 1 × MEM Non-essential Amino Acid Solution and 0.8 or 1.2 mg/mL glucose. All cell culture materials were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cells were maintained for a maximum of fifteen passages, in a humidified incubator with 5% of CO2, at 37 °C.
2.4. Anchorage-dependent growth assay
MDA-MB-231 cells were seeded at a density of 2.5 × 103 cells/well in 96-well plates. After overnight incubation, the medium was replaced with DMEM-RPF and cells were exposed to 5% of pre-exercise (t0), post-exercise (t1) or 2-h post-exercise (t2) HS supplementation. Two different concentrations of glucose (0.80 or 1.2 mg/mL) were tested. After 72 h of incubation, cell viability was assessed by the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA). This method is a colorimetric assay based on the ability of viable cells to convert soluble tetrazolium salt (MTS) into a formazan product. Data are expressed as relative viable cells (mean ± SEM of five experiments) compared to cells supplemented with at rest serum (t0).
2.5. Anchorage-independent transformation assay (soft-agar assay)
Soft agar assay was performed in 12-well plates as reported previously [7]. Briefly, 1 × 103 MDA-MB-231 cells were considered for each well and cultured in the central layer of agar, composed by a 0.5 mL of 0.3% agar and 0.8 mg/mL glucose-DMEM-RPF solution, supplemented with 5% of t0, t1 or t2 HS. The bottom layer was composed by a 0.5mL solution of 0.6% agar and 0.80 mg/mL glucose-DMEM-RPF, supplemented with 5% of t0, t1 or t2 HS and the top layer was composed by 0.8 mg/mL glucose-DMEM-RPF added by 5% of t0, t1 or t2 HS. After 18 days of incubation, cells were stained with 0.01% crystal violet and only colonies formed by more than 20 cells were considered and counted (Supplementary information). Data are expressed as the total number of colonies (mean ± SEM of four experiments) counted in each well.
2.6. Statistical analysis
Data are expressed as mean ± SEM of separate experiments. Data were analyzed with Prism5 software, using 1-way ANOVA followed by Bonferroni's multiple comparison test. Differences were considered significant at p < 0.05.
Acknowledgments
This work was supported by the University of Urbino Carlo Bo, Department of Biomolecular Sciences (under Grant “Progetti di Valorizzazione 2018” n. 28/2018).
The authors would like to thank Dr. S. Maggio and Dr. P. Ceccaroli for their technical contributions in this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104704.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Friedenreich C.M., Neilson H.K., Farris M.S., Courneya K.S. Physical activity and cancer outcomes: a precision medicine approach. Clin. Cancer Res. 2016;22(19):4766–4775. doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 2.Agostini D., Natalucci V., Baldelli G., De Santi M., Donati Zeppa S., Vallorani L., Annibalini G., Lucertini F., Federici A., Izzo R., Stocchi V., Barbieri E. New insights into the role of exercise in inhibiting mTOR signaling in triple-negative breast cancer. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/5896786. 2018:5896786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dethlefsen C., Pedersen K.S., Hojman P. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Canc. Res. Treat. 2017;162(3):399–408. doi: 10.1007/s10549-017-4129-4. [DOI] [PubMed] [Google Scholar]
- 4.Hojman P., Gehl J., Christensen J.F., Pedersen B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metabol. 2018;27(1):10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Borowicz S., Van Scoyk M., Avasarala S., Karuppusamy Rathinam M.K., Tauler J., Bikkavilli R.K., Winn R.A. The soft agar colony formation assay. J. Vis. Exp. 2014;92 doi: 10.3791/51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rundqvist H., Augsten M., Strömberg A., Rullman E., Mijwel S., Kharaziha P., Panaretakis T., Gustafsson T., Östman A. Effect of acute exercise on prostate cancer cell growth. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Santi M., Baldelli G., Diotallevi A., Galluzzi L., Schiavano G.F., Brandi G. Metformin prevents cell tumorigenesis through autophagy-related cell death. Sci. Rep. 2019;9(1):66. doi: 10.1038/s41598-018-37247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.