Abstract
Background
Globally, diabetic kidney disease (DKD) is the leading cause of end-stage renal disease. As the most common microvascular complication of diabetes, DKD is a thorny, clinical problem in terms of its diagnosis and management. Intensive glucose control in DKD could slow down but not significantly halt disease progression. Revisiting the tremendous advances that have occurred in the field would enhance recognition of DKD pathogenesis as well as improve our understanding of translational science in DKD in this new era.
Scope of review
In this review, we summarize advances in the understanding of the local microenvironmental changes in diabetic kidneys and discuss the involvement of genetic and epigenetic factors in the pathogenesis of DKD. We also review DKD prevalence changes and analyze the challenges in optimizing the diagnostic approaches and management strategies for DKD in the clinic. As we enter the era of ‘big data’, we also explore the possibility of linking systems biology with translational medicine in DKD in the current healthcare system.
Major conclusion
Newer understanding of the structural changes of diabetic kidneys and mechanisms of DKD pathogenesis, as well as emergent research technologies will shed light on new methods of dealing with the existing clinical challenges of DKD.
Keywords: Diabetic kidney disease, Genetics, Epigenetics, Metabolic memory, Systems biology
1. Introduction
The kidney is a vulnerable organ as well as the most important target of microvascular damage in both type 1 (T1DM) and type 2 diabetes mellitus (T2DM) [[1], [2], [3]]. The first description of the association between diabetes and kidney damage in humans was in 1552 BC [4,5]. As the disease spectrum has changed around the world, diabetic kidney disease (DKD) has become the single most frequent cause of end-stage renal disease (ESRD) at daunting rates over the past 30 years, in both developed and developing countries [[6], [7], [8], [9], [10], [11], [12]], It is no exaggeration to call DKD “a medical catastrophe of worldwide dimension” [13,14]. Every year, management of DKD is not only a comprehensive medical undertaking but also associated with substantial immediate and long-term health care costs in developed countries [[15], [16], [17]] as well as in emerging and developing economies [[18], [19], [20], [21]].
Previously known as diabetic nephropathy, DKD is the new medical term introduced in 2007 by the Kidney Disease Outcomes Quality Initiative (KDOQI) [22]. DKD is proposed as a presumptive diagnosis of kidney disease caused by diabetes, and physicians are now carefully screening diabetic patients to identify persons most likely to have secondary kidney diseases based on solid clinical evidence, in order to avoid invasive kidney biopsy [22,23]. Although new mechanisms [24], new approaches [[25], [26], [27]], and new biomarkers have been described in succession [[28], [29], [30], [31]], the accuracy of the DKD diagnosis without renal biopsy has been questioned in the clinical setting. Indeed, the pathogenesis of DKD is multifactorial. The interweaving of unpredictable clinical risk factors [5,32], difficulties in individual disease management [33], diversity in genetic background [34], kidney structure abnormalities [35], and aberrant activation of the intracellular signaling pathways determine the complexity of this multi-organ syndrome. Therefore, it is urgent that precise and effective translation of basic research findings on DKD into clinical applications has to be facilitated. As we enter a new era, burgeoning approaches such as multi-OMICS analyses and application of mathematical models, and even artificial intelligence, in biomedicine are becoming stronger weapons for dealing with DKD in the 21st century. In this review, we will dissect knowledge of translational research on DKD and common comorbidities in T2DM patients from bench work to bedside.
2. The focus on translational medicine of DKD: from the Nightmare to the light of dawn
Conventionally, DKD is a glomerulocentric clinical problem with features of diffuse or nodular glomerulosclerosis. Over a long period of time, hemodynamic changes including high intraglomerular pressure and hyperfiltration have become recognized as the dominant mechanisms in the onset and progression of DKD. In recent years, translational research has offered the field an updated understanding of the microenvironmental changes in diabetic kidneys, as well as further recognition of the multidimensional pathogenesis of DKD.
2.1. Structure changes build a ‘sophisticated’ microenvironment
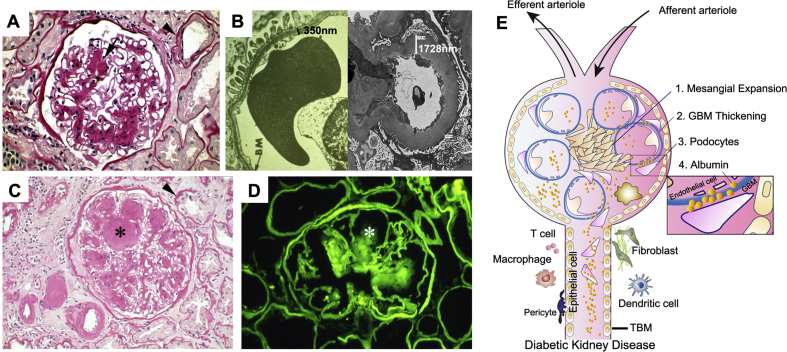
In the diabetic kidney, the glomerulus was previously thought to be the earliest diseased compartment after T2DM diagnosis (Figure 1A). Although hyperglycemia immediately causes an increase in the amount of glucose filtered through the glomerular filtration barrier [35], early damage in glomeruli normally takes 0–3 years to occur. The characteristic pathological glomerular changes in DKD have been well documented and include thickening of the glomerular basement membrane (GBM), podocyte injury, mesangial matrix expansion, and loss of glomerular endothelium fenestrations [36]. Subsequently, these cellular dysfunctions directly cause changes in the microvascular permeability and damage of glomerular filtration barrier and eventually result in excretion of the clinical hallmarks of DKD: microalbuminuria or albuminuria. Among these pathologic changes, thickening of the GBM is an early finding in DKD (Figure 1B). It is caused by the abnormal turnover and modification of the extracellular matrix (EMC) produced by endothelial cells and podocytes. GBM thickening is also considered an early sign of endothelial and podocyte activation in DKD [35]. DKD-caused podocytopathy features cellular hypertrophy, foot process effacement, and podocyte loss. Meanwhile, hyperglycemia also accelerates mesangial cell proliferation, by which excessive ECM is produced, resulting in glomerulosclerosis. Typically, hyperglycemia may lead to strikingly increased non-enzymatic glycosylation of proteins in the mesangial matrix. This nonenzymatic glycosylation of the matrix causes the most distinctive lesions of DKD, called Kimmelstiel-Wilson (K–W) nodules [37]. K–W nodules are pink hyaline materials surrounded by capillary loops in diseased glomerulus by which nodular glomerulosclerosis is formed (Figure 1C,D). They often present with microaneurysms in diseased glomeruli caused by DKD.
Figure 1.
Pathologic lesions in human DKD. (A) Nonnodular diabetic glomerulosclerosis. The black arrow indicates ECM deposition in the mesangial area. Black arrowhead indicates a thickening of tubular basement membrane (TBM). (B) Electric microscopy shows the thickness of the GBM in human diabetic kidneys compared with healthy control. (C) Nodular diabetic glomerulosclerosis, thickening of TBM, ECM accumulation, and interstitium expansion in diabetic kidneys. The black star denotes Kimmelstiel-Wilson (KW) nodule. Black arrowhead denotes thickening of TBM. (D) Immunofluorescence staining of immunoglobulin G shows KW nodule (white star) in diabetic kidney. (E) Schematic diagram depicting a ‘sophisticated’ microenvironment formation in diabetic kidneys. ECM: extracellular matrix.
In contrast to the meticulous exploration of glomerular damage, the renal tubulointerstitial compartment has been largely overlooked in DKD over the past years. Currently, ongoing research has shifted the glomerulocentric paradigm to tubulopathy in DKD. As the ‘prime mover’ of DKD [38], tubulointerstitial change is now thought to be a determining factor of the rate of attrition of renal function rather than diabetic glomerular damage [39]. In fact, there are often substantial changes in tubules, the largest resident cell population in the kidney, as well as in the interstitium, as observed on kidney biopsy specimens in DKD patients. In DKD, kidney tubule dysregulation may precede or at least accompany pathognomonic changes of the glomerulus.
Anatomically, the renal tubule is the portion of the nephron that contains the tubular fluid filtered through the glomerulus. The components of the renal tubules include the proximal convoluted tubule, loop of Henle, distal convoluted tubule, and collecting duct. The proximal tubule is the portion of the duct system contiguous with the parietal epithelium of Bowman's capsule and leads to the loop of Henle. In diabetic kidneys, tubular hypertrophy is observed after only a few days of hyperglycemia. In parallel with diabetic glomerular changes, increased tubular basement membrane (TBM) width is one of the earliest structural changes that can be readily quantitated in the diabetic kidney, even among patients with normoalbuminuria (Figure 1A,C). TBM may represent a better indicator of the severity of DKD than the thickening of the GBM [40,41]. Along with DKD progression, tubular atrophy, peritubular capillary rarefaction, and interstitial fibrosis are major pathologic changes in tubulointerstitial compartment. In addition, about 7% of T2DM patients were reported to have non-functioning atubular glomeruli because of atrophy occurring at the critical junction between Bowman's capsule and the proximal tubule [42]. Furthermore, as an independent risk factor, hyperglycemia itself could directly cause acute tubular necrosis, tubular cell apoptosis, epithelial–mesenchymal transition, and ECM deposition. In the advanced stage of DKD, tubulointerstitial and glomerular changes coalesce into fibrosis (Figure 1C).
Besides glomerular and tubular injuries, vascular changes and inflammatory cell infiltration are also indispensable components in the formation of the ‘sophisticated’ microenvironment in diabetic kidneys (Figure 1E). Vascular changes include endothelium and pericyte abnormalities. As a heterogeneous population of cells around the peritubular capillaries, the ratio of pericytes to endothelial cells is 1:2.5 [43]. Hyperglycemia causes peritubular pericyte migration away from the capillary into the interstitial space. The capillary becomes destabilized, resulting in microvascular rarefaction in T2DM DKD as well as kidney damage in T1DM [39]. The migrating peritubular pericytes are thought to undergo pericyte–myofibroblast transition, which accelerates tubulointerstitial changes [44]. In addition, DKD is associated with both systemic and local renal inflammation with the participation of crucial inflammatory cells such as macrophages, mast cells, and T lymphocytes. These infiltrated cells are tightly associated with albuminuria levels, a reduced rate of estimated glomerular filtration, and increased severity of DKD.
2.2. Re-recognition of the pathogenesis of DKD
In T2DM, β-cell failure, mitochondria dysfunction [[45], [46], [47]], abnormal endoplasmic reticulum stress [48,49], and declined autophagic activity have been implicated as potential causes of insulin resistance [50]. However, as the major microvascular complication of T2DM, the pathogenesis of DKD is complex and multifactorial. Traditionally, the initiation of DKD was thought to be induced mostly by hemodynamic changes and metabolic disorders. These alterations subsequently cause activation of the renin-angiotensin-aldosterone system (RAAS) [[51], [52], [53]], increased excretion of metabolic products, proinflammatory/profibrotic growth factors/chemo-cytokines, and dysregulation of a number of intracellular signalling cascades that are associated with oxidative stress [[54], [55], [56], [57], [58], [59]], inflammation [[60], [61], [62]], and fibrosis [[63], [64], [65], [66]], as well as the complement system [67]. On one hand, these local or circulating kidney events could themselves serve as independent risk factors for accelerating DKD progression. On the other hand, they also mediate cross-talk among podocyte-mesangium-endothelium-epithelium in diabetic kidneys [68]. These cell–cell communications ultimately contribute to irreversible kidney damage.
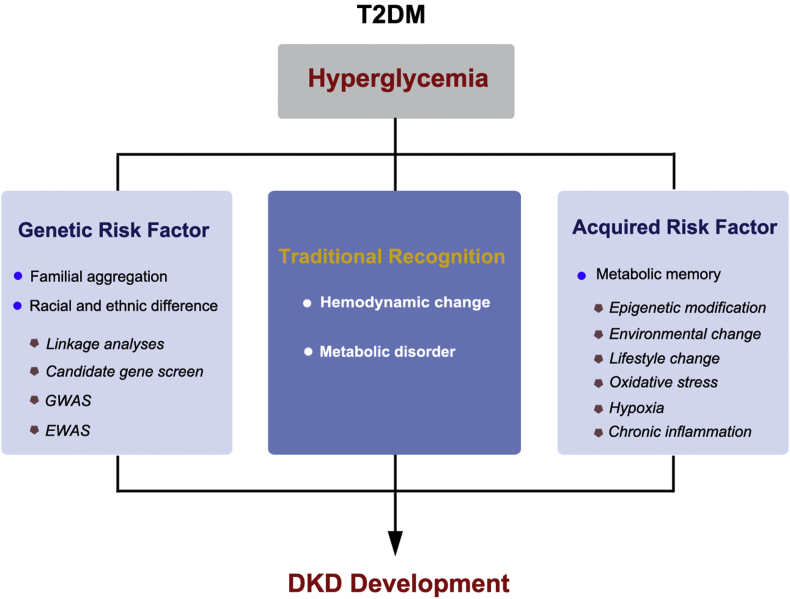
While kidney damage follows a typical course in the first two decades after the initial T1DM diagnosis [69,70], the clinical course of DKD in T2DM is far less predictable. The heterogeneity in the onset and progression of DKD is reflected in a lack of congruence among measures of glycemic control, the degree of albuminuria, the rate of decline in kidney function, and long-term clinical outcomes [71,72]. In recent years, a growing body of evidence has pointed to inherited factors together with an acquired factor of ‘metabolic memory’ playing an intricate role in the development of T2DM-induced DKD (Figure 2). It further enhances the understanding of the pathogenesis of DKD.
Figure 2.
The pathogenesis of DKD. Inherited and acquired risk factors have become hotspots in DKD research.
2.2.1. Genetic risk factors
From a genetic standpoint, diabetes can be classified into two categories: monogenic, including neonatal diabetes mellitus and maturity-onset diabetes of the young, and polygenic, including T1DM and T2DM. Until now, in the clinic, it was clear that T2DM-induced DKD does not develop in all T2DM patients, even those with poor long-term glycemic control and lifestyle intervention. It is noteworthy that diabetic patients who have a family history of hypertension or cardiovascular disease are more likely to develop DKD [[73], [74], [75], [76]]. This fact supports the idea that genetic factors may play central roles in the predisposition of T2DM-DKD. In T2DM-DKD, genetic susceptibility is mainly evidenced by familial aggregation, and the prevalence of DKD varies among different racial and ethnic groups [77]. In the 1980s, a pioneering genetic study of DKD was reported in a small study of families having two or more siblings diagnosed with T1DM [78]. The observation was soon replicated in other epidemiologic studies in both T1DM- and T2DM-DKD. Unfortunately, few of these initial research findings have remained positive, as knowledge and techniques of the genetic architecture underlying DKD have evolved. Over the past decades, from the application of traditional linkage analyses and candidate gene screen, to genome-wide association studies (GWAS), and now to the emerging next-generation sequencing (NGS) technology, tremendous progress in genome-wide studies has been observed to identify susceptible genetic loci or genes for dissecting DKD.
In early years, linkage analyses and candidate gene screen indeed identified a few T2DM risk genes, such as calpain 10 [79], transcription factor 7-like 2 [80], peroxisome proliferator-activated receptor gamma [81], insulin receptor substrate 1 [82,83], and angiotensin-converting enzyme [84,85]. It was found that the genetic peculiarity of T2DM was a high frequency of alleles with low or average effect for DKD predisposition [86]. Because the frequencies of these variants differ significantly among various ethnic groups, the population risk also differs [86]. However, the overall contributions of these approaches to the study of T2DM heritability remained underpowered, and some results were conflicting. For instance, different groups reported a discrepancy in the distribution of the angiotensin-converting enzyme genetic polymorphism between patients with and without nephropathy [[87], [88], [89]].
A significant advance in the study of genetic predisposition to diabetes and its complications was the use of GWAS [90,91]. GWAS was the first powerful tool for investigating the genetic architecture of human disease, and it has been proven to be extremely effective in identifying genetic loci that regulate the metabolic traits of T2DM and associated kidney disease [34]. To date, more than 100 genetic variants associated with T2DM and DKD have been identified [92]. These genes reveal the genetic architecture of T2DM and offer new insights into the pathogenesis of DKD, especially in the onset of T2DM, albuminuria, and declined renal function in various populations. Most recently, the largest GWAS of DKD in a population of European and Asian ancestry with T2DM reported that a novel signal near GABRR1 was associated with microalbuminuria in European T2DM case subjects; however, no replication of this signal was observed in Asian subjects with T2DM [93]. Certainly, GWAS has technique limitations, because it is based on the theory of an unbiased and hypothesis-free approach to scan for a ‘common variant,’ whereas it is possible that fewer common alleles also contribute to DKD. Moving forward, NGS approaches including whole-exome sequencing and whole-genome sequencing will help characterize the detailed genetic architecture of DKD by providing more information on common variants, rare variants, copy number variants, and the relationship between these changes for each individual.
Although the majority of these identified loci or genes have been robustly associated with the onset of DKD, they are unable to account for the heritability of DKD, which reflects the existence of risk factors beyond genetic sequence variations, such as epigenetics. Currently, rapid advances in epigenome-wide association studies by scanning the epigenetic modification of the whole genome have become strongly complementary to GWAS. Intriguingly, the epigenetic risk factor is recognized to be firmly linked to a critical acquired factor in the pathogenesis of DKD: metabolic memory.
2.2.2. Metabolic memory
The concept of ‘metabolic memory’ refers to an early glycemic environment that may cause long-term programming effects on retinopathy, nephropathy, and macrovascular diseases even with stringent metabolic controls in the years after diagnosed with diabetes. Patients can also benefit from intensive glucose controls in the occurrence of the above complications. It is reported that more than 5 years of intensive glucose control could significantly reduce the occurrence of DKD, retinopathy, and long-term microvascular complications in T2DM patients [94]. In contrast to genetic factors, metabolic memory, as an acquired risk factor, certainly originates from hyperglycemia in DKD. A 10-year follow-up study in T2DM patients found continued benefits of intensive glycemic control on micro- and macrovascular risks and death [95,96]. Over the years, increasing experimental and clinical evidence has supported this ‘legacy effect’ involving the consolidation of metabolic hints and transcriptional changes through historical mechanisms and epigenetic modifications [97,98].Epigenetics describes the study of heritable differences in gene expression without changing the underlying DNA sequence, which means a change occurred in phenotype without a change in genotype. At least three systems are involved in epigenetics, including DNA methylation, histone modification, and RNA interference [99]. In DKD, it is well known that epigenetic modifications affect gene expression related to ECM, transforming growth factor-β signaling pathway, RAAS system activation, and so on. In particular, the pathogenesis of DKD is associated with the interplay of non-enzymatic glycation of proteins, oxidative stress, chronic inflammation, hypoxia, and environmental and lifestyle changes. These pathologic factors also contribute to modifying the epigenetic profiles in the metabolic memory of DKD.
As the first epigenetic modification to be described decades ago, DNA methylation is the best-studied modification in transmitting epigenetic information. It is the process by which methyl groups are added to the DNA molecule. Cytosine and adenine, two of DNA's four bases, can be methylated. In T2DM, a most recent published clinical trial screened cytosine methylation levels at 397,063 genomic cytosine-phosphate-guanine sites to delineate the association between epigenetic modification and the estimated glomerular filtration rate (eGFR) change over a 6-year period in diabetic Pima Indians [100]. The results indicated that methylation levels at 77 sites were significantly associated with eGFR decline in blood leukocytes of DKD patients. Five of 77 regions showed an association between DNA methylation and changed gene expression and renal fibrosis [100]. This genome-wide, longitudinal study provides the first clues on the epigenetic link between lifestyle changes and the development of DKD in humans [100,101].
DNA methylation and histone modifications are highly interrelated and rely on each other mechanistically in maintaining normal chromatin functions [102]. Histone modification is a covalent posttranslational modification to histone proteins through multiple mechanisms including acetylation, methylation, phosphorylation, ubiquitylation, and sumoylation. In the field of T2DM, an increasing number of studies have shown that histone modifications are profoundly associated with metabolic memory, which could exacerbate diabetic complications. For example, the glycemic environment upregulates a proinflammatory gene that has been well recognized in the pathogenesis of DKD, thioredoxin-interacting protein (TXNIP) [103], through histone modifications. High glucose levels have persistent inhibitory effects on repressive histone markers (e.g., H3K9ac, H3K4me3, and H3K4me1) in the promoter of TXNIP [98,104]. In nondiabetic or new-onset T2DM kidneys, H3K23 acetylation, H3K4 dimethylation, and H3 phosphorylation at serine 10 are reduced. However, at serine 10, H3K9 and H3K23 acetylation, H3K4 dimethylation, and H3 phosphorylation are significantly increased in the advanced stage of DKD [105]. Modulation of plasma histone deacetylase activity and inflammatory status are at least partially related to exercise effects in T2DM patients [106]. This fresh evidence of epigenetic changes implies that the original changes in these markers might be due to the prior hyperglycemia. In DKD, it is clear that the modification of epigenetic status by metabolic memory and following microenvironmental changes plays a critical role in albuminuria change, renal function decline, and even more complex outcomes [98], and the features may be inherited through multiple generations.
3. DKD in the clinic: the arena to deal with the devil
Much of the basic research in DKD has offered a multitude of findings that are expected to be translated into revealing etiological mechanisms, supporting noninvasive diagnosis, or designing new strategies of DKD management. Yet, these individual findings are still far from being able to serve as clinical predictors that can counter the current recognition of glycemia, hyperlipidemia, obesity, aging, and other common complications of DKD. Therefore, providing a more comprehensive selection of factors for the diagnosis and management of DKD is a priority in the clinic.
3.1. Prevalence of DKD
Over the past few decades, the prevalence of T2DM has steadily increased worldwide. In 2017, the International Diabetes Federation (IDF) predicted that there were 451 million people with diabetes worldwide, and the number was expected to increase to 693 million by 2045 [12]. Among these diagnosed cases of diabetes, about 90% of patients have T2DM, and nearly half of T2DM patients eventually progress to chronic kidney disease (CKD) [16,[107], [108], [109]]. Impressively, it was estimated that, among people aged 20–79 years, 425 million had diabetes, 50% were undiagnosed, and approximately 4.0 million died, which accounted for 14.5% of global all-cause mortality among people in this age range [12,110]. In addition, the prevalence of diabetes in women is estimated to be 8.4%, which is slightly lower than the prevalence in men (9.1%). The top three countries or territories in terms of prevalence of diabetes are China, India, and the United States (Figure 3, http://www.diabetesatlas.org/) [12]. In China, DKD has surpassed glomerulonephritis and become the leading cause of ESRD. Specific to T2DM, about 35%–50% of patients will eventually develop various kidney damages defined as microalbuminuria, persistent albuminuria, renal function impairment, and others [16]. Although the overall prevalence of diabetes and its complications is increasing, reports have emerged indicating that the prevalence may be stabilizing in some DKD populations. In the United States, from 1988 to 2014, the overall prevalence of DKD among adults was kept at a relatively stable level, whereas the prevalence of albuminuria declined and the prevalence of reduced eGFR increased [6].
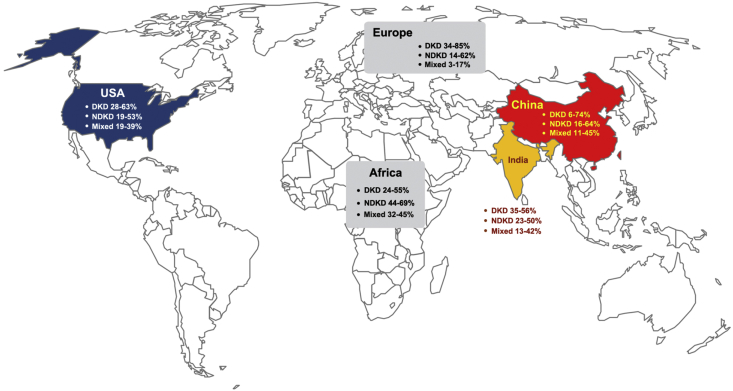
Figure 3.
Diagnostic data distributions of DKD, NDKD, or mixed forms in the clinic. The prevalence of DKD and NDKD varies among diabetes patients biopsied from different institutions worldwide. The diagnostic data of DKD in the top three countries (China, India, and the United States) of people with diabetes as well as the data of Europe and Africa are presented [35].
3.2. Challenges in the diagnosis and management of DKD
Apart from the updated understanding of the advances of the molecular signals involved in the development of DKD, physicians are focusing on two major concerns about DKD, optimizing the process of diagnosis and designing precise strategies for the management of DKD patients in the clinic. In general, the natural history of DKD could be divided into five stages: the initial increased GFR due to hyperfiltration, the ‘silent’ phase, the ‘incipient’ phase, the ‘overt’ phase, and eventual development of ESRD [111]. However, it is clear that not all T2DM patients exactly follow this classic pattern of the development of kidney complications. Based on the United Kingdom Prospective Diabetes Study (UKPDS) report, after a median of 15 years of follow-up after T2DM diagnosis, 38% of enrolled patients developed albuminuria and 29% developed renal dysfunction [112]. Impressively, of those participants who developed kidney impairment, 61% did not have preceding albuminuria and 39% never developed albuminuria during the study. It should be noted that some DKD patients present with impaired GFR and no albuminuria because the timing of T2DM onset in these patients is often unknown and early onset of DKD may reflect a long silent period. The potential GFR decline with age and albuminuria could be masked due to treatments of hypertension. Currently, although the overall prevalence of diabetes-associated kidney diseases is increasing, it is in part attributable to the presence of nondiabetic kidney diseases (NDKD) such as various primary or secondary forms of glomerulonephritis, which may not be suspected on the basis of clinical signs or urine abnormalities [35]. Therefore, CKD in a T2DM patient may represent true DKD, NDKD, or mixed forms of DKD and NDKD. Of note, the prevalence of DKD and NDKD varies significantly among T2DM-DKD patients receiving renal biopsy from different institutions worldwide (Figure 3) [35]. To some extent, these kinds of continuously evolving paradigms of development of kidney diseases in T2DM patients make it difficult for physicians to make a precise diagnosis of DKD. Renal biopsy remains the current most reliable approach to distinguish the above three entities. However, whether all T2DM patients need a kidney biopsy has become a tough topic in the field. A pooled meta-analysis which included 48 studies (4876 patients) found that NDKD (3–82.9%) is highly prevalent in patients with diabetes, and immunoglobulin A nephropathy is the most common NDKD [113]. The reality is that only a proportion of T2DM patients with kidney impairment are biopsied in the clinic, and an estimate of the rate of suspected DKD patients who receive renal biopsy is lacking. The T2DM-DKD patients who undergo renal biopsy constitute a heterogeneous group of renal damage. If the evidence were sufficient, physicians would be more willing to consider and analyze complex clinical factors, including family history, the existence of a variety of risk factors, and the onset timing of microalbuminuria, macroalbuminuria, anemia, and parathyroid hormone levels to aid the diagnosis rather than to adopt the method of invasive biopsy in suspect DKD patients. After all, as an invasive approach, renal biopsy is a real psychological burden for DKD patients. Furthermore, because of insulin deficiency, diabetic patients are more prone to anemia and bone and mineral metabolism disorders and enter the advanced stage of CKD earlier than patients with other types of CKD [[114], [115], [116]]. It is unfortunate that the time frame is difficult to determine the course of DKD, and monitoring the development of DKD based merely on pure clinical information might not be an ideal approach. Under this circumstance, seeking a noninvasive approach to aid DKD precise diagnosis is urgently needed.
In addition to the issues of diagnosis, another challenge is the management of DKD. In fact, a satisfying strategy for DKD management must be personalized. Recent research reported that T2DM patients could be stratified into five significantly different clusters based on six variables including glutamate decarboxylase antibodies, age at diagnosis, body mass index (BMI), Hemoglobin A1c (HbA1c), and homoeostatic model assessment 2 estimates of β-cell function and insulin resistance [117]. Among these five clusters, the individuals most resistant to insulin had higher risk to develop DKD. This finding was exciting step towards personalized medicine in the field. Meanwhile, the development of effective medications, precision nutrition, and systemic medical monitoring mechanisms are also critical for DKD management [33,118]. In the clinic, few effective drugs exist to hinder DKD progression. Based on more stringent metabolic controls of glycemia, blood pressure, body weight, and lipids, augmenting the efficacy of the renin-angiotensin system (RAS) blockade to ameliorate proteinuria is the current general consensus in DKD management [119]. Over the years, tremendous efforts have been made in seeking novel approaches that go beyond the current therapeutic strategies of DKD. Unfortunately, a number of randomized controlled trials (RCTs) for DKD have been terminated because of safety concerns or lack of efficacy; such trials included those using bardoxolone methyl [120], vitamin D receptor activators [121], and AGE inhibitors [[122], [123], [124]] among other treatments. Despite the incredibly high failure rate of new approaches (>90% overall and 50% in phase III trials) [119,125], ongoing trials are offering hopes for the field. Two newly emerged agents, sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists, have been incorporated into clinical practice for their renoprotective capacity in reducing glycemia, blood pressure, body weight, albuminuria, and GFR decline in T2DM patients, especially in those patients in moderate to severe stages of DKD [[126], [127], [128], [129], [130], [131], [132]]. Mechanistically, SGLT2 is a low-affinity, high capacity glucose transporter distributed in renal proximal tubules. It is responsible for 90% of glucose reabsorption [133]. Therefore, application of an SGLT2 inhibitor could block glucose reabsorption in diabetic kidneys to produce renoprotective potential in DKD patients. Meanwhile, SGLT2 inhibitors are able to increase insulin sensitivity, improve insulin release from β cells at the first phase, reduce SGLT2-mediated glucose uptake in smooth muscle cells, and decrease gluconeogenesis. In comparison, as a type of incretin-based medicine, GLP-1 receptor agonists act on the pancreas in order to provide indirect renal protection by improving blood glucose, blood pressure, and body weight control [134,135].
As illustrated in Table 1, recent landmark achievements in DKD clinical trials including Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) [132], Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) [136], Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) [137], and Dulaglutide With Insulin Glargine on Glycemic Control in Participants With Type 2 Diabetes and Moderate or Severe Chronic Kidney Disease (AWARD-7) [130] revealed that SGLT-2 inhibitors and GLP-1 receptor agonists were capable of improving long term renal outcomes of DKD in the clinic. The latest version of “the Standards of Medical Care in Diabetes” issued by the American Diabetes Association have graded the application of SGLT-2 inhibitors in T2DM-DKD treatment as evidence A (https://care.diabetesjournals.org/living-standards#June 3). In CREDENCE, after a median follow-up of 2.62 years, the relative risk of the primary outcome was 30% lower in 2202 patients received canagliflozin, compared with 2199 patients assigned to the placebo group [132]. Consistently, Empaglifozin in EMPA-REG Outcome also exhibits its capacity in reducing incident or worsening nephropathy (39%), progression to macroalbuminuria (38%), doubling of serum creatinine (44%), and risk in renal replacement therapy (55%) in DKD patients, compared with the placebo group [136]. In other words, SGLT2 inhibitors presented stronger renoprotective abilities than GLP-1 receptor agonists, Dipeptidyl peptidase 4, and Endothelin Receptor Antagonist in DKD treatment by thus far (Table 1). Nevertheless, the side effects and long-term safety of SGLT2 inhibitors or GLP-1 receptor agonists are needed to be further assessed [138]. In addition, several ongoing phase III trials include the studies focus on the efficacy of SGLT2 inhibitors (DAPA-CKD and EMPA-Kidney) and GLP1 receptor agonists (FLOW) are also expected to contribute to DKD prevention (https://clinicaltrials.gov/ct2/home).
Table 1.
The Selected Landmark Achievements of the Clinical Trials in Diabetic Kidney Disease.
| Name of the Trials | Tested Drugs | Brief Description of the Trial | Renal Outcomes | Ref. |
|---|---|---|---|---|
| SGLT2 Inhibitor | ||||
| CREDENCE | Canaglifozin | Patients recruited: 4,401 (690 sites, 34 countries); Median Follow-up:2.62years; eGFR:30 to <90 ml/min/1.73 m2; Urinary Albumin/Creatinine Ratio (UACR): 300–5000 mg/g; HbA1c:6.5–12%; Established cardiovascular disease; Treated with RAS blocker. |
30% lower relative risk of the primary outcome (a composite of ESRD, a doubling of the serum creatinine level, or death from renal or cardiovascular causes): 43.2 vs. 61.2 per 1000 patient-years | [132] |
| EMPA-REG OUTCOME | Empaglifozin | Patients recruited: 7,020 (590 sites, 42 countries); Median duration of treatment: 2.6 years; Median observation time: 3.1 years; eGFR:≥30 ml/min/1.73 m2; Established cardiovascular disease. |
39% relative risk reduction of incident or worsening nephropathy:12.7% vs. 18.8%; 38% relative risk reduction of progression to macroalbuminuria:11.2% vs. 16.2%; 44% relative risk reduction of doubling of Scr: 1.5% vs. 2.6%; 55% lower relative risk in replacement therapy: 0.3% vs. 0.6%. |
[136] |
| GLP-1 Receptor Agonist | ||||
| LEADER | Liraglutide | Patients recruited: 9,340; Median Follow-up:3.84years; eGFR: ≥30 ml/min/1.73 m2; A high risk of cardiovascular disease. |
Lower incidents of the renal outcome (new-onset persistent macroalbuminuria, persistent doubling of the Scr level and eGFR of 45 ml or less per min/1.73 m2, need renal-replacement therapy, or death):5.7% vs. 7.2%; New-onset persistent macroalbuminuria: 3.4% vs. 4.6%; eGFR decline:7.44 vs. 7.82 ml/min/1.73 m2. | [137] |
| AWARD-7 | Dulaglutide | Patients recruited: 577 (99 sites, 9 countries); Duration of treatment: 52 weeks; HbA1c:7.5–10.5%; Moderate-to-severe CKD; Treated with insulin or insulin plus an oral antihyperglycemic drug, RAS blocker, GLP-1 receptor agonist or DPP4 inhibitor. |
HbA1c-lowering effects persisted to 52 weeks; eGFR was higher at 52 weeks; No significant difference on UA reduction. |
[130] |
| Dipeptidyl Peptidase 4 (DPP-4) Inhibitor | ||||
| CARMELINA | Linagliptin | Patients recruited: 6,979 (605 sites, 27 countries); Median Follow-up:2.2years; HbA1c:7.5–10.5%; High CV risk and renal risk. |
No significant difference in kidney composite outcome: 9.4% vs. 8.8%. | [165] |
| SAVOR-TIMI 53 | Saxagliptin | Patients recruited: 16,492 (25 countries); Median Follow-up:2.1years; HbA1c:6.5–12%; Established cardiovascular disease. |
Improvement in and/or less deterioration in ACR, without affecting eGFR. | [166] |
| Selective Endothelin Receptor Antagonist | ||||
| SONAR | Atrasentan | Patients recruited: 2,648 (689 sites, 41 countries); Median Follow-up:2.2years; eGFR:25–75 ml/min/1.73 m2; Urinary Albumin/Cr: 300–5000 mg/g; Treated with RAS blocker at least 4 weeks. | Lower primary composite renal endpoint event: 6.0% vs. 7.9%; | [167] |
Of particular interest, a recent RCT of patients within 10 years of diagnosis of T2DM indicated that a lifestyle intervention actually could not meet the criterion for equivalence of glycemic control with standard care, although it did provide some benefits for DKD [139]. This conclusion does not mean that lifestyle change is not an important element in DKD prevention, but it indeed reflects the complexity and importance of establishing a precise DKD management system comprises monitoring, analysis, and translation sections to address the gap of translational, precision, and personalized medicine.
4. The promise of systems biology linking to translational medicine in DKD: one world, one dream
Despite the abundant information we have gained about single molecule in the regulation of DKD, we still do not completely understand how the renal system responds to DKD development and progression and could not resolve the existing conflicts. A major reason is that the current molecular reductionist approach is insufficient to illustrate the complex physiology of kidney as a whole system, although it is necessary to attain all the basic information about DKD. In the clinic, physicians indeed have difficulties in screening patients regularly with eGFR, urine albumin/creatinine ratio, and potential biomarkers, although it was recommended in all guidelines. Thus, identifying patients at risk or with disease is a problem even with the simple tools applied today. The field has realized the importance of constructing an integrative bridge to link systems biology to the current medical models including translational, precision, and personalized medicine, by which to systemically analyze the complicated spatial–temporal intercellular organization of DKD. The purpose of this connection is to identify novel non-invasive biomarkers for DKD diagnosis as well as to develop more effective therapeutic targets to slow down DKD development.
In general, two approaches, called bottom-up and top-down, are employed in systems biology for the understanding of complex systems within living organisms [140]. In the field of biomedicine, the bottom-up approach to systems biology normally starts with small regulatory networks based on the known chemical reactions and regulations in the biological systems. Through building sophisticated mathematical models based on these networks, the bottom-up approach is responsible for explaining current findings, resolving conflicts, and making new predictions that could provide comprehensive mechanisms for the systems and novel treatment designs for diseases. However, few studies using the bottom-up approach have been reported in DKD related research. In comparison, the top-down approach to systems biology is popularly applied in numerous biomedical studies. In principle, it starts with high-throughput OMICS data (e.g. transcriptomics, proteomics, and metabolomics) and ends with a very large, complex molecular regulatory/interaction network through analysis using appropriate bioinformatics methodologies [26].
Specifically, a typical function of linking the top-down approach to translational DKD research is biomarker identification, especially for the diagnosis of incipient DKD. As presented in Table 2, several well-known biomarkers such as Kidney Injury Molecule 1 (KIM-1), Neutrophil Gelatinase-Associated Lipocalin (NGAL), Monocyte Chemoattractant Protein-1 (MCP-1), Epidermal Growth Factor (EGF), N-acetyl-β-d-glucosaminidase (NAG), β2-microglobulin, Complement 7, IgG4, and Smad1 have already been identified as early signals of DKD by traditional experimental methods in various clinical cohorts studies over the past years [[141], [142], [143], [144], [145], [146], [147], [148], [149]]. However, few of these biomarkers have been truly implemented in the clinic so far because the specificity and sensitivity of most of these biomarkers lack rigorous external clinical validation. Microalbuminuria is still currently the most reliable predictor of DKD [150]. In recent years, advances in microarray and mass spectrometry have enhanced our capacity in identifying thousands of RNAs or proteins in a single experiment [[151], [152], [153], [154]]. A number of identified novel microRNAs or proteins, such as miR27-3b/1228-3p [155], α1-Antitrypsin [156], Transferrin [157], Haptoglobin [158]. and Vitamin D-binding protein [159] not only reflect defects in the glomerulus and tubules in diabetic kidneys [160], but also serve as tools to differentiate uncomplicated diabetes, incipient DKD, and overt DKD in the clinical setting [161]. Along with rapidly evolving metabolomics, large-scale analysis of small molecules to be interrogated in a targeted or untargeted manner has become a reality [29,162,163]. Our unpublished serum metabolomics data show a distinct profile of metabolites at different stages of T2DM-DKD, providing a means by which to monitor DKD development by using specific serum/urinary biomarkers rather than invasive biopsy. In particular, multi-OMICS combinational analyses and sophisticated computational algorithms allow scientists and physicians to identify the most accurate molecules, proteins, or metabolites to manage DKD in this new era.
Table 2.
List of Selected Promising Biomarkers for Incipient Diabetic Kidney Disease in Human.
| Biomarkers | Methods | Direction of Excretion | Biological Mechanisms | Ref. |
|---|---|---|---|---|
| KIM-1 | TEM | ↑(serum, urine) | Predicting renal function decline and prior to the changes of eGFR | [142,143] |
| NGAL | TEM | ↑(plasma, urine) | Tubular Damage in DKD | [144,147] |
| NAG | TEM | ↑(urine) | Predicting the severity of DKD | [149] |
| MCP-1 | TEM | ↑(serum, urine) | Promoting kidney local microenvironment | [168,169] |
| EGF/MCP-1 ratio | TEM | ↓(urine) | Tubular cell survival factor | [141] |
| Complement 7 | MiA | ↑(serum, kidney) | Early warning signal of DKD | [148] |
| miR126,155,29b | MiA | ↑ (urine, kidney) | Regulating response to proinflammatory molecules | [152] |
| miR362-3P,877-3P,150-5P | MiA | ↑ (urinary exosome) | miRNA candidates in incipient T2DM-DKD | [151] |
| miR15a-5P | MiA | ↓(urinary exosome) | miRNA candidates in incipient T2DM-DKD | [151] |
| miR27-3b,1228-3p | MiA | ↓ (urine, kidney) | Discriminating DKD from other nephritis in T2DM patient | [155] |
| Haptoglobin | Pro | ↑(urine) | Early indicator of DKD | [154] |
| AMBP | Pro | ↑(urine) | Proximal tubular dysfunction in DKD | [154] |
| TGOLN2 | Pro | ↑(serum) | Distinguishing T2DM and T2DM-DKD | Zhou unpublished |
| 7-methyluric acid | Metab | ↓ (urine) | Distinguishing T2DM and T2DM-DKD | [163] |
| Xanthosine | Metab | ↓ (urine) | Distinguishing T2DM and T2DM-DKD | [163] |
| Gluconic acid | Metab | ↑(serum) | Predicting the severity of DKD | Zhou unpublished |
Abbreviations: AMBP: α-1-microglobulin/bikunin precursor; TGOLN2: Trans-golgi network protein 2; TEM: traditional experimental method; MiA: microarray; Pro: proteomics; Metab: metabolomics.
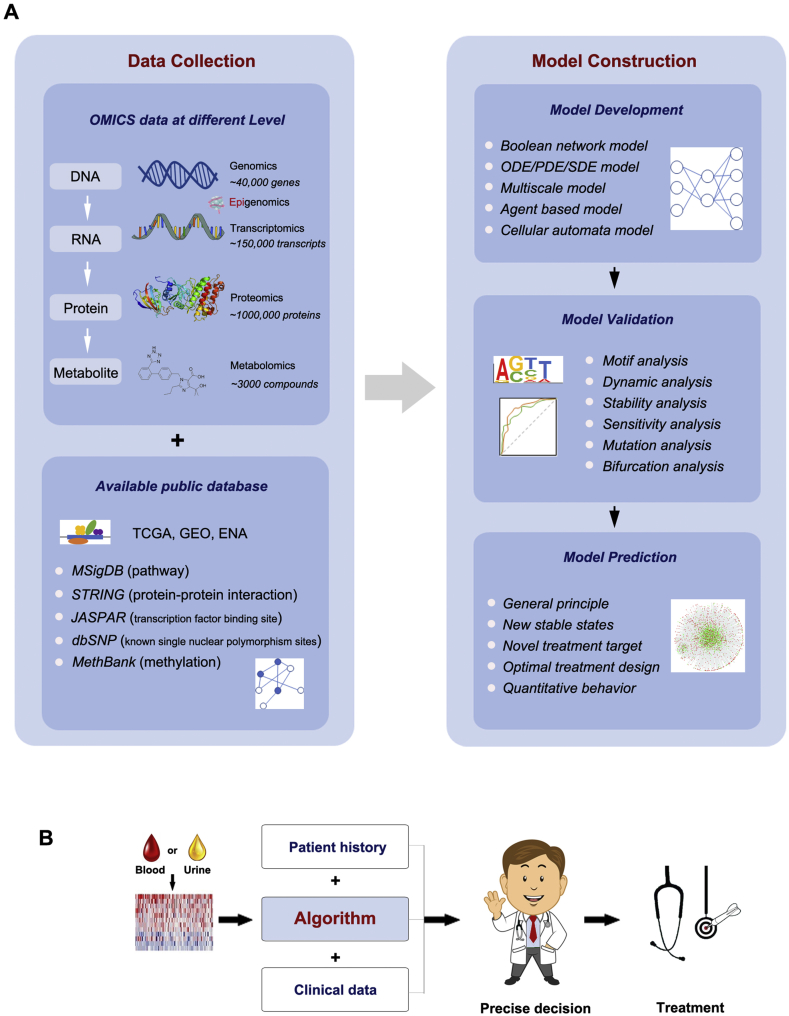
As we enter the ‘big data’ era, it is the right time for the confluence of the bottom-up and top-down approaches in the systems biology for DKD research (Figure 4). With the rapid development of experimental high-throughput techniques, big data is generated in an explosive way daily, but only a small part of it is analyzed and truly used in DKD related research. The paradigm shows how to converge the different approaches in DKD research. Diverse data such as OMICS databases (e.g. Gene Expression Omnibus, GEO; The Cancer Genome Atlas, TCGA), and software (e.g. Ingenuity Pathway Analysis, IPA; String Protein–Protein Interaction Networks, STRING) will be integrated and the data divided into four sets: initiation set, training set, test set, and validation set. The initiation set will be used to build models for DKD and train the model with the training set by searching the high-dimensional parameter space using sophisticated algorithms such as machine learning or the Metropolis–Hastings algorithm [164]. The models will be validated and refined with the validation set, and the predictions generated from the model will be verified with the test set. The goal is to find the self-consistent closure of these bottom-up and top-down analyses to understand the mechanism of the renal system and the misregulations in DKD. Although it will not be a short-term challenge, the clinical application of big data will likely be a milestone of translational, precision, and personalized medicine triumphs for DKD [29].
Figure 4.
Systems biology links to translational medicine lighting the way to prevent DKD. Schematic diagrams show (A) the methods of big data collection, including OMICS data at different levels in available public database, and precise model construction, including model development, validation, and prediction in the current systems biology medical research and (B) the translational applications in the clinic. GEO, Gene Expression Omnibus; TCGA, The Cancer Genome Atlas; ENA, European Nucleotide Archive; MSigDB, Molecular Signatures Database; STRING, String Protein–Protein Interaction Networks; dbSNP: Single Nucleotide Polymorphism Database; MethBank, Methylation Bank; ODE, Ordinary Differential Equation; PDE, Partial Differential Equation; SDE, Stochastic Differential Equations.
5. Summary
DKD is undoubtedly a worldwide medical catastrophe, with features of high prevalence, multifactorial pathogenesis, and lack of effective strategies in the treatment and management. Beyond a traditional understanding of the pathogenesis of hemodynamic changes and metabolic disorders, more attention is now paid to genetic risk factors and epigenetic modification in DKD development. Emerging valuable tools are ready for constructing multiple bridges among translational, precision, and personalize medicine of DKD in the clinic. However, translating these findings or recognition into the clinic and performing large-scale RCTs to validate identified biomarkers are the urgent missions that should be facilitated by physicians and scientists worldwide. We believe that new conceptions and novel techniques will light our way in efforts to prevent DKD in this new era.
Author contributions
All authors contributed equally to researching the data for the article and to discussions of the content. F.H. and Z.D. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Acknowledgments
We apologize to our colleagues whose important findings could not be cited in this article because of space limitations. Reviews were often cited at the expense of original work. This work is supported by National Institutes of Health grant 1K01DK116816 (to Zhou D) and National Science Foundation grant EF-1921412 (to Tian X-J). Fu H is supported by National Natural Science Foundation of China Grants 81970587 and 81770737. Fu H is the recipient of the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University, China. (2015J006).
Conflict of interest
None declared.
References
- 1.Thomas M.C., Brownlee M., Susztak K., Sharma K., Jandeleit-Dahm K.A., Zoungas S. Diabetic kidney disease. Nature Reviews Disease Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerg L., Hulman A., Carstensen B., Charles M., Jorgensen M.E., Witte D.R. Development of microvascular complications and effect of concurrent risk factors in type 1 diabetes: a multistate model from an observational clinical cohort study. Diabetes Care. 2018;41:2297–2305. doi: 10.2337/dc18-0679. [DOI] [PubMed] [Google Scholar]
- 3.Valencia W.M., Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ British Medical Journal. 2017;356 doi: 10.1136/bmj.i6505. [DOI] [PubMed] [Google Scholar]
- 4.Cameron J.S. The discovery of diabetic nephropathy: from small print to centre stage. Journal of Nephrology. 2006;19(Suppl 10):S75–S87. [PubMed] [Google Scholar]
- 5.Alicic R.Z., Rooney M.T., Tuttle K.R. Diabetic kidney disease: challenges, progress, and possibilities. Clinical Journal of the American Society of Nephrology. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afkarian M., Zelnick L.R., Hall Y.N., Heagerty P.J., Tuttle K., Weiss N.S. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low S.K.M., Sum C.F., Yeoh L.Y., Tavintharan S., Ng X.W., Lee S.B.M. Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Annals Academy of Medicine Singapore. 2015;44:164–171. [PubMed] [Google Scholar]
- 8.Thomas B., van Pelt M., Mehrotra R., Robinson-Cohen C., LoGerfo J. An estimation of the prevalence and progression of chronic kidney disease in a rural diabetic cambodian population. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas M.C., Weekes A.J., Broadley O.J., Cooper M.E., Mathew T.H. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Medical Journal of Australia. 2006;185:140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 10.Prasannakumar M., Rajput R., Seshadri K., Talwalkar P., Agarwal P., Gokulnath G. An observational, cross-sectional study to assess the prevalence of chronic kidney disease in type 2 diabetes patients in India (START -India) Indian J Endocrinol Metab. 2015;19:520–523. doi: 10.4103/2230-8210.157857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Wang F., Wang L., Wang W., Liu B., Liu J. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Gross M.L., Dikow R., Ritz E. Diabetic nephropathy: recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney International. 2005;67:S50–S53. doi: 10.1111/j.1523-1755.2005.09412.x. [DOI] [PubMed] [Google Scholar]
- 14.Ritz E., Rychlik I., Locatelli F., Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. American Journal of Kidney Diseases. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang W.Y., Dall T.M., Beronjia K., Lin J., Semilla A.P., Chakrabarti R. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41:917–928. [Google Scholar]
- 16.Saran R., Robinson B., Abbott K.C., Agodoa L.Y.C., Bhave N., Bragg-Gresham J. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. American Journal of Kidney Diseases. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alva M.L., Gray A., Mihaylova B., Leal J., Holman R.R. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84) Diabetic Medicine. 2015;32:459–466. doi: 10.1111/dme.12647. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M.C., Cooper M.E., Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nature Reviews Nephrology. 2016;12:73–81. doi: 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 19.Bommer C., Heesemann E., Sagalova V., Manne-Goehler J., Atun R., Barnighausen T. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endo. 2017;5:423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 20.Pradeepa R., Mohan V. Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. European Journal of Clinical Nutrition. 2017;71:816–824. doi: 10.1038/ejcn.2017.40. [DOI] [PubMed] [Google Scholar]
- 21.Men P., Liu T., Chu Y., Zhai S. Cost-effectiveness of empagliflozin in Chinese patients with type 2 diabetes and established cardiovascular disease: a discrete event simulation economic modelling study. Value in Health. 2018;21:S74. [Google Scholar]
- 22.KDOQI KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. American Journal of Kidney Diseases. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney F. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. American Journal of Kidney Diseases. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y.C., Chang Y.H., Yang S.Y., Wu K.D., Chu T.S. Update of pathophysiology and management of diabetic kidney disease. Journal of the Formosan Medical Association. 2018;117:662–675. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Gluck C., Ko Y.A., Susztak K. Precision medicine approaches to diabetic kidney disease: tissue as an issue. Current Diabetes Reports. 2017;17:30. doi: 10.1007/s11892-017-0854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komorowsky C.V., Brosius F.C., Pennathur S., Kretzler M. Perspectives on systems biology applications in diabetic kidney disease. Journal of Cardiovascular Translational. 2012;5:491–508. doi: 10.1007/s12265-012-9382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Y., Moradi H., Kalantar-Zadeh K. Emerging paradigms of treating diabetic nephropathy. Lancet Endocrinology and Diabetes. 2018;6:912–913. doi: 10.1016/S2213-8587(18)30304-8. [DOI] [PubMed] [Google Scholar]
- 28.Afkarian M., Zelnick L.R., Ruzinski J., Kestenbaum B., Himmelfarb J., de Boer I.H. Urine matrix metalloproteinase-7 and risk of kidney disease progression and mortality in type 2 diabetes. Journal of Diabetic Complications. 2015;29:1024–1031. doi: 10.1016/j.jdiacomp.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darshi M., Van Espen B., Sharma K. Metabolomics in diabetic kidney disease: unraveling the biochemistry of a silent killer. American Journal of Nephrology. 2016;44:92–103. doi: 10.1159/000447954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You Y.H., Quach T., Saito R., Pham J., Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. Journal of the American Society of Nephrology. 2016;27:466–481. doi: 10.1681/ASN.2015030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Li C., Gong W., Lou T. New urinary biomarkers for diabetic kidney disease. Biomark Research. 2013;1:9. doi: 10.1186/2050-7771-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKnight A.J., McKay G.J., Maxwell A.P. Genetic and epigenetic risk factors for diabetic kidney disease. Advances in Chronic Kidney Disease. 2014;21:287–296. doi: 10.1053/j.ackd.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Gloyn A.L., Drucker D.J. Precision medicine in the management of type 2 diabetes. Lancet Endocrinology and Diabetes. 2018;6:891–900. doi: 10.1016/S2213-8587(18)30052-4. [DOI] [PubMed] [Google Scholar]
- 34.Ahlqvist E., van Zuydam N.R., Groop L.C., McCarthy M.I. The genetics of diabetic complications. Nature Reviews Nephrology. 2015;11:277–287. doi: 10.1038/nrneph.2015.37. [DOI] [PubMed] [Google Scholar]
- 35.Anders H.J., Huber T.B., Isermann B., Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nature Reviews Nephrology. 2018;14:361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 36.Reidy K., Kang H.M., Hostetter T., Susztak K. Molecular mechanisms of diabetic kidney disease. Journal of Clinical Investigation. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimmelstiel P., Wilson C. Intercapillary lesions in the glomeruli of the kidney. American Journal of Pathology. 1936;12:83–98.7. [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert R.E. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes. 2017;66:791–800. doi: 10.2337/db16-0796. [DOI] [PubMed] [Google Scholar]
- 39.Humphreys B.D. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Seminars in Nephrology. 2012;32:463–470. doi: 10.1016/j.semnephrol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto Y., Yamagishi S., Mizukami H., Yabe-Nishimura C., Lim S.W., Kwon H.M. Polyol pathway and diabetic nephropathy revisited: early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase. Journal of Diabetes and Investigations. 2011;2:111–122. doi: 10.1111/j.2040-1124.2010.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas M.C., Burns W.C., Cooper M.E. Tubular changes in early diabetic nephropathy. Advances in Chronic Kidney Disease. 2005;12:177–186. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 42.White K.E., Marshall S.M., Bilous R.W. Prevalence of atubular glomeruli in type 2 diabetic patients with nephropathy. Nephrology Dialysis Transplantation. 2008;23:3539–3545. doi: 10.1093/ndt/gfn351. [DOI] [PubMed] [Google Scholar]
- 43.Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circulation Research. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 44.Ferland-McCollough D., Slater S., Richard J., Reni C., Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacology & Therapeutics. 2017;171:30–42. doi: 10.1016/j.pharmthera.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasi U.S.S., Sindhu G., Raj P.S., Raghu K.G. Mitochondria associated membranes (MAMs): emerging drug targets for diabetes. Current Medicinal Chemistry. 2019 doi: 10.2174/0929867326666190212121248. [DOI] [PubMed] [Google Scholar]
- 46.Sharma K., Karl B., Mathew A.V., Gangoiti J.A., Wassel C.L., Saito R. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. Journal of the American Society of Nephrology. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czajka A., Ajaz S., Gnudi L., Parsade C.K., Jones P., Reid F. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBio Medicine. 2015;2:499–512. doi: 10.1016/j.ebiom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindenmeyer M.T., Rastaldi M.P., Ikehata M., Neusser M.A., Kretzler M., Cohen C.D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. Journal of the American Society of Nephrology. 2008;19:2225–2236. doi: 10.1681/ASN.2007121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brosius F.C., Kaufman R.J. Is the ER stressed out in diabetic kidney disease? Journal of the American Society of Nephrology. 2008;19:2040–2042. doi: 10.1681/ASN.2008090959. [DOI] [PubMed] [Google Scholar]
- 50.Barlow A.D., Thomas D.C. Autophagy in diabetes: beta-cell dysfunction, insulin resistance, and complications. DNA and Cell Biology. 2015;34:252–260. doi: 10.1089/dna.2014.2755. [DOI] [PubMed] [Google Scholar]
- 51.Gurley S.B., Coffman T.M. The renin-angiotensin system and diabetic nephropathy. Seminars in Nephrology. 2007;27:144–152. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Molnar A., Koszegi S., Lenart L., Hodrea J., Balogh D., Szkibinszkij E. The renin-angiotensin-aldosterone system inhibitors ameliorate hyperglycaemia induced tubulointersitial fibrosis in diabetic nephropathy. Nephrology Dialysis Transplantation. 2018;33:179. [Google Scholar]
- 53.Rahimi Z. The role of renin angiotensin aldosterone system genes in diabetic nephropathy. Canadian Journal of Diabetes. 2016;40:178–183. doi: 10.1016/j.jcjd.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Sharavana G., Joseph G.S., Baskaran V. Lutein attenuates oxidative stress markers and ameliorates glucose homeostasis through polyol pathway in heart and kidney of STZ-induced hyperglycemic rat model. European Journal of Nutrition. 2017;56:2475–2485. doi: 10.1007/s00394-016-1283-0. [DOI] [PubMed] [Google Scholar]
- 55.Kumar Pasupulati A., Chitra P.S., Reddy G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomolecular Concepts. 2016;7:293–309. doi: 10.1515/bmc-2016-0021. [DOI] [PubMed] [Google Scholar]
- 56.Buse M.G. Hexosamines, insulin resistance, and the complications of diabetes: current status. American Journal of Physiology, Endocrinology and Metabolism. 2006;290:E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geraldes P., King G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation Research. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Idris-Khodja N., Ouerd S., Mian M.O.R., Gornitsky J., Barhoumi T., Paradis P. Endothelin-1 overexpression exaggerates diabetes-induced endothelial dysfunction by altering oxidative stress. American Journal of Hypertension. 2016;29:1245–1251. doi: 10.1093/ajh/hpw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y.N., Zhou J.W., Li T.T., Wu J., Xie S.H., Liu H.F. Sulodexide protects renal tubular epithelial cells from oxidative stress-induced injury via upregulating klotho expression at an early stage of diabetic kidney disease. Journal of Diabetes Research. 2017;2017:4989847. doi: 10.1155/2017/4989847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrero M.B., Banes-Berceli A.K., Stern D.M., Eaton D.C. Role of the JAK/STAT signaling pathway in diabetic nephropathy. American Journal of Physiology – Renal Physiology. 2006;290:F762–F768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 61.Anderberg R.J., Meek R.L., Hudkins K.L., Cooney S.K., Alpers C.E., Leboeuf R.C. Serum amyloid A and inflammation in diabetic kidney disease and podocytes. Laboratory Investigation. 2015;95:250–262. doi: 10.1038/labinvest.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suryavanshi S.V., Kulkarni Y.A. NF-kappabeta: a potential target in the management of vascular complications of diabetes. Frontiers in Pharmacology. 2017;8:798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deshpande S.D., Putta S., Wang M., Lai J.Y., Bitzer M., Nelson R.G. Transforming growth factor-beta-induced cross talk between p53 and a MicroRNA in the pathogenesis of diabetic nephropathy. Diabetes. 2013;62:3151–3162. doi: 10.2337/db13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L., Chen L., Zang J., Tang X., Liu Y., Zhang J. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64:597–610. doi: 10.1016/j.metabol.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Li S.Y., Huang P.H., Tarng D.C., Lin T.P., Yang W.C., Chang Y.H. Four-and-a-Half LIM domains protein 2 is a coactivator of Wnt signaling in diabetic kidney disease. Journal of the American Society of Nephrology. 2015;26:3072–3084. doi: 10.1681/ASN.2014100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn S.H., Susztak K. Getting a notch closer to understanding diabetic kidney disease. Diabetes. 2010;59:1865–1867. doi: 10.2337/db10-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nature Reviews Nephrology. 2017;13:311–318. doi: 10.1038/nrneph.2017.31. [DOI] [PubMed] [Google Scholar]
- 68.Hasegawa K., Wakino S., Simic P., Sakamaki Y., Minakuchi H., Fujimura K. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nature Medicine. 2013;19:1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hovind P., Tarnow L., Rossing P., Jensen B.R., Graae M., Torp I. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katsarou A., Gudbjornsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J. Type 1 diabetes mellitus. Nature Reviews of Disabilities in Primers. 2017;3:17016. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 71.Florez J.C. Genetics of diabetic kidney disease. Seminars in Nephrology. 2016;36:474–480. doi: 10.1016/j.semnephrol.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Ma R.C., Cooper M.E. Genetics of diabetic kidney disease-from the worst of nightmares to the light of dawn? Journal of the American Society of Nephrology. 2017;28:389–393. doi: 10.1681/ASN.2016091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeung E.H., Pankow J.S., Astor B.C., Powe N.R., Saudek C.D., Kao W.H. Increased risk of type 2 diabetes from a family history of coronary heart disease and type 2 diabetes. Diabetes Care. 2007;30:154–156. doi: 10.2337/dc06-1463. [DOI] [PubMed] [Google Scholar]
- 74.Fava S., Hattersley A.T. The role of genetic susceptibility in diabetic nephropathy: evidence from family studies. Nephrology Dialysis Transplantation. 2002;17:1543–1546. doi: 10.1093/ndt/17.9.1543. [DOI] [PubMed] [Google Scholar]
- 75.Tedla F.M., Brar A., Browne R., Brown C. Hypertension in chronic kidney disease: navigating the evidence. International Journal of Hypertension. 2011;2011:132405. doi: 10.4061/2011/132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canani L.H., Gerchman F., Gross J.L. Increased familial history of arterial hypertension, coronary heart disease, and renal disease in Brazilian type 2 diabetic patients with diabetic nephropathy. Diabetes Care. 1998;21:1545–1550. doi: 10.2337/diacare.21.9.1545. [DOI] [PubMed] [Google Scholar]
- 77.Davis T.M.E., Coleman R.L., Holman R.R., Grp U. Ethnicity and long-term vascular outcomes in Type 2 diabetes: a prospective observational study (UKPDS 83) Diabetic Medicine. 2014;31:200–207. doi: 10.1111/dme.12353. [DOI] [PubMed] [Google Scholar]
- 78.Seaquist E.R., Goetz F.C., Rich S., Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. New England Journal of Medicine. 1989;320:1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- 79.Horikawa Y. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus(vol26, pg 163-175, 2000) Nature Genetics. 2000;26:502. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 80.Grant S.F.A., Thorleifsson G., Reynisdottir I., Benediktsson R., Manolescu A., Sainz J. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nature Genetics. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 81.Mori Y., Kim-Motoyama H., Katakura T., Yasuda K., Kadowaki H., Beamer B.A. Effect of the Pro12Ala variant of the human peroxisome proliferator-activated receptor gamma 2 gene on adiposity, fat distribution, and insulin sensitivity in Japanese men. Biochemical and Biophysical Research Communications. 1998;251:195–198. doi: 10.1006/bbrc.1998.9421. [DOI] [PubMed] [Google Scholar]
- 82.Clausen J.O., Hansen T., Bjorbaek C., Echwald S.M., Urhammer S.A., Rasmussen S. Insulin resistance: interactions between obesity and a common variant of insulin receptor substrate-1. Lancet. 1995;346:397–402. doi: 10.1016/s0140-6736(95)92779-4. [DOI] [PubMed] [Google Scholar]
- 83.Imai Y., Fusco A., Suzuki Y., Lesniak M.A., D'Alfonso R., Sesti G. Variant sequences of insulin receptor substrate-1 in patients with noninsulin-dependent diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 1994;79:1655–1658. doi: 10.1210/jcem.79.6.7989470. [DOI] [PubMed] [Google Scholar]
- 84.Tarnow L., Gluud C., Parving H.H. Diabetic nephropathy and the insertion/deletion polymorphism of the angiotensin-converting enzyme gene. Nephrology Dialysis Transplantation. 1998;13:1125–1130. doi: 10.1093/ndt/13.5.1125. [DOI] [PubMed] [Google Scholar]
- 85.Kunz R., Bork J.P., Fritsche L., Ringel J., Sharma A.M. Association between the angiotensin-converting enzyme-insertion/deletion polymorphism and diabetic nephropathy: a methodologic appraisal and systematic review. Journal of the American Society of Nephrology. 1998;9:1653–1663. doi: 10.1681/ASN.V991653. [DOI] [PubMed] [Google Scholar]
- 86.Vikulova O.K., Zheleznyakova A.V., Lebedeva N.O., Nikitin A.G., Nosikov V.V., Shestakova M.V. Genetic factors in the development of chronic kidney disease in patients with diabetes mellitus. Russian Journal of Genetics. 2017;53:420–432. [Google Scholar]
- 87.Doi Y., Yoshizumi H., Yoshinari M., Iino K., Yamamoto M., Ichikawa K. Association between a polymorphism in the angiotensin-converting enzyme gene and microvascular complications in Japanese patients with NIDDM. Diabetologia. 1996;39:97–102. doi: 10.1007/BF00400419. [DOI] [PubMed] [Google Scholar]
- 88.Ohno T., Kawazu S., Tomono S. Association analyses of the polymorphisms of angiotensin-converting enzyme and angiotensinogen genes with diabetic nephropathy in Japanese non-insulin-dependent diabetics. Metabolism. 1996;45:218–222. doi: 10.1016/s0026-0495(96)90057-8. [DOI] [PubMed] [Google Scholar]
- 89.Wong T.Y.H., Chan J.C.N., Poon E., Li P.K.T. Lack of association of angiotensin-converting enzyme (DID/II) and angiotensinogen M235T gene polymorphism with renal function among Chinese patients with type II diabetes. American Journal of Kidney Diseases. 1999;33:1064–1070. doi: 10.1016/S0272-6386(99)70143-5. [DOI] [PubMed] [Google Scholar]
- 90.Wang X., Strizich G., Hu Y., Wang T., Kaplan R.C., Qi Q. Genetic markers of type 2 diabetes: progress in genome-wide association studies and clinical application for risk prediction. Journal of Diabetes. 2016;8:24–35. doi: 10.1111/1753-0407.12323. [DOI] [PubMed] [Google Scholar]
- 91.Li M., Pezzolesi M.G. Advances in understanding the genetic basis of diabetic kidney disease. Acta Diabetologica. 2018;55:1093–1104. doi: 10.1007/s00592-018-1193-0. [DOI] [PubMed] [Google Scholar]
- 92.Fuchsberger C., Flannick J., Teslovich T.M., Mahajan A., Agarwala V., Gaulton K.J. The genetic architecture of type 2 diabetes. Nature. 2016;536:41–47. doi: 10.1038/nature18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Zuydam N.R., Ahlqvist E., Sandholm N., Deshmukh H., Rayner N.W., Abdalla M. A genome-wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes. 2018;67:1414–1427. doi: 10.2337/db17-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zoungas S., Arima H., Gerstein H.C., Holman R.R., Woodward M., Reaven P. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431–437. doi: 10.1016/S2213-8587(17)30104-3. [DOI] [PubMed] [Google Scholar]
- 95.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 96.Chalmers J., Cooper M.E. UKPDS and the legacy effect. New England Journal of Medicine. 2008;359:1618–1620. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 97.Reddy M.A., Zhang E.L., Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58:443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mimura I. Epigenetic memory in kidney diseases. Kidney International. 2016;89:274–277. doi: 10.1016/j.kint.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 99.Egger G., Liang G.N., Aparicio A., Jones P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 100.Qiu C.X., Hanson R.L., Fufaa G., Kobes S., Gluck C., Huang J. Cytosine methylation predicts renal function c for updates decline in American Indians. Kidney International. 2018;93:1417–1431. doi: 10.1016/j.kint.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bomsztyk K., Denisenko O., Wang Y.L. DNA methylation yields epigenetic clues into the diabetic nephropathy of Pima Indians. Kidney International. 2018;93:1272–1275. doi: 10.1016/j.kint.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 102.Rose N.R., Klose R.J. Understanding the relationship between DNA methylation and histone lysine methylation. Biochimica et Biophysica Acta. 2014;1839:1362–1372. doi: 10.1016/j.bbagrm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shalev A. Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Molecular Endocrinology. 2014;28:1211–1220. doi: 10.1210/me.2014-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Marinis Y., Cai M.Y., Bompada P., Atac D., Kotova O., Johansson M.E. Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney International. 2016;89:342–353. doi: 10.1016/j.kint.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 105.Sayyed S.G., Gaikwad A.B., Lichtnekert J., Kulkarni O., Eulberg D., Klussmann S. Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrology Dialysis Transplantation. 2010;25:1811–1817. doi: 10.1093/ndt/gfp730. [DOI] [PubMed] [Google Scholar]
- 106.Korb A., Bertoldi K., Lovatel G.A., Delevatti R.S., Elsner V.R., Meireles L.C.F. Acute exercise and periodized training in different environments affect histone deacetylase activity and interleukin-10 levels in peripheral blood of patients with type 2 diabetes. Diabetes Research and Clinical Practice. 2018;141:132–139. doi: 10.1016/j.diabres.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 107.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 108.Holman N., Young B., Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabetic Medicine. 2015;32:1119–1120. doi: 10.1111/dme.12791. [DOI] [PubMed] [Google Scholar]
- 109.Najafian B., Fogo A.B., Lusco M.A., Alpers C.E. AJKD Atlas of renal pathology: diabetic nephropathy. American Journal of Kidney Diseases. 2015;66:e37–e38. doi: 10.1053/j.ajkd.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 110.Karuranga S., Huang Y., Moura A.F., Rathmann W., Malanda B. Diabetes prevalence, mortality and healthcare expenditure in 2017 and 2045 in Europe: data from the IDF Diabetes Atlas. Diabetologia. 2018;61:S139–S140. [Google Scholar]
- 111.Zagkotsis G., Markou M., Paschou E., Papanikolaou P., Sabanis N. Preventing the development and progression of diabetic kidney disease: where do we stand? Diabetes Metab Syndr. 2018;12:585–590. doi: 10.1016/j.dsx.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 112.Retnakaran R., Cull C.A., Thorne K.I., Adler A.I., Holman R.R., Group U.S. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 113.Fiorentino M., Bolignano D., Tesar V., Pisano A., Biesen W.V., Tripepi G. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrology Dialysis Transplantation. 2017;32:97–110. doi: 10.1093/ndt/gfw070. [DOI] [PubMed] [Google Scholar]
- 114.Idris I., Tohid H., Muhammad N.A., MR A.R., Mohd Ahad A., Ali N. Anaemia among primary care patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD): a multicentred cross-sectional study. BMJ Open. 2018;8:e025125. doi: 10.1136/bmjopen-2018-025125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas M.C., Cooper M.E., Rossing K., Parving H.H. Anaemia in diabetes: is there a rationale to TREAT? Diabetologia. 2006;49:1151–1157. doi: 10.1007/s00125-006-0215-6. [DOI] [PubMed] [Google Scholar]
- 116.Moseley K.F. Type 2 diabetes and bone fractures. Current Opinion in Endocrinology Diabetes and Obesity. 2012;19:128–135. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahlqvist E., Storm P., Karajamaki A., Martinell M., Dorkhan M., Carlsson A. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 118.Wang D.D., Hu F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Endocrinology and Diabetes. 2018;6:416–426. doi: 10.1016/S2213-8587(18)30037-8. [DOI] [PubMed] [Google Scholar]
- 119.Fernandez-Fernandez B., Ortiz A., Gomez-Guerrero C., Egido J. Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nature Reviews Nephrology. 2014;10:325–346. doi: 10.1038/nrneph.2014.74. [DOI] [PubMed] [Google Scholar]
- 120.Pergola P.E., Raskin P., Toto R.D., Meyer C.J., Huff J.W., Grossman E.B. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. New England Journal of Medicine. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 121.de Zeeuw D., Agarwal R., Amdahl M., Audhya P., Coyne D., Garimella T. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 122.Williams M.E., Bolton W.K., Khalifah R.G., Degenhardt T.P., Schotzinger R.J., McGill J.B. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. American Journal of Nephrology. 2007;27:605–614. doi: 10.1159/000108104. [DOI] [PubMed] [Google Scholar]
- 123.Lewis E.J., Greene T., Spitalewiz S., Blumenthal S., Berl T., Hunsicker L.G. Pyridorin in type 2 diabetic nephropathy. Journal of the American Society of Nephrology. 2012;23:131–136. doi: 10.1681/ASN.2011030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dwyer J.P., Greco B.A., Umanath K., Packham D., Fox J.W., Peterson R. Pyridoxamine dihydrochloride in diabetic nephropathy (PIONEER-CSG-17): lessons learned from a pilot study. Nephron. 2015;129:22–28. doi: 10.1159/000369310. [DOI] [PubMed] [Google Scholar]
- 125.Himmelfarb J., Tuttle K.R. New therapies for diabetic kidney disease. New England Journal of Medicine. 2013;369:2549–2550. doi: 10.1056/NEJMe1313104. [DOI] [PubMed] [Google Scholar]
- 126.Cooper M., Warren A.M. A promising outlook for diabetic kidney disease. Nature Reviews Nephrology. 2019;15:68–70. doi: 10.1038/s41581-018-0092-5. [DOI] [PubMed] [Google Scholar]
- 127.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 128.Johnston R., Uthman O., Cummins E., Clar C., Royle P., Colquitt J. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation. Health Technology Assessment. 2017;21:1–218. doi: 10.3310/hta21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perkovic V., de Zeeuw D., Mahaffey K.W., Fulcher G., Erondu N., Shaw W. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 130.Tuttle K.R., Lakshmanan M.C., Rayner B., Busch R.S., Zimmermann A.G., Woodward D.B. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 131.Wang W., Nevarez L., Filippova E., Song K.H., Tao B., Gu L. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes, Obesity and Metabolism. 2019;21:234–243. doi: 10.1111/dom.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 133.Preiss D., Sattar N. Research digest: SGLT2 inhibition in kidney and liver disease. Lancet Diabetes Endocrinol. 2019;7:427. doi: 10.1016/S2213-8587(19)30160-3. [DOI] [PubMed] [Google Scholar]
- 134.Dieter B.P., Alicic R.Z., Tuttle K.R. GLP-1 receptor agonists in diabetic kidney disease: from the patient-side to the bench-side. American Journal of Physiology - Renal Physiology. 2018;315:F1519–F1525. doi: 10.1152/ajprenal.00211.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryan D., Acosta A. GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring) 2015;23:1119–1129. doi: 10.1002/oby.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M. Empagliflozin and progression of kidney disease in type 2 diabetes. New England Journal of Medicine. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 137.Mann J.F.E., Orsted D.D., Brown-Frandsen K., Marso S.P., Poulter N.R., Rasmussen S. Liraglutide and renal outcomes in type 2 diabetes. New England Journal of Medicine. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 138.Filippas-Ntekouan S., Filippatos T.D., Elisaf M.S. SGLT2 inhibitors: are they safe? Postgraduate Medicine. 2018;130:72–82. doi: 10.1080/00325481.2018.1394152. [DOI] [PubMed] [Google Scholar]
- 139.Johansen M.Y., MacDonald C.S., Hansen K.B., Karstoft K., Christensen R., Pedersen M. Effect of an intensive lifestyle intervention on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318:637–646. doi: 10.1001/jama.2017.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Edwards L.M. Metabolic systems biology: a brief primer. Journal of Physiology. 2017;595:2849–2855. doi: 10.1113/JP272275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Satirapoj B., Dispan R., Radinahamed P., Kitiyakara C. Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC Nephrology. 2018;19:246. doi: 10.1186/s12882-018-1043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Colombo M., Looker H.C., Farran B., Hess S., Groop L., Palmer C.N.A. Serum kidney injury molecule 1 and beta2-microglobulin perform as well as larger biomarker panels for prediction of rapid decline in renal function in type 2 diabetes. Diabetologia. 2019;62:156–168. doi: 10.1007/s00125-018-4741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Curovic V.R., Hansen T.W., Eickhoff M.K., von Scholten B.J., Reinhard H., Jacobsen P.K. Urinary tubular biomarkers as predictors of kidney function decline, cardiovascular events and mortality in microalbuminuric type 2 diabetic patients. Acta Diabetologica. 2018;55:1143–1150. doi: 10.1007/s00592-018-1205-0. [DOI] [PubMed] [Google Scholar]
- 144.Kim S.Y., Jeong T.D., Lee W., Chun S., Sunwoo S., Kim S.B. Plasma Neutrophil gelatinase-associated Lipocalin as a marker of tubular damage in diabetic nephropathy. Annals of Laboratory Medicine. 2018;38:524–529. doi: 10.3343/alm.2018.38.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Satirapoj B., Pooluea P., Nata N., Supasyndh O. Urinary biomarkers of tubular injury to predict renal progression and end stage renal disease in type 2 diabetes mellitus with advanced nephropathy: a prospective cohort study. Journal of Diabetes and its Complications. 2019;33:675–681. doi: 10.1016/j.jdiacomp.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 146.Doi T., Moriya T., Fujita Y., Minagawa N., Usami M., Sasaki T. Urinary IgG4 and Smad1 are specific biomarkers for renal structural and functional changes in early stages of diabetic nephropathy. Diabetes. 2018;67:986–993. doi: 10.2337/db17-1043. [DOI] [PubMed] [Google Scholar]
- 147.Tang X.Y., Zhou J.B., Luo F.Q., Han Y.P., Zhao W., Diao Z.L. Urine NGAL as an early biomarker for diabetic kidney disease: accumulated evidence from observational studies. Renal Failure. 2019;41:446–454. doi: 10.1080/0886022X.2019.1617736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sircar M., Rosales I.A., Selig M.K., Xu D.H., Zsengeller Z.K., Stillman I.E. Complement 7 is up-regulated in human early diabetic kidney disease. American Journal Of Pathology. 2018;188:2147–2154. doi: 10.1016/j.ajpath.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sheira G., Noreldin N., Tamer A., Saad M. Urinary biomarker N-acetyl-beta-D-glucosaminidase can predict severity of renal damage in diabetic nephropathy. Journal of Diabetes and Metabolic Disorders. 2015;14 doi: 10.1186/s40200-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mogensen C.E. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. New England Journal of Medicine. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 151.Xie Y.J., Jia Y.J., Xie C.H., Hu F., Xue M., Xue Y.M. Urinary exosomal MicroRNA profiling in incipient type 2 diabetic kidney disease. Journal of Diabetes Research. 2017;2017:6978984. doi: 10.1155/2017/6978984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beltrami C., Simpson K., Jesky M., Wonnacott A., Carrington C., Holmans P. Association of elevated urinary mir-126, mir-155, and mir-29b with diabetic kidney disease. American Journal Of Pathology. 2018;188:1982–1992. doi: 10.1016/j.ajpath.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 153.Yang X.Y., Liu S.H., Zhang R., Sun B., Zhou S.J., Chen R. Microribonucleic acid-192 as a specific biomarker for the early diagnosis of diabetic kidney disease. Journal of Diabetes Investigation. 2018;9:602–609. doi: 10.1111/jdi.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liao W.L., Chang C.T., Chen C.C., Lee W.J., Lin S.Y., Liao H.Y. Urinary proteomics for the early diagnosis of diabetic nephropathy in Taiwanese patients. Journal of Clinical Medicine. 2018;7:E483. doi: 10.3390/jcm7120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Conserva F., Barozzino M., Pesce F., Divella C., Oranger A., Papale M. Urinary miRNA-27b-3p and miRNA-1228-3p correlate with the progression of kidney fibrosis in diabetic nephropathy. Scientific Reports-UK. 2019;9:11357. doi: 10.1038/s41598-019-47778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim M., Cai Q., Oh Y. Therapeutic potential of alpha-1 antitrypsin in human disease. Annals of Pediatric Endocrinology. 2018;23:131–135. doi: 10.6065/apem.2018.23.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gluhovschi C., Gluhovschi G., Petrica L., Timar R., Velciov S., Ionita I. Urinary biomarkers in the assessment of early diabetic nephropathy. Journal of Diabetes Research. 2016;2016:4626125. doi: 10.1155/2016/4626125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhensdadia N.M., Hunt K.J., Lopes-Virella M.F., Tucker J.M., Mataria M.R., Alge J.L. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney International. 2013;83:1136–1143. doi: 10.1038/ki.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fawzy M.S., Abu AlSel B.T. Assessment of vitamin D-binding protein and early prediction of nephropathy in type 2 Saudi diabetic patients. Journal of Diabetes Research. 2018;2018:8517929. doi: 10.1155/2018/8517929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zylka A., Dumnicka P., Kusnierz-Cabala B., Gala-Bladzinska A., Ceranowicz P., Kucharz J. Markers of glomerular and tubular damage in the early stage of kidney disease in type 2 diabetic patients. Mediators of Inflammation. 2018;2018:7659243. doi: 10.1155/2018/7659243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van J.A.D., Scholey J.W., Konvalinka A. Insights into diabetic kidney disease using urinary proteomics and bioinformatics. Journal of the American Society of Nephrology. 2017;28:1050–1061. doi: 10.1681/ASN.2016091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ibarra-Gonzalez I., Cruz-Bautista I., Bello-Chavolla O.Y., Vela-Amieva M., Pallares-Mendez R., Nevarez D.R.D.Y. Optimization of kidney dysfunction prediction in diabetic kidney disease using targeted metabolomics. Acta Diabetologica. 2018;55:1151–1161. doi: 10.1007/s00592-018-1213-0. [DOI] [PubMed] [Google Scholar]
- 163.Chen C.J., Liao W.L., Chang C.T., Lin Y.N., Tsai F.J. Identification of urinary metabolite biomarkers of type 2 diabetes nephropathy using an untargeted metabolomic approach. Journal of Proteome Research. 2018;17:3997–4007. doi: 10.1021/acs.jproteome.8b00644. [DOI] [PubMed] [Google Scholar]
- 164.Chib S., Greenberg E. Understanding the metropolis-hastings algorithm. The American Statistician. 1995;49:327–335. [Google Scholar]
- 165.Rosenstock J., Perkovic V., Johansen O.E., Cooper M.E., Kahn S.E., Marx N. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mosenzon O., Leibowitz G., Bhatt D.L., Cahn A., Hirshberg B., Wei C. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40:69–76. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 167.Heerspink H.J.L., Parving H.H., Andress D.L., Bakris G., Correa-Rotter R., Hou F.F. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 168.Wu L., Li X.Q., Chang D.Y., Zhang H., Li J.J., Wu S.L. Associations of urinary epidermal growth factor and monocyte chemotactic protein-1 with kidney involvement in patients with diabetic kidney disease. Nephrology Dialysis Transplantation. 2018 doi: 10.1093/ndt/gfy314. [DOI] [PubMed] [Google Scholar]
- 169.Wada T., Furuichi K., Sakai N., Iwata Y., Yoshimoto K., Shimizu M. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney International. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]