Abstract
Seprehvir (HSV1716) is an oncolytic herpes simplex virus-1 (HSV-1) previously demonstrated to be well tolerated in pediatric patients when administered intratumorally. To determine the safety of administering Seprehvir systemically, we conducted the first-in-human phase I trial of intravenous injection in young patients with relapsed or refractory extra-cranial solid cancers. We delivered a single dose of 5 × 104 infectious units (iu)/kg (maximum dose of 2 × 106) or 2.5 × 105 iu/kg (maximum dose of 1 × 107 iu) of Seprehvir via the peripheral vein, monitored adverse events, and measured tumor responses by imaging. We monitored HSV-1 serology as well as viremia and shedding by PCR and culture. We administered a single dose of Seprehvir to seven patients and multiple doses to two patients. We did not observe any dose-limiting toxicities. All five HSV-1 seronegative patients seroconverted by day 28. Four of nine patients had detectable HSV-1 genomes in peripheral blood appearing on day +4 consistent with de novo virus replication. Two patients had stable disease in response to Seprehvir. Intravenous Seprehvir is well tolerated without viral shedding in children and young adults with late-stage cancer. Viremia consistent with virus replication holds promise for future Seprehvir studies at higher doses and/or in combination with other anti-neoplastic therapies.
Keywords: oncolytic virus, herpes simplex type 1, pediatrics, immunotherapy, solid tumors
Oncolytic viruses hold promise for the field of oncology as novel targeted therapies. Streby et al. report the first-in-human intravenous administration of an oncolytic herpes simplex virus and demonstrate its safety in young patients with relapsed or refractory extra-cranial solid cancers.
Introduction
Children with recurrent and/or refractory solid tumors urgently need novel targeted and less toxic therapy to improve survival and limit treatment-related toxicities. Oncolytic viruses offer a new platform for cancer therapy because of their large therapeutic index and limited toxic effects. The majority of cancer patients entering phase I clinical trials have metastatic disease. Because preclinical studies of oncolytic viruses given intratumorally suggest that virus is mostly contained within the tumor with minimal systemic spread, localized virus injection may be efficacious for patients with metastatic disease only if there is a significant abscopal-like effect. Thus, systemically administered virus is likely to be the most useful as a cancer therapy for patients with metastases.
Herpes simplex virus-1 (HSV-1) is an attractive oncolytic vector for multiple reasons. The pathogenesis of natural HSV-1 infections is well known, and diagnostic assays are commonly available.1, 2 In particular, clinicians are accustomed to treating HSV-1 infections, and it is one of the few human viral pathogens for which there are safe and clinically proven anti-viral therapies. Attenuating mutations have been well described that render the virus safe yet retain replication competency in cancer cells, as demonstrated by the clinical experience with talimogene laherparepvec, which was safe to administer by intralesional injection in melanoma patients and prolonged survival in patients with local and regional disease.3
Seprehvir (HSV1716) is genetically altered to replicate in and lyse dividing tumor cells, but fails to replicate in normal non-cancer cells by virtue of deletion of the gene encoding ICP34.5.1 This virus additionally maintains its expression of thymidine kinase, serving as a safety net whereby the virus can be stopped with administration of acyclovir. In our previous study, we demonstrated the safety of Seprehvir, an oncolytic herpes simplex type 1 virus, delivered by direct intratumoral injection to children and young adults with non-CNS solid tumors.4 Prior to our intratumoral trial, no one had prior experience administering oncolytic HSV to children and young adults. Other oncolytic viruses, such as Seneca Valley virus, reovirus, and vaccinia virus, have been studied in children with few toxicities but also little evidence of disease response.5, 6, 7
In younger patients, intratumoral injection has more limitations because of patient anxiety, need for sedation and/or coordination with multiple providers, radiation exposure for image-guided injection, and cost. Intravenous administration eliminates these limitations in addition to potentially exposing more tumor sites to oncolytic virus than with direct intratumoral injection.
Here we report our single-institution phase 1 clinical trial aiming to determine the safety of intravenous injection of Seprehvir in children and young adults with non-CNS solid tumors by recording adverse events and any dose-limiting toxicities (DLTs) of intravenous Seprehvir at the doses given. We secondarily aimed to measure the antiviral immune response in patients with relapsed and/or refractory cancers by serologies, to measure the systemic viremia and viral shedding after intravenous Seprehvir injection by PCR and viral culture, and to preliminarily define the antitumor activity of Seprehvir within the confines of a phase 1 trial (https://clinicaltrials.gov/; ClinicalTrials.gov: NCT00931931) by modified RECIST (response evaluation criteria in solid tumors) criteria and metabolic activity on positron emission tomography (PET) scan. We also compared the findings of our previous intratumoral trial with the results of administering Seprehvir systemically.
Results
Patient Characteristics
A total of nine patients aged 11–30 years were enrolled and fully evaluable for safety and toxicity. Three patients were accrued to each of two dose levels, and an additional three patients were enrolled at dose level one after the first patient had a potential serious adverse event (SAE; see below). The patients had varying pediatric cancer diagnoses, including osteosarcoma (n = 2), Ewing sarcoma, rhabdomyosarcoma (n = 2), neuroblastoma, chondrosarcoma, cholangiocarcinoma, and pancreatic neuroendocrine tumor (see Table 1). Most patients received multiple courses of therapy for relapsed or refractory disease prior to enrollment in this trial (chemotherapy and/or radiation therapy).
Table 1.
Patient Demographics
| Patient No. | Diagnosis | Age (years) | Prior Chemotherapy Regimens (n) | Previous Radiation Tx | Disease at Trial Entry | Time from Dx to Tx (months) | Seprehvir Dose (iu) |
|---|---|---|---|---|---|---|---|
| HSV01 | pleomorphic rhabdomyosarcoma | 30 | VCR/Irino/Doxo/CTX/Etop, VCR/Dactino/CTX, Ifos/Carbo/Etop, liposomal Doxo/temsirolimus (4) | 45 Gy retroperitoneum | multiple pleural and diaphragmatic nodules, paraspinal mass, lung mets | 19 | 2 × 106 |
| HSV02 | cholangiocarcinoma | 18 | cisplatin/gemcitabine, bevacizumab/5-FU/leucovorin/oxaliplatin, cisplatin/gemcitabine (3) | none | intrahepatic biliary mass, multiple liver mets, mediastinal node | 18 | 2 × 106 |
| HSV03 | pancreatic neuroendocrine tumor | 28 | Temodar/Xeloda, everolimus, Doxo (3) | none | mass in bed of pancreatic head, three liver lesions, mass near SMA, mesenteric nodes | 27 | 2 × 106 |
| HSV04 | Ewing sarcoma | 25 | VCR/Doxo/CTX/Ifos/Etop, Irino/Temodar, VCR/CTX/Topo/bevacizumab (3) | 56 Gy right knee, 18 Gy lung | right tibial mass with two distal lesions and multiple pulmonary nodules | 50 | 2 × 106 |
| HSV05 | osteosarcoma | 17 | MTX/Doxo/Cisplat, high-dose ifosfamide, liposomal Doxo, gemcitabine/docetaxel, gemcitabine, and nab-paclitaxel (5) | none | multiple pleural, lung, and diaphragmatic nodules | 26 | 2 × 106 |
| HSV06 | rhabdomyosarcoma | 11 | VCR/Irino/Doxo/Dactino/CTX/Etop, vinorelbine/CTX/temsirolimus (2) | 37.5 Gy humeri; 41.4 Gy cervical, thoracic, and sacral spine; 37.5 Gy foot | pelvic mass, abdominal and inguinal nodes, thigh mass, bilateral lung and hilar nodules, liver nodules | 26 | 1.6 × 106 |
| HSV07 | osteosarcoma | 19 | Doxo/cisplatin/Zometa/HD-MTX/Ifos/Etop, high-dose ifos, gemcitabine/Taxotere, sorafenib, liposomal doxo (5) | none | right upper lung mass with satellite nodules and right subcarinal node | 53 | 1 × 107 |
| HSV08 | neuroblastoma | 16 | CTX/Doxo/VCR/Cisplat/Etop; Carbo/Etop/Mel, retinoic acid, Irino/Temodar, CTX/Topo, anti-GD2 immunotherapy, oral etoposide, AZD1775/oral Irino (8) | 21.6 Gy abdomen and skull, XRT to thigh (dose unknown) | entire right hip bone, right hip mass, left femoral head, right liver lobe mets, and L4/S1 vertebral bodies | 132 | 1 × 107 |
| HSV09 | chondrosarcoma | 29 | MTX/Doxo/Cisplat, Doxo, Ifos/Etop, pazopanib, Ifos/Etop (5) | proton XRT to thigh (dose unknown) | large pelvic/sacral mass into the sacral canal, paraspinal, and gluteal muscles | 54 | 1 × 107 |
5-FU, fluorouracil; Carbo, carboplatin; Cisplat, cisplatin; CTX, cyclophosphamide; Dactino, dactinomycin; Doxo, doxorubicin; Dx, diagnosis; Etop, etoposide; HD-MTX, high-dose methotrexate; Ifos, ifosfamide; Irino, irinotecan; Mel, melphalan; SMA, superior mesenteric artery; Tx, therapy; VCR, vincristine; XRT, radiation therapy.
Serologic Responses and Toxicities
All nine patients were serologically negative for anti-HSV-1 antibodies at baseline, and all six patients for whom we had collected data seroconverted following injection by day 28 (Table 2). Seroconversion data were not available for HSV03 because the patient became acutely ill due to disease progression and was admitted to another institution. Patients HSV05 and HSV06 declined having these labs drawn. Of note, HSV03 was serologically positive for HSV-2 by immunoglobulin G (IgG) and immunoglobulin M (IgM) prior to Seprehvir, but had no evidence of HSV-1 immunity. In addition, HSV08 and HSV09 had evidence of HSV-1 PCR in the blood on day 0 after injection, and HSV08 had persistent evidence of HSV-1 DNA in the peripheral blood. HSV08 also was the only patient to seroconvert in both IgG and IgM by day 28. The seroconversion or detection of HSV-1 DNA in peripheral blood did not appear to depend on white blood cell count (WBC), absolute neutrophil count (ANC), or absolute lymphocyte count (ALC).
Table 2.
Patient Serologic Responses to a Single Dose of Intravenous Seprehvir
| Patient No. | WBC | ANC | ALC | HSV-1 PCR Blood | HSV-1 Seroconversion (IgG/IgM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | S | S | S | S | 0a | 1 | 4 | 7 | 14 | 21 | 28 | S | 28 |
| HSV01 | 9.1 | 5,600 | 1,638 | − | − | − | + | + | + | NA | NA | − | NA |
| HSV02 | 6.2 | 4,030 | 1,612 | − | − | − | + | − | − | − | − | − | +IgM |
| HSV03 | 13.5 | 9,585 | 1,620 | − | − | − | − | ND | ND | ND | ND | − | ND |
| HSV04 | 4.2 | 2,772 | 966 | − | − | − | − | − | − | − | + | − | +IgM |
| HSV05 | 5.4 | 3,078 | 864 | − | − | − | − | − | ND | ND | ND | − | ND |
| HSV06 | 5.8 | 5,046 | 348 | − | − | − | + | + | + | + | − | − | ND |
| HSV07 | 3.5 | 2,100 | 910 | − | − | − | − | − | − | − | − | − | +IgM |
| HSV08 | 8.8 | 5,280 | 2,376 | − | + | + | + | + | − | + | + | − | +IgM, +IgG |
| HSV09 | 4.6 | 3,634 | 414 | − | − | − | − | − | − | − | − | − | +IgM |
| HSV09-II | 4.7 | 3,384 | 705 | − | +b | − | − | − | − | − | − | +IgM | +IgM, +IgG |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; NA, not applicable (patient deceased); ND, not done; S, screening; WBC, white blood cell count.
Measured at 3, 6, and 18 h after virus injection.
Measured only at 3 h after virus injection.
No dose-limiting toxicities (DLTs) were noted in any of the patients. One patient had grade 3 hypotension and flu-like symptoms that were possibly attributed to Seprehvir or its administration. Grade 1 and 2 adverse events possibly or probably attributable to Seprehvir included laboratory abnormalities such as anemia, leukopenia, lymphopenia, and mild elevations in liver enzymes (Table 3). One patient had mild bleeding, and one patient had a pneumothorax after a biopsy of a lung parenchyma tumor was taken on day 7 as per clinical trial guidelines. One patient had a grade 5 gastrointestinal (GI) hemorrhage (HSV01) that was determined by the data safety monitoring board and by the US Food and Drug Administration (FDA) to be unrelated to Seprehvir and related to disease progression. However, because this patient was the first to receive intravenous Seprehvir, the FDA requested expansion of the first dose level to six patients, and the remaining doses in those patients were well tolerated.
Table 3.
Adverse Events Possibly, Probably, or Definitely Attributable to Intravenous Seprehvir or Study Procedures
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Attribution to Seprehvir | Attribution to Study Procedure |
|---|---|---|---|---|---|
| Blood/Bone Marrow | |||||
| Anemia | 2 | 1 | possibly | possibly | |
| Leukopenia | 4 | 2 | possibly | ||
| Lymphopenia | 3 | 4 | possibly | ||
| Neutropenia | 1 | 1 | possibly | ||
| Thrombocytopenia | 3 | possibly | |||
| Cardiac | |||||
| Hypotension | 1 | possibly | |||
| Constitutional Symptoms | |||||
| Chills | 1 | possibly | |||
| Fatigue | 3 | possibly | possibly | ||
| Gastrointestinal | |||||
| Anorexia | 1 | possibly | |||
| Dehydration | 1 | possibly | |||
| Nausea | 1 | 1 | 1 | possibly | |
| Injury, Poisoning, and Procedural Complications | |||||
| Postoperative hemorrhage | 1 | definitely | |||
| Investigations | |||||
| Acidosis | 1 | possibly | |||
| ALT increased | 2 | possibly | |||
| AST increased | 1 | possibly | possibly | ||
| Hyperbilirubinemia | 1 | possibly | |||
| Serum bicarbonate decreased | 1 | possibly | possibly | ||
| Pain | |||||
| Neck pain | 1 | possibly | |||
| Headache | 1 | possibly | |||
| Respiratory | |||||
| Pleural effusion | 2 | possibly | |||
| Pneumothorax | 1 | 1 | definitely | ||
| Syndromes | |||||
| Flu-like symptoms | 1 | possibly | |||
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
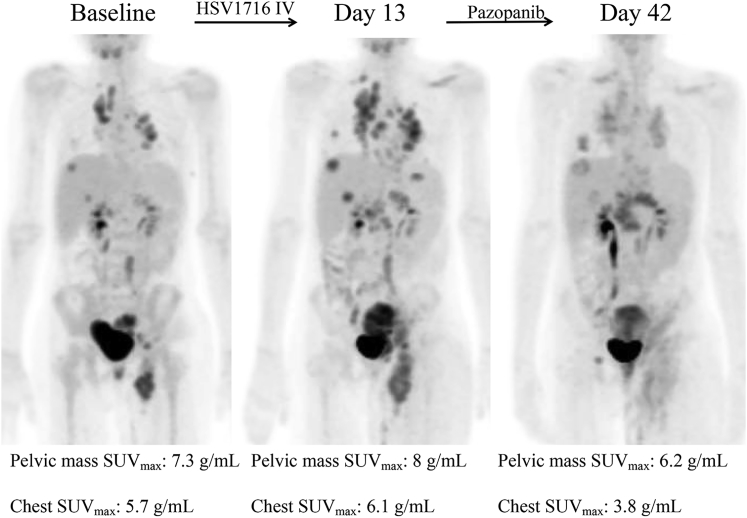
Three patients met eligibility for part two of the trial (up to three additional doses of Seprehvir) based on stable disease at days +14 or +28. Two patients declined further injections because of the treating oncologist’s preference or concern for significant disease progression. Patient HSV09 received one additional dose of Seprehvir, with no significant adverse events noted, but declined further dosing after a 19% increase in overall tumor burden. Patient HSV06 had evidence of disease progression on day 14 imaging. This patient was removed from the study and started on oral pazopanib (450 mg/m2/day) on what would have been day 20 of the trial. Pazopanib is a small-molecule tyrosine kinase angiogenesis inhibitor shown to have potent activity against sarcomas.8 Thirty days after Seprehvir and 10 days after starting pazopanib, imaging done at her local institution by computed tomography (CT)/MRI revealed stable disease (as well as a clinically relevant decrease in lymphedema secondary to vascular compression by the tumor). Although it was unclear whether this lack of further disease progression was due to pazopanib or Seprehvir, an expanded access use (EAU) protocol was submitted to and approved by the FDA on IND 13196 Serial No. 00058 for three doses of weekly Seprehvir at the same dose as previously, in combination with oral pazopanib at 450 mg/m2/day. In screening for the EAU protocol, the PET scan, obtained 42 days after her initial Seprehvir injection, showed a significant decrease in metabolic activity of her tumors diffusely (see Figure 1). HSV06 continued on Seprehvir injections weekly ×2 more doses while continuing to take oral pazopanib. She tolerated the combination therapy well with no dose-limiting toxicities attributable to either therapy, but unfortunately had further progression of her disease, which ultimately led to her death 1 month after starting the EAU protocol.
Figure 1.
Transient Increase in PET Avidity after Systemic HSV1716 Administration
Patient HSV06 PET scans at baseline, day 13 after a single intravenous dose of Seprehvir (which showed increase in number, size, and SUV of metastatic lesions), and on day 42 after coming off Seprehvir study and starting pazopanib 450 mg/m2/day on day +20 after Seprehvir infusion (which showed decreased size and SUV of the pelvic mass and of the chest metastases).
Viremia and Virus Shedding
No viral shedding was observed in any patient on this trial because all HSV-1 cultures, including blood, buccal swab, rectal swab, and urine, at all study visits through day 28 were negative. PCR for HSV-1 genomes were also negative in all urine samples and all buccal and rectal swabs. Blood PCRs for HSV-1 genomes were negative at baseline in all patients, and four of the nine patients had positive HSV-1 PCRs on day 4. Three of the four patients had persistence of the positive PCR beyond day 4, and HSV06 and HSV08 had positive PCRs throughout the remainder of the 28-day evaluation. HSV06 became PCR-negative during the EAU part of her treatment, and PCRs were negative in follow-up for HSV08. We obtained tumor biopsies on two patients over the age of 18 years (HSV07 and HSV09) but could not detect any evidence of virus by PCR or immunohistochemical staining within the tumor on day 7 after treatment.
Disease Responses
Two of the seven patients had stable disease; one had stable PET maximum standardized uptake value (SUVmax; HSV04), and the other had a slight decrease in PET SUVmax on day 28 (HSV09). As shown in Table 4, patient survival did not differ significantly by the dose of Seprehvir given, and a few patients lived more than 6 months. These patients did go on to receive other therapies such as erlotinib and bevacizumab in HSV02; liposomal doxorubicin, autologous tumor cell vaccine therapy, gemcitabine and nab-paclitaxel, pazopanib, and trabectedin and irinotecan in HSV04; tumor embolization in HSV05; bevacizumab and zoledronic acid in HSV07; and temozolomide, irinotecan, and dinutuximab, ceritinib and ribociclib, alectinib and oral cyclophosphamide, and 131I-mIBG (meta-iodobenzylguanidine) in HSV08. Because this is a very small number of patients and all were treated with different therapies before and/or after Seprehvir, we are unable to draw any conclusions about the role Seprehvir might have played in patient survival.
Table 4.
Disease Response and PET SUV Changes after a Single Dose of Intravenous Seprehvir
| Patient No. | Day 14 CT/MRI | Day 14 PET | Day 28 CT/MRI | Day 28 PET | Time from Tx to Death (months) |
|---|---|---|---|---|---|
| HSV01 | stable disease | SUV stable | NA | NA | 0.5 |
| HSV02 | PD | SUV ↑ | PD | SUV ↑ | 11.5 |
| HSV03 | stable disease | ND | PD | ND | 1 |
| HSV04 | stable disease | SUV stable | stable disease | SUV stable | 26a |
| HSV05 | PD | ND | ND | ND | 3.5 |
| HSV06 | PD | SUV ↑ | NA | NA | 2 |
| HSV07 | NEb | NEb | PD | PD | 7 |
| HSV08 | PD | SUV ↑↑ | PD | SUV ↑ | 11 |
| HSV09-I | stable disease | SUV stable | stable disease | SUV ↓ | |
| HSV09-II | NA | NA | PD | SUV ↑ | 6a |
NA, not applicable; ND, not done; NE, not evaluable; PD, progressive disease; SUV, standardized uptake valuemax; Tx, treatment.
Patient is still alive so time from treatment to follow-up.
Tumor resected on day 7 for biopsy specimen and was the only site of active disease at the time.
Discussion
We found that intravenous administration of Seprehvir in children and young adults with relapsed and/or refractory non-CNS solid tumors is well tolerated. We saw no evidence of neurological toxicities or any other significant treatment-related toxicities in this clinical trial. All of the patients enrolled in this trial were HSV-1 seronegative, suggesting that pediatric and young adult patients may benefit the most from HSV virotherapy if pre-existing anti-HSV-1 immunity is ultimately found to diminish anti-tumor efficacy.
Systemic administration of any oncolytic HSV by intravenous injection has never been used in humans prior to this clinical trial. However, other types of viruses have been administered systemically for cancer therapy. Arterial infusion (via the hepatic artery) of the oncolytic HSV NV1020 was safe in 13 adult subjects in a phase I dose-escalation study and 22 adult subjects in a subsequent phase II expanded study (using the highest “optimal biologic dose” determined from the phase I study of 1 × 108 plaque-forming units [PFU]/dose × 4 doses).9 Based on similar logic, there are a number of other virus studies that utilize systemic delivery, including loco-regional infusion of an oncolytic HSV (rRp450 via hepatic artery; ClinicalTrials.gov: NCT01071941) and intravenous infusions of reovirus (pediatric study ClinicalTrials.gov: NCT01240538 and other adult studies), Seneca valley virus (pediatric study ClinicalTrials.gov: NCT01048892 and other adult studies), Newcastle disease virus (ClinicalTrials.gov: NCT01174537), and vaccinia virus (ClinicalTrials.gov: NCT01380600 and others).
Systemic administration of Seprehvir induced an anti-viral immune response in every patient for whom we had data, as evidenced by the development of HSV-1 IgM antibodies. In addition, the patients who were given repeated injections developed PCR+ viremia only after the first, but not after subsequent injections. These observations suggest the optimal dosing of systemic virotherapy is likely early, before anti-viral immunity develops. The anti-viral immunity could prove beneficial if combined with intratumoral dosing because it could boost the local immune response within the tumor microenvironment where the oncolytic virus was being injected. We previously showed that not all of the patients treated with intratumoral Seprehvir had seroconversion.4 All of these data together suggest patients may receive maximum benefit from a combination of intravenous and intratumoral virus administration. Further research into the functionality of the immune system at various time points in cancer treatment may be warranted to guide immunotherapeutic clinical trials.
Intravenous Seprehvir resulted in systemic viremia as evidenced by initially negative and subsequent appearance of HSV-1 by PCR in the peripheral blood in several patients. The lack of a PCR signal in the peripheral blood of the other patients may reflect insufficient dosing for systemic delivery, impaired delivery of virus because of tumor location, inadequate vascular supply of the tumor, or their particular tumors did not support robust virus replication. HSV08 had early evidence suggestive of viral replication. Whether or not neuroblastoma is particularly susceptible to oncolytic HSV infection is unknown, but our group and others have published on the anti-tumor efficacy in vitro and in vivo in human and murine models of neuroblastoma preclinically.10, 11, 12 Unlike what was reported in our previous study with intratumoral administration of Seprehvir, the absolute lymphocyte count (ALC) did not appear to influence virus replication because two of the three patients with persistent viral DNA had a normal ALC. We previously hypothesized that the prolonged persistence of HSV detection could be caused by the inhibition of immunosuppressor cells within the tumor microenvironment, such as regulatory T cells. This idea could still hold true, because we were unable to examine the tumor microenvironment directly in either study, but there was a persistence of HSV-1 DNA with low and high ALC when Seprehvir was delivered by intravenous injection. ANC also does not appear to impact DNA replication. Due to the small number of patients accrued, definitive conclusions cannot be drawn.
There are several potential explanations for the negative biopsies at 7 days after HSV1716 intravenous administration. We may have had a sampling error in geography or time, such that the region of the large tumors we tested or the time point at which it was collected were not optimal. It is also possible that virus was inactivated in the bloodstream and unable to reach the tumor site; we think this is less likely because both patients were negative for pre-existing anti-HSV antibodies and we were able to detect virus genomes in the blood at later time points. Another possibility is that the dose we used might have been insufficient to reach and intravasate into the tumor site, because the majority of intravenous HSV is taken up in the liver (at least in mice).13 To that point, we used a similar dose of HSV1716 as we used intratumorally because our preclinical data showed efficacy of systemic delivery at an equivalent dose level (albeit with two doses given instead of one as in our trial).14 Finally, it is possible that these particular tumors were not susceptible or permissive to virus replication, so that the virus particles that did reach the tumor did not spread and were rapidly cleared. Preclinical studies demonstrate the ability of HSV1716 to home in to cancer cells. Braidwood et al.14 treated mice bearing subcutaneous human hepatocellular carcinoma (huH7) xenograft tumors with HSV1716 at a dose of 1 × 106 or 1 × 107 PFU by tail vein injection on days 1 and 4. Mice treated with intravenous HSV1716 had significant tumor growth delays and prolonged survival when compared with the controls. No significant difference was noted between the two dose levels of HSV1716. Virus was detected only within the tumors of the mice (not in any other major organ as determined by titration and luciferase studies from tissues harvested after animals were sacrificed). HSV1716 was detected within the tumors by virus plaque assay on days 1 and 4 after injection. In a second experiment, mice received three doses (days 1, 14, and 29) of HSV1716 at 1 × 105 and 1 × 106 PFU by tail vein injection and again had significant tumor growth delays and prolonged survival when compared with controls. Once again, no significant difference was noted between these two dose levels, including the lower dose of virus. Also, virus was detected within the tumors on days 1, 14, 29, and even up to 35 days after the initial virus treatment. In addition, Braidwood et al.14 showed evidence of intratumoral virus replication after systemic intravenous administration.
It is worth noting that other oncolytic viruses have been shown to reach tumor sites by protein and RNA or DNA detection at various time points after systemic virus administration. Samson et al.15 showed evidence of reovirus within tumors at 3–17 days after intravenous virus delivery in patients with brain tumors. The vast majority of the virus homed to the tumor cells, and 0%–6% of the virus was detected within the local endothelial cells. Vaccinia virus also is known to home to tumors and showed evidence of prolonged virus survival and replication in tumors.16 In that study, two of eight patients had vaccinia virus genomes (as evaluated by qRT-PCR) present in their tumors at days 8 and 22 after virus administration. Breitbach et al.17 demonstrated the presence of JX-594 virus in metastatic tumors 8–10 days after virus administration by PCR and immunohistochemistry in 87% of patients. They noted patients treated with a JX-594 dose of less than 1 × 109 PFU had no evidence of virus within the tumors after systemic administration. We are unsure why we did not detect virus in the two patient tumor samples we analyzed, but we suspect the doses used were not sufficient to achieve readily detectable virus particles, especially if those particular tumor cells did not support robust virus replication. We suspect the doses we used were not sufficient to achieve detectable levels in the two samples we tested, because those examples used one or more log higher doses (1e+10 median tissue culture infectious dose [TCID50] for reovirus and >3e+8 PFU for vaccinia virus), and in the study by Breitbach et al.,17 there was a clear dose-dependent effect. Further studies in pediatric and adult patients could validate Seprehvir’s ability to survive, replicate, and home to tumors as we have seen in preclinical studies.
Although this study cannot answer the comparative question of which route is more efficacious, intravenous delivery is more practical for widespread application because it is less cumbersome than intratumoral delivery. In comparison with intratumoral delivery, intravenous Seprehvir was slightly less costly (no anesthesia or radiology-guidance charges), carried fewer risks to the patients (due to decreased anesthesia exposure), and was better tolerated. Additionally, patients treated with intravenous Seprehvir had a similar overall disease response to those treated with intratumoral Seprehvir (two patients with stable disease, one partial response versus three patients with stable disease, respectively). No difference was noted in time from treatment to time of death in patients treated with intratumoral or intravenous Seprehvir. However, intratumoral virus delivery, especially with more than one dose, would afford pediatric clinical scientists the opportunity to biopsy the tumor at the same time as the dose is delivered, because it is considered unethical to perform a biopsy on a child solely for research in the setting of increased risk (as patients are already undergoing sedation, imaging, and a needle injection, any additional risk is abrogated). Tumor biopsies are vital to enable an assessment of the tumor microenvironment, which may be the most critical aspect of immunotherapy response.
It remains unclear from this study if direct tumor injection is better than intravenous in light of seroconversion and lack of virus in the tumors. Seroconversion could be a benefit to viral therapy and could elicit a greater anti-tumor immune response with direct intratumoral dosing administered after the intravenous dose. Repeat intravenous dosing is unlikely to be more successful because the immune system could inactivate and/or remove any active virus systemically before it would reach the tumor. There are certainly several benefits (cost, ease of administration, etc.) to intravenous versus intratumoral dosing, but it remains to be seen if systemic administration or intratumoral administration has a benefit over one another, or if they will work best in synchrony or in interval dosing.
One of the primary reasons we remain enthusiastic about this type of treatment is the remarkable efficacy seen in animal models, both as a stand-alone therapeutic and in combination with other agents including immunotherapies. For example, HSV1716 given in combination with an aurora kinase inhibitor, a PD1 antibody, or a transforming growth factor β (TGF-β) inhibitor shows significant anti-tumor responses, prolonged survival, and even complete responses in murine models.11, 18, 19 Thus, either our dosing strategy is not sufficient to achieve the same effect in humans (hence our proposal for testing higher doses given that we did not encounter a dose-limiting toxicity [DLT]), or the mouse models do not faithfully recapitulate the interplay between the virus, tumor, and the immune system. Regarding the latter, it may be that testing in mice with humanized immune systems may be better predictors of clinical activity.20 Finally, it may be that only a subset of patients respond, in which case future studies would benefit from patient selection with an appropriate predictive biomarker, which at this point remains elusive. The doses utilized in this initial clinical trial were limited by manufacturing constraints, and we hope to test higher doses in future studies.
In conclusion, systemic administration of Seprehvir is well tolerated and shows promise of anti-cancer efficacy for children and young adults without evidence of any neurotoxicity. Further studies with higher doses of virus and/or combination therapies are warranted, especially in the pediatric and young adult population, where the majority of patients are seronegative. Additionally, this study in a challenging pediatric and/or young adult population supports testing of intravenous Seprehvir in more common adult cancers. Well-designed studies should also investigate the tumor microenvironment and immune system as a whole to evaluate the optimal timing for intervening with immune therapy in children with cancer. We posit that the ideal combination protocol would be designed to optimize the initial oncolysis followed by the development of anti-tumor immunity for long-term survivorship.
Materials and Methods
This trial received a waiver regarding the need for public discussion from the NIH Recombinant DNA Advisory Committee. The Nationwide Children’s Hospital local Institutional Review Board approved the trial. It was conducted under FDA Investigational New Drug BB-13196 and registered on ClinicalTrials.gov: NCT00931931. Informed consent was obtained from patients 18 years or older and/or from parents or legal guardians of patients younger than 18 years. Assent was obtained in children 9–18 years of age in accordance with our institutional policies.
Eligibility: Inclusion Criteria
The trial population included patients with recurrent or refractory incurable non-CNS solid tumors and patients who were aged ≥7 to ≤30 years at the time of virus injection. The FDA required the first three patients to be ≥18 years old. Patients were required to have a Karnofsky (age ≥ 16 years) or Lansky (age < 16 years) performance score of >50%. Organ function requirements included adequate bone marrow function (absolute neutrophil count ≥ 750/mL in the absence of granulocyte-colony stimulating factor (G-CSF) for 72 h or pegylated G-CSF (PEG-GCSF) for 14 days, platelet count > 100,000/mL, and hemoglobin ≥ 9 g/dL), adequate renal function (serum creatinine ≤ 1.5× upper limit of normal [ULN] for age or creatinine clearance or radioisotope glomerular filtration rate (GFR) ≥ 70 mL/min/1.73 m2), adequate hepatic function (total bilirubin ≤ 2× ULN for age, alanine transaminase [ALT] ≤ 2.5× ULN for age, and albumin ≥ 2 g/dL), adequate hemostatic function (prothrombin time [PT]/international normalized ratio [INR] and activated partial thromboplastin time [aPTT] < 1.5× ULN for age), adequate CNS function (baseline CNS conditions grade ≤2 per National Cancer Institute [NCI] Common Toxicity Criteria [CTCAE] v.3.0), and adequate cardiac function (shortening fraction > 25% by echocardiogram, no focal wall motion abnormalities, and no evidence of ischemia or significant arrhythmia on electrocardiogram). Patients with primary brain malignancies were excluded from the trial, but asymptomatic patients with previously treated brain metastases were eligible for enrollment. We required patients to test negative for hepatitis B surface antigen, hepatitis C antibody, and HIV-1 and HIV-2 antibodies at or within 3 months prior to trial entry. Patients also must have fully recovered from the acute toxicities of previous therapies prior to trial enrollment. Patients could not have received myelosuppressive chemotherapy within 28 days prior to study entry or non-myelosuppressive therapy within 14 days; could not have received biologic agents within 7 days prior to trial entry; no local palliative radiation therapy within 14 days and no myeloablative radiation therapy within 42 days prior to trial entry; no immunoablative or myeloablative stem cell transplant within 6 months prior to trial entry; and no investigational agent within 28 days prior to trial entry.
In addition, patients needed to either be ineligible for intratumoral dosing, or the intratumoral arm of the study had to be closed prior to enrolling in the intravenous administration arm. The response of the target lesion(s) determined whether a patient was eligible for part two of the trial in which patients could consent to receive up to three additional monthly doses of Seprehvir. Participation in part two of the trial required a second consent and/or assent. The requirement of the 28-day interval between virus doses and between patients was mandated by the FDA as a safety measure because this was the first study of an oncolytic herpes virus in children. To be eligible, all target lesions were required to be characterized as stable disease or better using a modified version of the RECIST.
Eligibility: Exclusion Criteria
Exclusion criteria included a history of allogeneic stem cell transplant, currently pregnant or breastfeeding, unable or unwilling to give voluntary informed consent and/or assent, significant infection or other severe systemic disease or medical and/or surgical condition deemed significant by the principal investigator, PEG-GCSF within 14 days or G-CSF within 72 h of trial entry, and planned use of antiviral therapy between 2 days prior to Seprehvir administration up to 28 days after Seprehvir administration.
Clinical Trial Design and Treatment
ClinicalTrials.gov: NCT00931931 continued enrollment at Nationwide Children’s Hospital (Columbus, OH, USA), affiliated with the Ohio State University NCI-designed Comprehensive Cancer Center. The dose-escalation portion of the trial enrolled patients in a 3+3 fashion. Baseline assessments included organ function, HSV serologies, and relevant imaging studies such as CT and/or MRI and 18fluorine-deoxyglucose PET/CT imaging. Patients received a single infusion of Seprehvir through a peripheral intravenous line over 1 h. Patients were monitored in the inpatient unit overnight for any adverse events. Peripheral blood was collected for bacterial culture, HSV-1 PCR, and HSV culture prior to injection on day 0, for HSV-1 PCR and culture on day 0 at 3, 6, 18, and 24 h after injection, and again on days 4, 7, 14, 21, and 28. On these same days, patients had a swab of their oral mucosa, swab of the rectal mucosa, and a urine sample collected for HSV culture and HSV PCR to detect any evidence of viral shedding. The HSV-1 PCR assay was our standard hospital clinical laboratory assay, which utilizes a primer for a 148-bp fragment for the gene encoding glycoprotein B that is present in both wild-type HSV and Seprehvir. Patients were discharged after the 24-h laboratory draw and/or when it was medically appropriate to discharge the patient home. They returned on days 4, 7, 14, 21, and 28 for laboratory tests and physical examinations to monitor adverse events, organ function, immune response, and virus studies.
We amended the protocol to require patients aged 18 years or older to have a biopsy of an active tumor on day 7 after intravenous virus administration to evaluate for the presence of Seprehvir within the tumor. We limited this biopsy to patients over the age of 17 years because the biopsy carried additional risk without clear benefit and would be unethical to perform in children. In choosing which site to biopsy if more than one was available, we planned to target most actively growing areas based on PET scan imaging. The biological correlative studies were limited to blood work and tissue studies for HSV-1 and did not include immunologic analyses.
Dose-Limiting Toxicities
Toxicity was graded according to the NCI Common Toxicity Criteria (CTCAE) v.3.0. DLT was any grade 3 or 4 toxicity, or grade 2-4 neurologic or allergic toxicity that was possibly, probably, or definitely attributable to participation in the study (with the exclusion of grade 3 flu-like symptoms, grade 3 anorexia, and grade 3 pain in tumors). The highest tested and tolerated dose was predefined as the highest dose level of Seprehvir administered at which no more than one out of six patients experienced a DLT.
Evaluation of Clinical Activity
Baseline imaging was obtained within 14 days prior to the first Seprehvir dose. After injection, imaging was obtained after 14 days, 28 days, 3 months, 6 months, and then as clinically indicated until withdrawal from the trial. All measurable lesions were deemed target lesions and were followed for response as appropriate for cancer type and location. We evaluated response according to modified RECIST guidelines at days 14 and 28. The modification varied from RECIST v.1.0 because we measured the longest diameter instead of the sum of the longest diameters.
Virus Production, Handling, and Administration
Vials of Seprehvir were manufactured by UK BioReliance and administered at a dose of 5 × 104 infectious units (iu)/kg or a maximum of 2 × 106 iu/kg used in dose level 1, or 2.5 × 105 iu/kg or a maximum of 1 × 107 iu used in dose level 2. Infectious units are defined as the equivalent of PFU per milliliter. Seprehvir was stored in an ultralow freezer (−80°C) until patient arrival.
Frozen vial(s) of Seprehvir were transported on dry ice to the investigational drug pharmacy clean room and hand thawed. Vials were checked immediately for clarity and particulate matter, sprayed, and wiped down with 70% ethanol. The vials were re-suspended before the virus was injected into a 250-mL bag of lactated Ringer’s solution for intravenous infusion. The bag was gently mixed using a backward and forward rocking motion. Once the label was placed on the bag containing lactated Ringer’s and Seprehvir, the virus was administered via a peripheral vein within 3 h of preparation of the Seprehvir product.
All vials contained an additional 0.1 mL of Seprehvir for quality assurance testing. Immediately following injection, vials containing residual Seprehvir were transported on ice to the laboratory for post-procedure virus titer assessment using the standard plaque assay procedure as previously described.21 In parallel, 10 Seprehvir control vials were thawed and assayed for quality assurance. We followed standard biosafety level 2 precautions. The acceptable range established for 10 control vials at 2 × 106 iu was 6.3 × 105 to 6.3 × 106 iu (2 SDs). All post-injection titers were within the expected range except for HSV02, whose HSV post-injection titer was 2.4 × 105 iu. This was the only sample not measured immediately, and the lower value was likely due to freeze-thaw in lactated Ringer’s solution prior to testing (Table S1).
Author Contributions
Conceptualization, T.P.C., M.A.C., and J.C.; Methodology, T.P.C., M.A.C., and J.C.; Investigation, T.P.C., M.A.C., and K.A.S.; Writing – Original Draft, K.A.S.; Writing – Review and Editing, K.A.S., J.C., and T.P.C.; Resources, J.C., K.S., M.A.R., B.S., M.A.S., S.W., N.D.Y., K.B.H., and T.P.C.; Visualization, K.A.S. and T.P.C.; Supervision, T.P.C.; Project Administration, M.T., K.O., D.J.D., and M.R.V.; Funding Acquisition, T.P.C.
Conflicts of Interest
J.C. and K.S. are employees of Virttu Biologics, Ltd. K.A.S. is a consultant/advisory board member for Amgen Inc. The other authors declare no competing interests.
Acknowledgments
Support for this research was provided by the OSU Comprehensive Cancer Center using Pelotonia funds and Alex's Lemonade Stand Foundation. We thank members of the Nationwide Children’s Hospital Division of Hematology/Oncology/BMT Clinical Research Office including Myeshia Harmon, Melissa Jaskiewicz, Amy Yekisa, and members of the H12 nursing staff including Deborah Hockett, Sam Howorka, and Emily Smith. We thank nurse navigators Paula Sanborn and Jennifer English. We thank Jill Blind in the NCH investigative pharmacy. We thank the referring oncologists as well as the patients and their families who participated in this trial. Finally, we thank Robert Arceci (in memorium), Paul Sondel, E. Anders Kolb, and Eileen King for serving on the data safety monitoring board and reviewing the safety and toxicity data.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.08.020.
Supplemental Information
References
- 1.McGeoch D.J., Dalrymple M.A., Davison A.J., Dolan A., Frame M.C., McNab D., Perry L.J., Scott J.E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 2.Perry L.J., McGeoch D.J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 1988;69:2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- 3.Andtbacka R.H., Ross M., Puzanov I., Milhem M., Collichio F., Delman K.A., Amatruda T., Zager J.S., Cranmer L., Hsueh E. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 2016;23:4169–4177. doi: 10.1245/s10434-016-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streby K.A., Geller J.I., Currier M.A., Warren P.S., Racadio J.M., Towbin A.J., Vaughan M.R., Triplet M., Ott-Napier K., Dishman D.J. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin. Cancer Res. 2017;23:3566–3574. doi: 10.1158/1078-0432.CCR-16-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke M.J., Ahern C., Weigel B.J., Poirier J.T., Rudin C.M., Chen Y., Cripe T.P., Bernhardt M.B., Blaney S.M. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children’s Oncology Group. Pediatr. Blood Cancer. 2015;62:743–750. doi: 10.1002/pbc.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb E.A., Sampson V., Stabley D., Walter A., Sol-Church K., Cripe T., Hingorani P., Ahern C.H., Weigel B.J., Zwiebel J., Blaney S.M. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr. Blood Cancer. 2015;62:751–758. doi: 10.1002/pbc.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cripe T.P., Ngo M.C., Geller J.I., Louis C.U., Currier M.A., Racadio J.M., Towbin A.J., Rooney C.M., Pelusio A., Moon A. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015;23:602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glade Bender J.L., Lee A., Reid J.M., Baruchel S., Roberts T., Voss S.D., Wu B., Ahern C.H., Ingle A.M., Harris P. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children’s oncology group phase I consortium report. J. Clin. Oncol. 2013;31:3034–3043. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y., Kim T., Bhargava A., Schwartz L., Brown K., Brody L., Covey A., Karrasch M., Getrajdman G., Mescheder A. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol. Ther. 2009;17:389–394. doi: 10.1038/mt.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P.Y., Swain H.M., Kunkler A.L., Chen C.Y., Hutzen B.J., Arnold M.A., Streby K.A., Collins M.H., Dipasquale B., Stanek J.R. Neuroblastomas vary widely in their sensitivities to herpes simplex virotherapy unrelated to virus receptors and susceptibility. Gene Ther. 2016;23:135–143. doi: 10.1038/gt.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currier M.A., Sprague L., Rizvi T.A., Nartker B., Chen C.Y., Wang P.Y., Hutzen B.J., Franczek M.R., Patel A.V., Chaney K.E. Aurora A kinase inhibition enhances oncolytic herpes virotherapy through cytotoxic synergy and innate cellular immune modulation. Oncotarget. 2017;8:17412–17427. doi: 10.18632/oncotarget.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillory L.A., Megison M.L., Stewart J.E., Mroczek-Musulman E., Nabers H.C., Waters A.M., Kelly V., Coleman J.M., Markert J.M., Gillespie G.Y. Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of neuroblastoma. PLoS ONE. 2013;8:e77753. doi: 10.1371/journal.pone.0077753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schellingerhout D., Bogdanov A., Jr., Marecos E., Spear M., Breakefield X., Weissleder R. Mapping the in vivo distribution of herpes simplex virions. Hum. Gene Ther. 1998;9:1543–1549. doi: 10.1089/hum.1998.9.11-1543. [DOI] [PubMed] [Google Scholar]
- 14.Braidwood L., Learmonth K., Graham A., Conner J. Potent efficacy signals from systemically administered oncolytic herpes simplex virus (HSV1716) in hepatocellular carcinoma xenograft models. J. Hepatocell. Carcinoma. 2014;1:149–161. doi: 10.2147/JHC.S71019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samson A., Scott K.J., Taggart D., West E.J., Wilson E., Nuovo G.J., Thomson S., Corns R., Mathew R.K., Fuller M.J. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018;10:eaam7577. doi: 10.1126/scitranslmed.aam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downs-Canner S., Guo Z.S., Ravindranathan R., Breitbach C.J., O’Malley M.E., Jones H.L., Moon A., McCart J.A., Shuai Y., Zeh H.J., Bartlett D.L. Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients With Advanced Solid Cancers. Mol. Ther. 2016;24:1492–1501. doi: 10.1038/mt.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q., Nieva J., Hwang T.H., Moon A., Patt R. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 18.Hutzen B., Chen C.Y., Wang P.Y., Sprague L., Swain H.M., Love J., Conner J., Boon L., Cripe T.P. TGF-β Inhibition Improves Oncolytic Herpes Viroimmunotherapy in Murine Models of Rhabdomyosarcoma. Mol. Ther. Oncolytics. 2017;7:17–26. doi: 10.1016/j.omto.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C.Y., Wang P.Y., Hutzen B., Sprague L., Swain H.M., Love J.K., Stanek J.R., Boon L., Conner J., Cripe T.P. Cooperation of Oncolytic Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Sci. Rep. 2017;7:2396. doi: 10.1038/s41598-017-02503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsoneva D., Minev B., Frentzen A., Zhang Q., Wege A.K., Szalay A.A. Humanized Mice with Subcutaneous Human Solid Tumors for Immune Response Analysis of Vaccinia Virus-Mediated Oncolysis. Mol. Ther. Oncolytics. 2017;5:41–61. doi: 10.1016/j.omto.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshun F.K., Currier M.A., Gillespie R.A., Fitzpatrick J.L., Baird W.H., Cripe T.P. VEGF blockade decreases the tumor uptake of systemic oncolytic herpes virus but enhances therapeutic efficacy when given after virotherapy. Gene Ther. 2010;17:922–929. doi: 10.1038/gt.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.