Graphical abstract
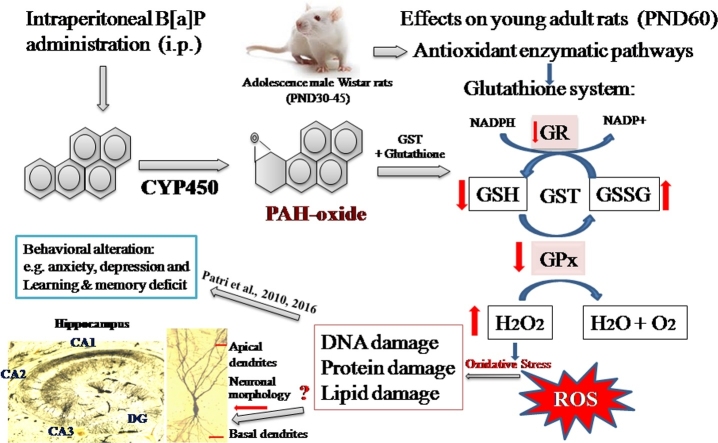
Schematic diagram for the mechanism B[a]P metabolism by activation that occurs through cytochrome P450 enzymes. During cellular metabolism large amounts of reactive oxygen species (ROS) are generated. The resulting oxidative stress induces the alteration of antioxidant enzymes (GST, GPx, GR and total glutathione) leading towards behavioral changes with altered neuronal morphology in the hippocampus of adult wistar rat brain. Early exposure to B[a]P correlates with impaired learning and memory in adults, and reduction in dendrite population in CA1 regions of hippocampus during development.
Keywords: PAHs, ROS, Oxidative stress, Antioxidant enzymes, Anxiolytic like behavior, Depression, Learning and memory
Highlights
-
•
Intraperitoneal B[a]P administration induces anxiolytic like behavior in rats.
-
•
B[a]P induces oxidative stress and reduces antioxidant enzyme activity.
-
•
Exposure to B[a]P-induces decrease in dendrite length and spine density through oxidative stress affecting antioxidant defence system.
-
•
Alteration in the neuronal architecture of the hippocampal cells after B[a]P administration is associated with learning and memory defict.
Abstract
Exposure to benzo[a]pyrene (B[a]P), a prototype of polycyclic aromatic hydrocarbons (PAHs) easily cross blood brain barrier (BBB) and is associated with impaired learning and memory in adult rats. However, there is no symmetric study reported on association between B[a]P exposure during the early development and hippocampal dendritic architecture causing behavioral changes like learning and memory deficit of adult rats. We investigated a fourteen day consecutive B[a]P administration, intraperitonial (i.p.), with two different doses (0.1 and 1μM) during early adolescence at PND30-44 and learning behavior assessed between PND 45-60 in adult male rats. The anxiolytic like behavioural analysis was done by LDPT. Depressive like behaviour was estimated through sucrose preference and learning and memory by T-maze. After B[a]P administration oxidative stress markers like glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx), reduced (GSH) and oxidized glutathione (GSSG) were evaluated. To parallel these behavioral and antioxidant level changes to alteration in dendritic morphology, Golgi-Cox staining was performed in the hippocampus. Our study showed anxiolytic like behavioral response with significant increase in time spent in light zone and significant (p < 0.05) decrease in preference for sucrose and a reduction in percentage of spontaneous responses in T-maze test in B[a]P administered group as compared to vehicle control. B[a]P exposed male rats showed significant increase in GST activity (p < 0.05) and concentration of GSSG with a decay in GSH, GPx and GR in both the groups as compared to control. B[a]P administered rats showed significant loss in total dendritic length and number (28%) with reduced spine density (18%) in both higher and lower doses. These results suggested that B[a]P administration can be associated with an increase ROS production showing altered antioxidant defence system through glutathione biosynthesis and causing profound alterations in dendritic length and spine density of hippocampal neurons leading towards learning and memory deficits in adult rats.
1. Introduction
The environmental pollutants like polycyclic aromatic hydrocarbons (PAHs) generated from the incomplete combustion of fossil fuels are found in ambient air, food, water and sediments [1]. Benzo[a]pyrene, (B[a]P) has been classified as prototype of PAHs and the most studied member of PAHs having high molecular weight [2]. The lipophilic property of B[a]P enable this compound to easily cross the BBB and placenta during pregnancy and directly access the central nervous system [3]. Previous studies advocated the susceptibility of neonates to chronic PAHs exposure which might be due to postnatal developmental immaturity leading to slower clearance of the toxicants from the body [4]. B[a]P is a potent neurotoxicant with high affinity for lipid-rich tissues such as brain, it enters into the brain tissues and enhances the generation of reactive oxygen species (ROS), resulting significant changes in mood behaviour [5]. Moreover, when exposed to high levels, B[a]P affects thyroid function in fish [6] and its metabolites tend to accumulate both in the blood and brain [7], thus causing neurological impairments [8]. Early exposure to PAHs may adversely affect children’s IQ, cognitive development and increased behavioral problems [[8], [9], [10], [11]]. Blood levels of PAHs in children are correlated with oxidative stress and altered antioxidant status, during metabolism [[12], [13], [14], [15]]. PAHs were also detected in smoked fish with high concentration of B[a]P inducing serious health effects in humans [16,17], but the cellular mechanism of action is not clear. Due to rapid biotransformation only minimal levels of unmetabolized B[a]P could be detected.
Exposure to B[a]P is associated with many adverse biological effects due to oxidative stress, that may disrupt oxidant-antioxidant balance within the brain affecting antioxidant enzyme function [18]. Antioxidant enzymes play a central role in cell homeostasis by maintaining the cellular antioxidant status [19,20]. This phenomenon sometimes leads to depletion in glutathione antioxidant system and increases in production of ROS causing oxidative stress [21,22], whereas the biological basis for B[a]P-induced neurobehavioral toxicity is as yet unclear. Some previous studies in cell line models have proposed that B[a]P neurotoxic effects are due to the severe levels of oxidative stress [23]. During cellular metabolism B[a]P can undergo intracellular biotransformation to reactive intermediates due to metabolic activation to diol-epoxides by cytochrome P450 (CYP) enzymes leading to production of ROS [24]. These studies suggest a possible mechanism by which B[a]P could affect neuronal behavior of adult rats through cellular oxidative stress and disturbance in the balance between ROS and antioxidants production [25]. However, previous studies mostly focuses on the effects of prenatal stress in adulthood and relatively little is known about the early adolescence exposure, during a critical phase of neonatal brain development.
Antioxidant enzymes play a major role in eliminating the active oxygen to protect the cells against adverse effects of environmental toxic radicals [26], notably glutathione system by reacting with the free radicals [27]. Induction of the antioxidant enzymes can be considered as a self-defence process of organisms to adapt the adverse environment and to reduce toxicity [26,27]. B[a]P is known to enhance the production of ROS (H2O2 and OH+) initiate reactions with cellular biomolecules like DNA [28] and the enzymatic glutathione system plays vital role in detoxification of this compound through disposal of peroxides by brain cells [[28], [29], [30]]. The enzyme glutathione-S-transferase (GST) plays a major role in the detoxification of many environmental chemicals by catalyzing the conjugation of glutathione (GSH) to electrophilic compounds (e.g. epoxides of B[a]P), rendering them less reactive and more water-soluble [31]. Mostly, glutathione exists in the reduced (GSH) as well as oxidized (GSSG) forms, which can be interconvert by glutathione peroxidase (GPx) and glutathione reductase (GR) respectively. Glutathione dependent enzymes such as GPx, GR can scavenge the free radicals thus neutralizing ROS, and maintaining redox balance [31,32]. The cycling between GSH and GSSG serves to remove ROS for protection of cells though protecting the cells from oxidative stress [33,34]. Therefore, the antioxidant enzymes and antioxidant molecules are believe to play major role in the prevention of oxidative stress and enhance the survival of neurons in brain through the activation of GSH system and other antioxidant enzymes [35]. Many studies provide a causal link between the prenatal B[a]P exposure and neurodegeneration involving production of free radicals contributing to behavioural deficits by altering antioxidant defence system in adult rats [36,37]. But the molecular mechanisms underlying B[a]P-induced oxidative stress causing spine loss and dendritic retraction in adult during adolescence in the hippocampus are not well understood.
Identifying measures of brain function during adolescence is critical, particularly in relation to stress, because majority of human adolescents reported mood disturbances and anxiety [38]. It is also a time period during which hormonal changes and alterations in lifestyle can affect neuronal proliferation and survival within the dentate gurus [39]. In human, the formation of neuronal networks and synaptic connections occurs during early adolescent period and environmental exposure to B[a]P may alter spine density, number of granule cells and overall volume of the hippocampal layers [40]. Dendritic complexity is an integral index which depends on the sum of terminal orders, number of ends, total dendritic length and number of primary dendrites and is a marker directly related to the functional complexity and connectivity of neurons [41]. Changes in their density and morphology are indicative of cellular processes like excessive activation of receptors [42,43] related to and have been in various neuropsychiatric disorders [44,45].
Communication between neurons primarily takes place at synapses and there are several dynamic processes to control the morphology, number and strength of brain synapses [46]. Therefore, understanding the dynamics of the dendritic spine growth and elimination can provide key insights to the mechanisms of structural plasticity. In addition to learning and memory formation, the connectivity of neural networks also affects cognition, perception, and behavior [47]. There are well known differences in the learning ability and the memory performance between young children and adults [48]. Earlier studies have mainly focuses on the adult behavior with the role of neurogenesis in hippocampus showing alteration in cognitive function after exposure to environmental stress [46,49], but it is also important to consider the effect across the lifespan to know the differential effects. Therefore, in the present study we have focused on the impact of early adolescence B[a]P exposure that could affect hippocampal neuronal morphology like dendritic spine density due to oxidative stress and antioxidant defence system altering the cognitive function of adult male wistar rats.
2. Materials and methods
2.1. Ethics statement
Male Wistar rat (Rattus norvegicus) pups at postnatal day 21 (PND21) were obtained from the animal house of Ravenshaw University, Cuttack. The experiments were carried out according to Ravenshaw University ethical guidelines for the care and use of laboratory animals. The experiments were approved by the Institutional Animal Ethics Committee (Regd. No.1927/Go/Re/S/16/CPCSEA).
2.2. Chemicals and reagents
The chemicals used in this experimentation such as B[a]P were purchased from Sigma-Aldrich Chemicals Co., St. Louis, MO, USA,. Other chemicals unless otherwise mentioned like dimethylsulfoxide (DMSO) and paraformaldehyde, etc. were purchased from Sisco Research Laboratories (SRL, India).
2.3. Experimental animals and treatment
The male Wistar rat pups (weight of 55 g–65 g) weaned on PND21 were obtained from animal house of Ravenshaw University and acclimated to laboratory conditions for 7 days under standard condition at a controlled temperature 22 °C (±3 °C), 60% humidity and 12 h light/12 h dark cycle in home cages with free access to tap water and standard diet ad libitum. We performed fourteen day consecutive B[a]P administration (i.p.) to adolescence rats between PND30-45. Rats were randomly divided into four experimental groups (n = 6 in each group); Naïve (without any treatment), control (vehicle treated) received 10 μl of DMSO (<0.1%) and two different B[a]P treated groups; 1μM (higher) and 0.1μM (lower) doses B[a]P suspended in 10 μl of DMSO.
Before B[a]P administration (i.p.) rats were anesthetized with mild ether to ensure unwanted side effects. The behavioural tests were performed from PND45 to PND60 under close observation and left undisturbed until early adulthood. The 24 h time interval between B[a]P treatment and the start of the behavioural tests (PND 45-60) was given in the time line of experiments (Supplementary Fig. 1). All animal handling procedures with respect to food and water supply, cage material changes and B[a]P administration were performed in the time window of light cycle (10:00 to 11:30 a.m.) to avoid experimental deviations due to diurnal cycle and behavioural tests were conducted between 9.00 a.m. and 1.00 p.m. in standard conditions [48].The animals were sacrificed after completion of behavioural test (PND60) and brain sections of hippocampal region were snap frozen, washed in normal saline and stored at -80 °C for biochemical analysis.
2.4. Behavioural analysis
2.4.1. Light and Dark Preference Test (LDPT)
Light and Dark box test is a method to test the anxiolytic like behaviour of rats. In Light and Dark box, the time spent in the light chamber was assessed between PND 45-60 for anxiolytic like activity with slight modifications of our previous study [20]. The light and dark box is made of glass (32 × 40 × 30) and divided into two equal compartments i.e. light and dark chamber by a partition. The one side of the box is coloured black and covered from the top so that no light could entered into it, whereas, the other part is transparent and exposed to bright light. There is an opening within the box at the base for the exploratory behaviour. During the test session the rat was placed in the light zone and allowed to move between both the compartments inside the apparatus placed on the floor of the experimental room and a camera was mounted above the apparatus to record the total time spent in light and dark section with transition between two sections during 5 min test session (ANY maze video tracking System). The rat then was taken out of the box and then the box was swipe with 70% ethanol to avoid experimental error.
2.4.2. T-maze test
T-maze test especially used in rodent models for nervous system disorders was performed according to the protocol of previous studies [49,50] with some modifications. This behavioral test is based on the exploratory behavior of rodents to a new environment, i.e. they prefer to visit a new arm of the maze rather than a familiar arm. The T-maze is made up of acrylic material with two arms left arm and right arm. Two arms of the maze are (50 cm long, 10 cm in width, with walls of 25 cm height) and the starting arm is (70 cm) separated by an acrylic partition. The maze was placed on a table with a height of 80 cm. The start arm was (20 cm in length) separated from the maze by an acrylic partition.
On Day 1 of the training each rat was placed in the centre of the T-maze and was free to explore the each arm to habituate the rats to the T-maze. On training Day 2 and Day 3, food pellets were placed as a reward in left arm of the maze and rats were placed on the start arm and then allowed to explore through the maze for 5 min test session and the number of arm entries and the number of triads were recorded (ANY maze video tracking System) to calculate the percentage of spontaneous alternation. On Day 4 and Day 5, the actual test was conducted at with food deprivations and adult rats at PND 45-60 were placed on the start arm allowed to explore the maze for 5 min test session. The number of turns in each goal arm with the percentage of spontaneous alterations, total time spent in each arm with trial duration of the rat was recorded and calculated as spontaneous alternation/(total number of arm entries-2) x100. Between each consecutive test the maze was cleaned thoroughly with a 70% ethanol solution and dried between animals.
2.4.3. Sucrose Preference Test
The sensitivity to reward was assessed by a sucrose preference test according to Forbes et al [51] to check depressive like mood behaviour in rats. Rats are born with an interest for sweet food and water, any deviation from the normal physiological behaviour indicates altered behavioural response. Here, reduced preference for sweet solution in sucrose preference test was a putative indicator of anhedonia in rats. Anhedonia is the decreased ability to experience pleasure and depressive mood behaviour. The adult male rats between PND 45-60 kept in starvation for food and water for 24 h before the start of experiment was brought to the testing room approximately 30 min before the test. Then fresh water and 1% sucrose solution was poured in separate feeding bottles and rats were allowed to feed. After 8 hs duration the volume of the liquid consumed per bottle was noted. Again the same procedure continued by altering the position of the bottle till 6 consecutive readings were taken for 4 days. Weight of the bottle and volume of the liquid consumed per bottle was even noted. Preference for sucrose was calculated in ml sucrose solution consumed/total ml of sucrose solution respectively.
2.5. Biochemical analysis
The total protein content was calculated according to Lowry et al [52] using the bovine serum albumin as standard. The homogenate of hippocampus, 10% (w/v) was prepared in ice cold homogenizing buffer (Phosphate Buffer, 50 mM, pH 7.4) for assay of antioxidant glutathione biosynthesis system (GST, GR, GPx, GSH, GSSG and OSI) according to our previous study by Patel et al [20] and for redox potential of GSH/GSSG the protocol of Jones DP [53,54]. For these experimental assays samples of hippocampus of brain was passed in sephadex G-25 column before estimation of protein.
2.5.1. Glutathione S-transferase assay (GST)
The enzyme (GST) was measured by procedure described previously [53]. GST in tissue sample was quantified by monitoring conjugation reaction of GSH and a broad spectrum substrate of GST called as 1-chloro-2,4-dinitro benzene (CDNB), observing increase in absorbance at 340 nm by UV-VIS double beam spectrophotometer (Systronics, India). The enzyme activity was calculated using molar extinction coefficient for CDNB (9.6 × 103M-1cm-1) and expressed as nmol CDNB conjugated formed/min/mg protein.
2.5.2. Glutathione Reductase Assay (GR)
The enzyme glutathione reductase catalyse the reduction of oxidized glutathione (GSSG) into its reduced form i.e., GSH [54]. This chemical reaction was simultaneously accompanied by oxidation of NADPH to NADP + . The enzyme activity of tissue sample was assayed by decrease in absorbance of the reaction mixture at 340 nm (UV-VIS double beam spectrophotometer, Systronics, India) as NADPH is progressively converted to NADP + . The enzyme activity was calculated using molar extinction coefficient (6.22 × 103M-1cm-1) and expressed as nmol NADPH oxidized/min/mg protein.
2.5.3. Glutathione Peroxidase Assay (GPx)
The GPx enzyme was assayed as per the protocol previously suggested with certain modifications [54,55]. GPx enzyme catalyses detoxification of hydrogen peroxide and other organic peroxides by oxidising reduced glutathione (GSH). Total GPx activity was measured using cumene-hydro peroxide as substrate whereas selenium-dependent GPx (Se-D GPx) was determined using tert-butylhydroperoxide as substrate at 340 nm by UV-VIS double beam spectrophotometer (Systronics, India). The activity of Se-independent GPx (Se-I GPx) was calculated by subtracting activity of Se-D GPx from that of total GPx. The enzyme activity was also calculated using molar extinction coefficient for NADPH (6.22 × 103M-1cm-1) and expressed as nmol NADPH oxidized/min/mg protein.
2.5.4. Estimation of glutathione content (GSH, GSSG, Total glutathione)
The total glutathione assayed was carried out as per our previous protocol with certain modifications [20,53]. The content of intracellular GSH was detected by the enzymatic recycling method. In short the Sephadex G-25 column passed supernatant sample was taken. Samples (0.2 ml) were first treated with equal volume of TCA/HCL solution (final concentration 5% /0.001 N). The samples were first centrifuged at 1000 g for 15 min; pellets were discarded, precipitated proteins and supernatants samples were used for the assay. The reaction rate and the standard curves prepared earlier were used to calculate concentrations of GSSG and redox ratio is determined by the calculating the ratio of GSH to GSSG. First the total Glutathione content was calculated by taking 1.4 ml NADPH in pipette into the quartz cuvette with 0.2 ml DTNB suitably added to the 0.1 ml diluted supernatant sample to the quartz cuvette and mixed thoroughly. A mixture of potassium phosphate buffer along with EDTA of 0.28 ml was added simultaneously. GR 20 μl was then suitably added to the cuvette and mixed gently to start the reaction. Increase in absorbance was recorded at 412 nm every 1 min interval of 6 min in a UV-VIS spectrophotometer.
2.6. Histopathological study by Golgi-cox staining
The Golgi-Cox solution contained 5 parts of 5% potassium dichromate, 5 parts of 5% mercuric chloride, 4 parts of 5% potassium chromate in 10 parts of double distilled water were freshly prepared and the rat brains were removed and placed in a brain slicer (WPI, USA) for 5 mm thick coronal sections. Then blocks of brain sections containing the antero-posterior extension of hippocampus were taken out from each rat brain. The freshly prepared Golgi-Cox stain was kept at 37 °C for 72 h and each brain tissue block was placed in separate cotton-lined dark colored glass bottle containing 25–30 ml solution and slides were prepared as reported earlier [56]. In brief, coronal sections through hippocampus (200 μm thick) were cut using a cryostat (Leica CM3050 S, Germany). The brain sections were first rinsed and then dehydrated with 50% ethanol and then transferred into ammonia solution (3:1, 25% ammonia: distilled water) for staining. After stained sections were rinsed and treated with 5% sodium thiosulfate in dark and it was again rinsed in distilled water. Then the sections were dehydrated through increasing grades of alcohol (70, 80, 95% of ethanol and 99% of 1-butanol), cleared in toluene, and mounted in DPX on gelatine-coated slides. The slides were allowed to dry for 72 h at room temperature and observed under microscope (Olympus, BX43 F, Japan) with CCD camera attached at low and high magnifications. Spine density of the CA1 apical proximal dendrites and basal dendrites was calculated as the average number of spines per micrometer of dendritic length. The neuronal dendrite and spine measurement was carried out by using ImageJ software (NIH, Bethesda, MD, USA).
2.7. Statistical analysis
The data were expressed as the means ± standard error of mean (SEM) of five independent experimental groups (n = 6). All data observed from the number of replicates for each experiment was three and each set contain four experimental groups, i.e., naive, vehicle control (DMSO < 0.1%), higher (1μM/10 μl) and lower (0.1μM/10 μl) doses of B[a]P. The statistical analysis for behavioral studies in all experimental groups was carried out by using one-way ANOVA and post hoc analysis was done by the Newman-Keuls test (Sigma Stat software, Jandel Scientific USA). In all groups the difference below the probability level p < 0.05 was also considered statistically significant.
3. Results
3.1. Behavioural test
3.1.1. B[a]P exposure induced an anxiolytic like behaviour in adult rats
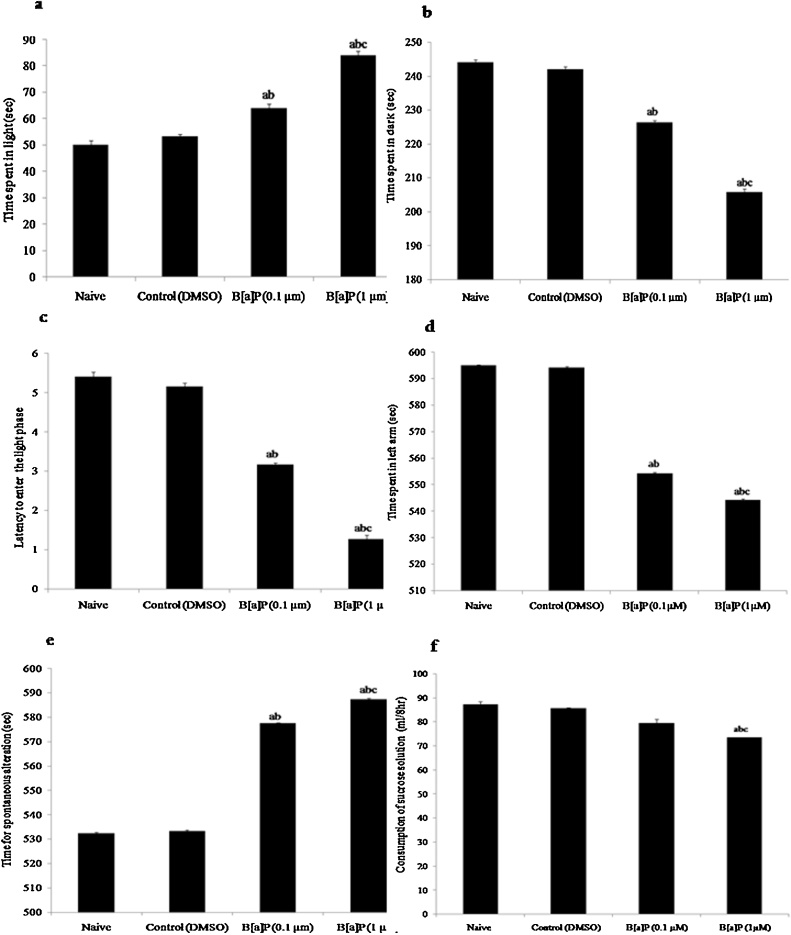
The ANOVAs on the B[a]P higher dose administered groups revealed main effects in light and dark box test with significant increase in the time spent in light phase (F3,20 = 59.82, p < 0.05). The time spent in the dark zone (F3,20 = 33.46, p < 0.05) were also observed in higher dose of B[a]P treated groups showing decrease time duration as compared to naive and vehicle control groups [Fig. 1: (b)]. Similarly, transfer latency to light (F3,20 = 21.59, p < 0.05) significantly decreased in higher dose of B[a]P treated groups (1μM/10 μl DMSO) as compared to naïve and vehicle control (DMSO) groups in LDPT [Fig. 1: (a)–(c)].
Fig. 1.
Behavioural studies: Effects of early adolescence intraperitoneal (i.p.) administration of B[a]P on adult wistar rats behavior.
(a) Time spent in the light zone (sec), (b) Time spent in the dark zone (sec), (c) Latency to enter into light phase in LDPT (d) Time spent in left arm (sec) and (e) Time for the spontaneous alteration (sec) in T-maze test (f) Consumption of sucrose solution (ml/8 h) in sucrose preference test in naive, control (DMSO), B[a]P treated groups (0.1 μM/10 μl) and B[a]P (1 μM/10 μl). Values are expressed as mean ± SEM, n = 6. ‘a’ denotes p < 0.05 when compared to naïve group, ‘b’ denotes p < 0.05 when compared to control (DMSO) group and ‘c’ denotes p < 0.05 when compared to B[a]P (0.1 ⁎μM).
3.1.2. B[a]P impaired learning and memory in adult rats
The T-maze test was used to assess the effects of B[a]P on the learning and memory of these two groups of B[a]P treated rats. The statistical analysis showed significant decrease in the time spent in left arm (F3, 20 = 5690, p < 0.05), with significant increase in the time for spontaneous exploration performance (F3,20 = 6725, p < 0.05) in higher dose of B[a]P treated groups (1μM/10 μl DMSO) as compared to naive and vehicle control (DMSO) groups [Fig. 1: (d) and (e)], implicating that exposure to the higher doses of B[a]P impaired learning and memory in adult rats.
3.1.3. B[a]P-indued depression-like behavior
Sensitivity to reward can be assessed by a simple sucrose preference test in which animals have access to water without and with different concentrations of sucrose, and the preference rate is then analyzed. The sucrose preference test showed decrease in preference for sucrose solution (F3,20 = 101.2, p < 0.05) in higher dose of B[a]P treated groups (1μM/10 μl DMSO) as compared to naive and vehicle control (DMSO) groups However, the lower dose of B[a]P treatment to healthy adolescence rats had no effect on the preference for sucrose solution in sucrose preference test in early adult male rats. [Fig. 1 : (f)].
3.2. Estimation of total protein content
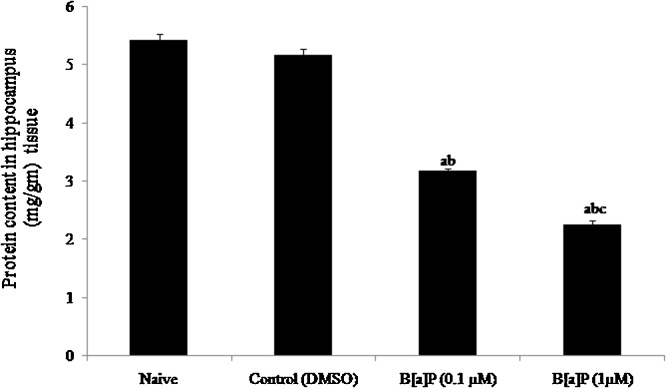
Brain lysates were prepared from the brain tissue in ice cold homogenizing buffer (Phosphate Buffer, 50 mM, pH 7.4) and the total protein content was estimated by Lowry et al [49]. The result showed significant reduction (F3, 20 = 323.9, p < 0.05) in the protein content in hippocampus of adult rats in the subsequent higher and lower doses of B[a]P treated male wistar rat groups as compared to naive and vehicle control (DMSO) groups (Fig. 2).
Fig. 2.
Protein content in hippocampus: The representative graph indicates significantly reduced protein content in the hippocampus of adult Wistar rats in the B[a]P treated groups after administration (i.p.) at PND30-45 rats as compared to naive and control groups.
3.3. Antioxidant activity
3.3.1. Glutathione-S-Transferase (GST) activity
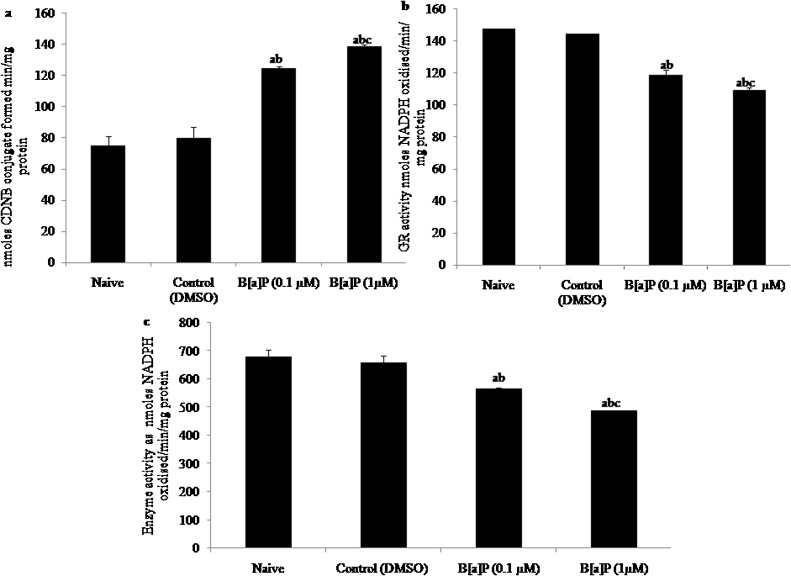
Administration (i.p.) of B[a]P during early adolescences period showed significant increase in Glutathione-S-Transferase (GST) activity (F3,20 = 47.88, p < 0.05) during early adulthood in both the higher and loer dose of B[a]P treated groups as compared to naive and control (DMSO) groups [Fig. 3: (a)].
Fig. 3.
Antioxidant enzymatic activity : Effects of early adolescence intraperitoneal administration of B[a]P to Wistar rats on Glutathione system (a) Glutathione-S-Transferase (GST) in nmol CDNB conjugate formed/min/mg protein, (b) Glutathione Reductase (GR), and (c) Glutathione peroxidase (GPX) in nmol NADPH oxidised/min/mg protein in Hippocampus of adult Wistar rats in naive, control (DMSO), B[a]P treated groups (0.1 μM/10 μl) and B[a]P (1 μM/10 μl).
Values are expressed as mean ± SEM, n = 6. ‘a’ denotes p < 0.05 when compared to naïve group, ‘b’ denotes p < 0.05 when compared to control (DMSO) group and ‘c’ denotes p < 0.05 when compared to B[a]P (0.1 ⁎μM).
3.3.2. Glutathione Reductase (GR) activity
Early B[a]P administration (i.p.) to adolescence rats showed significant decrease in Glutathione Reductase (GR) activity (F3,20 = 99.42, p < 0.05) in early adulthood of male rats in the higher as well as lower dose of B[a]P treatment as compared to naive and vehicle control (DMSO) groups [Fig. 3: (b)].
3.3.3. Glutathione Peroxidase (GPX) activity
Administration (i.p.) of B[a]P during adolescences showed significant decrease in Glutathione Reductase (GR) activity (F3,20 = 31.43, p < 0.05) in early adult male wistar rats in the higher and lower dose of B[a]P treated groups as compared to naive and vehicle control (DMSO) groups [Fig. 3: (c)].
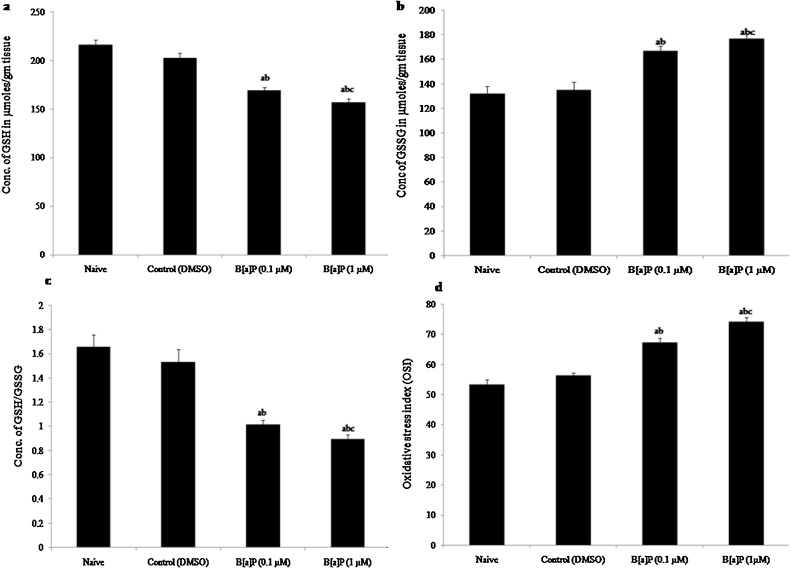
3.3.4. Estimation of glutathione (GSH, GSSG, redox ratio and oxidative stress index)
The GSH enzyme activity (F3,20 = 58.44, p < 0.05) and redox ratio (GSH/GSSG) (F3,20 = 24.98, p < 0.05) were decreased significantly in the higher dose of B[a]P treated group as compared to naive and vehicle control (DMSO) groups [Fig.4 (a) and (c)]. But, the oxidized glutathione (GSSG) (F3,20 = 23.80, p < 0.05) and oxidative stress index (OSI) (F3,20 = 4204, p < 0.05) increased significantly in the higher dose of B[a]P treated group as compared to naive and vehicle control (DMSO) groups [Fig. 4 (b) and (d)].
Fig. 4.
Concentration of Glutathione: Effects of early adolescence intraperitoneal (i.p.) administration of B[a]P to Wistar rats on total glutathione level (a) Concentration of reduced glutathione (GSH) in μmol/gm tissue, (b) Concentration of oxidised glutathione (GSSG) in μmol/gm tissue, (c) Redox ratio (GSH/GSSG), (d) Oxidative stress index (OSI) in Hippocampus of adult wistar rats in naive, control (DMSO), B[a]P treated groups (0.1 μM/10 μl) and B[a]P (1 μM/10 μl).
Values are expressed as mean ± SEM, n = 6. ‘a’ denotes p < 0.05 when compared to naïve group, ‘b’ denotes p < 0.05 when compared to control (DMSO) group and ‘c’ denotes p < 0.05 when compared to B[a]P (0.1 ⁎μM).
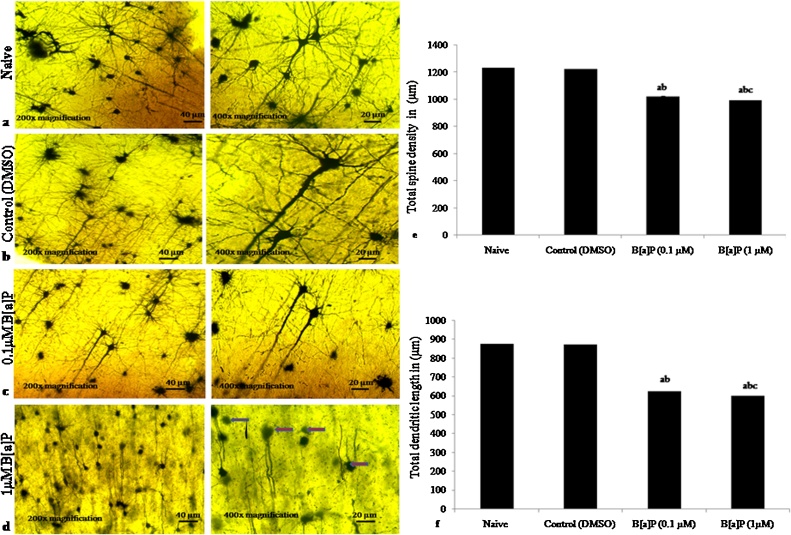
3.4. Histopathological study by Golgi-cox staining
Representative images of Golgi-Cox staining sections showing dendritic length and dendritic spine density of rat hippocampus during adult stage at PND60; naive [Fig. 5:(a)], control [Fig. 5:(b)], lower dose of B[a]P (0.1μM) [Fig. 5: (c)] and higher dose of B[a]P (1μM) [Fig. 5: (d)] after B[a]P administration (i.p.) to Wistar rats in early adolescences observed at 200x (Scale bar = 40 μm) and 400× magnifications (Scale bar =20 μm). Histogram showed change in total spine density (18%) and reduction in dendritic length (28%) of hippocampal neurons in Golgi-Cox stained brain sections (p < 0.05) in higher doses of B[a]P treated groups, as well as the lower doses of B[a]P with reduction in spine length and density as compared to naive and vehicle control groups [Fig. 5(d) and (e)]. The percentage change in dendritic length, spine number, density and nodes were also measured on the secondary branches of apical and basal dendrites of pyramidal neurons located in the CA1 subfield of the dorsal hippocampus (Supplementary Fig. 2). Number of fields examined = 30; with 3 independent experiments.
Fig. 5.
Effects of early adolescence intraperitoneal administration (i.p.) of B[a]P and measurement of dendrite morphology and spine density in adult wistar rats using Golgi-cox staining. Representative images of Golgi-Cox staining sections of adult rat hippocampus in naïve [Fig. 5:(a)], control (DMSO) [Fig. 5:(b)] and two different dose levels, B[a]P (0.1 ⁎μM) [Fig. 5:(c)] and B[a]P (1μM) [Fig. 5:(d)] of adult after intraperitoneal administration (i.p.) to early adolescence male wistar rats with (Scale bar = 100 μm) at 20x magnifications and (Scale bar =20 μm) at 40x magnifications (Fig.5 a).
Histogram representing alteration of spine length, spine number, density [Fig. 5: (e)] and dendrite nodes of neurons in hippocampus of two different dose levels of B[a]P treated groups [Fig. 5:(f)]; (0.1 μM/10 μl) and B[a]P (1 μM/10 μl) as compared to naïve and control (DMSO) groups. Values are expressed as mean ± SEM, n = 6. ‘a’ denotes p < 0.05 when compared to naïve group, ‘b’ denotes p < 0.05 when compared to control (DMSO) group and ‘c’ denotes p < 0.05; when compared to B[a]P (0.1 ⁎μM).
4. Discussion
In the present study, we evaluated B[a]P-induced oxidative stress during early adolescence on dendritic spine growth and neurobehavior development in adult male wistar rats following several typical neurobehavior including exploration and anxiety activities, learning and memory and depressive-like behavior, with standardized tests. We observed that B[a]P administration during early adolescence impaired short-time memory and behavioral performance and induces anxiety and depressive-like behavior with both higher and lower doses of B[a]P in adult male rats. Earlier studies has shown that with an oral dose of 100 mg per Kg body weight, 40% of B[a]P is available in the brain [2,3]. Moreover, cognitive deficits also have been reported in rats administered with 25 mg/kg body weight of 3-methylcholanthrene [5]. Therefore, to study the neurotoxic effect of B[a]P through i.p. administration, it is desirable to limit the dose to that of the environmental level (5–100 μg/kg). There are reports of high level of B[a]P exposure at industrial sites (200 mg/kg dry weight in contaminated sites) [57]. Workers in industrial areas exposed to 6–8 working hours per day for at least 4–6 months prior to blood analysis showed maximum 4–5 μg/B[a]P/m3 [58]. The highest environmental concentrations of B[a]P are found in soil samples and usually range from 0.8 ng kg-1 to 100 mg kg-1. B[a]P content in food stuffs and plants is between 0.1 and 150 μg kg-1, the air has a concentration of around 1.3 to 500 ng/m3 and drinking water generally contains 2.5 to 9 ng/L-1 [13].
The behavioral analysis after early B[a]P administration showed an anxiolytic-like traits having susceptibility to affective disorder and depressive symptoms with a significantly increased stay time in light and decreased latency to light phase in LDPT [48]. T-maze test showed significantly decrease time spent in left arm and increased exploratory behavior with time taken for spontaneous alteration indicative of altered learning and memory behavior in male adult rats. The results found in T- maze test suggested that lower doses of B[a]P exposure during early adolescence also showed learning disability as well as higher doses of B[a]P with impaired learning and memory functions at PND60. Reduced preference for sucrose solution in sucrose preference test showed a depressive-like behavior of adult rats at PND60. Previous studies have shown sub-chronic exposure of B[a]P also causes behavioral alteration of learning and memory in hippocampus of male rats [[10], [11], [12]]. Prenatal exposure to inhaled B[a]P causes deficits in learning and memory performance [35]. In a separate report of chronic B[a]P exposure found that a B[a]P dose of 6.25 mg/kg significantly impacted learning and memory [59].This study supports B[a]P-induced effects on the central nervous system of male rats are dose-dependent.
Cigarette smoking is a powerful risk factor for atherosclerotic cardiovascular disease and is associated with the increased incidence of stroke and coronary artery disease [4,58]. Light/dark cycle is also an important factor in studying the anxiolytic like behavior in rats. Not many reports have evaluated the age-dependent expression of anxiety-related behavior and even fewer studies available regarding the influence of diurnal cycles. Our observation showed that rats treated with B[a]P affects behavioral performance in light cycle, in particular altering neuronal architecture that supported by previous studies [[45], [46], [47]]. To the best of our knowledge, no study has analyzed the relationships between B[a]P-induced anhedonia and other behavioral endpoints, which is also associated with the dendrite spine alterations in adult rats [50]. However, to our knowledge, this is the first report documenting the change in dendritic morphology and behavioral phenotypes of two different dose levels of B[a]P exposure during major developmental time points of male wistar rats. Males tended to be more vulnerable to the long-term effects of early life and adolescent stress [59]. Recent studies also showed subtle changes in anxiolytic like mood behaviour and cognitive functioning of adult wistar rats at PND60 following administration to B[a]P (i.p.) during adolescence as the findings supported by previous studies [11,12,47].
Cellular proteins are the targets of oxidation and our results clearly indicate that the brain tissue protein content significantly reduced in hippocampus showing suffered reactive-oxygen intermediate mediated damage induced by early exposure to B[a]P. In the present study B[a]P-induced oxidative stress and the antioxidant glutathione system reflects significant increase in GST activity and concentration of GSSG, whereas the concentration of GR, GPx and GSH were decreased significantly in subsequent higher doses at PND60. Earlier studies shown decreased GST activity after B[a]P exposure which supports our findings [19,20,55]. The intracellular GSH/GSSG ratio is highly regulated. However, an increase in ROS levels can decrease the cell’s ability to convert GSSG to GSH. Under such conditions, GSSG accumulates in cells and there is a concomitant decrease of the GSH/GSSG ratio [54]. Thus, this ratio is a dynamic indicator of oxidative stress and imbalance in GSH/GSSG ratio in turn can affect intracellular GPx and GR activities [54,55]. The glutathione level is regulated by the activity of glutathione metabolizing enzymes. The key functional element of glutathione is the cysteinyl moiety, which provides the reactive thiol group [60]. In the presence of GPx, GSH is oxidized to GSSG, which in turn is rapidly reduced back to GSH by GSSG reductase at the expense of NADPH [53,60]. Saunders et al also found significant decrease in the concentration of both GSH and GPX activity showing more prone to oxidative stress that may lead to possible health effects in the B[a]P treated group as compared to control [18].
In the present study, the activity of the glutathione dependent enzyme, GR was found to be reduced significantly in hippocampus of experimental rats, administered with higher doses of B[a]P. The formation of sulfhydryl complex with SH groups of the GR might lead to a decrease in the activity of the enzyme [54]. Our data also showed early administration of B[a]P to male rats elevate GST activity. The elevated activity of GST may be an attempt by the tissues to counteract the increased peroxides or toxic electrofiles [61]. The activity of GPx was found to be decreased that indicates that GSH metabolizing pathway is disturbed in B[a]P-treated rats. It seems clear that B[a]P decreases intracellular GSH level not only by binding to its thiol group, but also by decreasing the activity of GR [[53], [54], [55]]. The present work indicates that disruption of the cellular glutathione system is a key element in the mechanism of B[a]P-induced oxidative stress in brain. However, the exact mechanisms underlying B[a]P-induced neurotoxic effects through altered neuronal morphology remain unknown. One possible explanation for the phenomenon behavioral alteration may be due to the hippocampal lesions with loss of dendritic spine density and length of hippocampus [62].
To address this aim, our present study focused on the hypothesis that early adolescence B[a]P exposure can affect many aspects of neuronal functions including dendritic spine morphology during a critical phase of neonatal brain development and to cause morphological abnormalities in adulthood. It is well known that the hippocampus plays an essential role in spatial learning and memory processes in animals. Early B[a]P exposure may promote the morphological alteration of dendritic spine dysgenesis and density, thus opening the possibility that behavioural phenotypes that occur due to neurodegeneration in hippocampus. B[a]P can easily cross the BBB to forms a stable complex and accumulates in different regions particularly in the striatum, hippocampus and cortex causing neuronal and glial disorders [10,11]. The Golgi-Cox staining methods we followed in the present study provide a clue towards the neuronal degeneration in hippocampus leading to plasticity [56]. Quantification of dendrite structure in this study showed that, in adult rats at PND60, a significant reduction in dendrite spine density occurred due to early adolescence exposure to B[a]P. The higher doses of B[a]P can function to regulate mature spine morphology and behavioral phenotype like anxiety-related and depression-related learning behavior. This study proves to be a novel concept suggesting that B[a]P has direct action on developing neuronal cells with consequences on brain maturation and development during early adolescences, inducing anxiolytic like behavioral alteration and neuromorphological changes in hippocampus with serious neurological disorders in the male wistar adult rats.
5. Conclusion
In conclusion, early exposure to B[a]P during neonatal brain development can cause delayed neurobehavioral impairments in a dose dependent manner in adult male wistar rats. This results provide an additional evidence for effects of B[a]P like polycyclic aromatic compounds during development to assess the neurotoxicity of exposure following the technique of early administration (i.p.) of B[a]P which can help in overcoming the difficulty experienced in assessing the cytotoxicity to know the real consequences of cellular metabolism through liver functioning. The neurotoxic effects of B[a]P-induced altered neuronal morphology causing decreased dendritic population in hippocampus may lead to learning and memory impairment in adulthood. Further work will be needed to elucidate whether these alterations in neuronal spine structure and density are contributing directly to behavioral deficits during development.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by research grant to MP from (BRNS, Mumbai, 37(1)/14/27/2015/BRNS), Department of Science and Technology (DST), Odisha, (2762800402014/20/665) and DRDO, New Delhi DG(TM)/81(48222/LSRB-294/PEE & BS/2017) The funds supported to Lipsa Das (UGC-RGNF National Fellow-RGNF-2015-17-SC-ORI-10772) awarded by University Grant Commission (UGC), Govt. of India.
References
- 1.Atlanta G.A. Agency for Toxic substances and Disease Registry (ATSDR), US Department of Health & Human service; 1995. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) [PubMed] [Google Scholar]
- 2.IARC Polynuclear aromatic compounds, Part 1, chemical, environmental and experimental data. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1983;32:1–453. [PubMed] [Google Scholar]
- 3.Balansky R., Ganchev G., Iltcheva M. Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis. 2007;28:2236–2243. doi: 10.1093/carcin/bgm122. [DOI] [PubMed] [Google Scholar]
- 4.Das M., Mukhtar H., Seth P. Distribution of benzo[a]pyrene in discrete regions of the rat brain. Bull. Environ. Contam. Toxicol. 1985;35:500–504. doi: 10.1007/BF01636545. [DOI] [PubMed] [Google Scholar]
- 5.Tang D., Li T.Y., Liu J.J., Zhou Z.J., Yuan T., Chan Y.H., Rauh V.A., Xieand J., Perera F. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ. Health Perspect. 2008;116(May (5)):674–679. doi: 10.1289/ehp.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movahedinia A., Salamat N., Kheradmand P. Effects of the environmental endocrine disrupting compound benzo[a]pyrene on thyroidal status of abu mullet (Liza abu) during short-term exposure. Toxicol. Rep. 2018;5:377–382. doi: 10.1016/j.toxrep.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramesh A., Inyang F., Hood D.B., Archibong A.E., Knuckles M.E., Nyanda A.M. Metabolism bioavailability and toxicokinetics of benzo[a]pyrene in F-344 rats following oral administration. Exp. Toxicol. Pathol. 2001;53(4):275–290. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- 8.Konstandi M., Pappas P., Jhonson E.O., Lecklin A., Marcelos M. Suppression of the acquisition of conditioned avoidance behavior in the rat by 3-Methylcholanthrene. Pharmacol. Biochem. Behav. 1997;56(April (4)):637–641. doi: 10.1016/s0091-3057(96)00407-8. [DOI] [PubMed] [Google Scholar]
- 9.Gao M., Li Y., Long J., Shah W., Fu L., Lai B., Wang Y. Induction of oxidative stress and DNA damage in cervix in acute treatment with benzo[a]pyrene. Mutat. Res. 2011;719:52–59. doi: 10.1016/j.mrgentox.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Genuis S.J. Elimination of persistent toxicants from the human body. Hum. Exp. Toxicol. 2011;30(Jan (1)):3–18. doi: 10.1177/0960327110368417. [DOI] [PubMed] [Google Scholar]
- 11.Perera F.P., Rauh V., Whyatt R.M., Tsai W.Y., Tang D., Diaz D., Hoepner L., Barr D., Tu Y.H., Camann D.E. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perera F.P., Li Z., Whyatt R., Hoepner L., Wang S., Camann D.E., Rauh V. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124(August (2)):e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards S.C., Jedrychowski W., Butscher M., Camann D.E., Kieltyka A., Mroz E., Flak E., Li Z., Wang S., Rauh V. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ. Health Perspect. 2010;118:1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grova N., Schroeder H., Farinelle S., Prodhomme E., Valley A., Muller C.P. Sub- acute administration of benzo[a]pyrene (B[a]P) reduces anxiety-related behaviour in adult mice and modulates regional expression of N-methyl-D-aspartate (NMDA) receptors genes in relevant brain regions. Chemosphere. 2008;73(1 Suppl):S295–302. doi: 10.1016/j.chemosphere.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Chen C., Tang Y., Jiang X., Qi Y., Cheng S., Qiu C., Peng B., Tu B. Early postnatal Benzo[a]pyrene exposure in Sprague-Dawley rats causes persistent neurobehavioral impairments that emerge postnatally and continue into adolescence and adulthood. Toxicol. Sci. 2012;125:248–261. doi: 10.1093/toxsci/kfr265. [DOI] [PubMed] [Google Scholar]
- 16.Potratz S., Jungnickel H., Grabiger S., Tarnow P., Otto W., Fritsche E., von Bergen M., Lucha A. Differential cellular metabolite alterations in HaCaT cells caused by exposure to the aryl hydrocarbon receptor-binding polycyclic aromatic hydrocarbons chrysene, benzo[a]pyrene and dibenzo[a,l]pyrene. Toxicol. Rep. 2016;3:763–773. doi: 10.1016/j.toxrep.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tongo I., Ogbeide O., Ezemonye L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017;4:55–61. doi: 10.1016/j.toxrep.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders C.R., Das S.K., Ramesh A., Shockley D.C., Mukherjee S. Benzo[a]pyrene- induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J. Appl. Toxicol. 2006;26:427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- 19.Singh V.K., Patel D.K., Ram S., Mathur N., Siddiqui M.K.J. Blood levels of polycyclic aromatic hydrocarbons in children and their association with oxidative stress indices, an Indian perspective. Clin. Biochem. 2008;41(3):152–161. doi: 10.1016/j.clinbiochem.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Patel B., Das S.K., Das S., Das L., Patri M. Neonatal exposure to Benzo[a]pyrene induces oxidative stress causing altered hippocampal cytomorphometry and behaviour during early adolescence period of male Wistar rats. Int. J. Dev. Neurosci. 2016;38(2):150–162. doi: 10.1016/j.ijdevneu.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Uno S., Dalton T.P., Derkenne S. Oral exposure to benzo[a]pyrene in the mouse: detoxification by inducible cytochrome P450 is more important than metabolic activation. J. Mol. Pharmacol. 2004;65(5):1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- 22.Sims P., Grover P.L., Swaisland A., Pal K., Hewer A. Metabolic activation of benzo[a]pyrene proceeds by a diol-epoxide. Nature. 1974;252:326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- 23.Simon B.R., Wilson M.J., Wickliffe J.K. The RPTEC/TERT1 cell line models key renal cell responses to the environmental toxicants, benzo[a]pyrene and cadmium. Toxicol. Rep. 2014;1:231–242. doi: 10.1016/j.toxrep.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nebert D.W., Shi Z., Galvez-Peralta M. Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences–Cyp1 knock out mouse lines as a paradigm. J. Mol. Pharmacol. 2013;84(3):304–313. doi: 10.1124/mol.113.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Briede J.J., Godschalk R.W., Emans M.T., De Kok T.M., Van Agen E., Van Maanen J., Van Schooten F.J., Kleinjans J.C. In vitro and in vivo studies on oxygen free radical and DNA adduct formation in rat lung and liver during benzo[a]pyrene metabolism. J. Free Radic Res. 2004;38(9):995–1002. doi: 10.1080/10715760400000976. [DOI] [PubMed] [Google Scholar]
- 27.Poli G., Albano E., Dianzani M.U. Birkhauser Verlag; Basel, Switzerland: 1993. Free Radicals: From Basic Science to Medicine; pp. 365–373. [Google Scholar]
- 28.Kumar G., Tajpara P., Bukhari B.A., Asha G.R., De A., Maru G.B. Dietary curcumin post-treatment enhances the disappearance of B(a)P-derived DNA adducts in mouse liver and lungs. Toxicol. Rep. 2014;1:1181–1194. doi: 10.1016/j.toxrep.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2006;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patri M., Padmini A., Babu P.P. Polycyclic aromatic hydrocarbons in air and their neurotoxic potency in association with oxidative stress. Ann. Neurosci. 2010;16:22–30. [Google Scholar]
- 31.Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. [Review] Mutat. Res. 1988;202:343–361. doi: 10.1016/0027-5107(88)90197-2. [DOI] [PubMed] [Google Scholar]
- 32.Locigno R., Castronovo V. Reduced glutathione system: role in cancer development, prevention and treatment (review) Int. J. Oncol. 2001;19:221–236. doi: 10.3892/ijo.19.2.221. [DOI] [PubMed] [Google Scholar]
- 33.Rojas M.I., Cascorbi I., Alexandrov K., Kriek E., Auburtin G., Mayer L., Kopp-Schneider A., Roots I., Bartsch H. Modulation of benzo[a]pyrene diolepoxide-DNA adduct levels in human white blood cells by CYP1A1, GSTM1 and GSTT1 polymorphism. Carcinogenesis. 2000;21:35–41. doi: 10.1093/carcin/21.1.35. 1. [DOI] [PubMed] [Google Scholar]
- 34.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James S., Sheeladevi R., Ravindran R. Oxidative stress in brain and antioxi-dant activity of Ocimum sanctum in noise exposure. Neurobehav. Toxicol. Teratol. 2007;28:679–684. doi: 10.1016/j.neuro.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Sheline C.T., We L. Free radical-mediated neurotoxicity may be caused by inhibition of mitochondrial dehydrogenases in vitro and in vivo. Neuroscience. 2006;140:235–246. doi: 10.1016/j.neuroscience.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Sheng L., Ding X., Ferguson M., McCallister M., Rhoades R., Maguire M., Ramesh A., Aschner M., Campbell D., Levitt P., Hood D.B. Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioural deficits and down regulation of receptor tyrosine kinase. MET. Toxicol. Sci. 2010;118:625–634. doi: 10.1093/toxsci/kfq304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z., Chadalapaka G., Ramesh A., Khoshbouei H., Maguire M., Safe S., Rhoades R.E., Clark R., Jules G., McCallister M., Aschner M., Hood D.B. PAH particles perturb prenatal processes and phenotypes: protection from deficits in object discrimination afforded by dampening of brain oxido-reductase following in utero exposure to inhaled benzo[a]pyrene. Toxicol. Sci. 2012;125:233–247. doi: 10.1093/toxsci/kfr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustamante C., Bilbao P., Contreras W., Martinez M., Mendoza A., Reyes A. Effects of prenatal stress and exercise on dentate granule cells maturation and spatial memory in adolescent mice. Int. J. Dev. Neurosci. 2010;28:605–609. doi: 10.1016/j.ijdevneu.2010.07.229. [DOI] [PubMed] [Google Scholar]
- 40.Wormley D.D., Chirwa S., Nayyar T., Wu J., Johnson S., Brown L.A., Harris E., Hood D.B. Inhaled benzo[a]pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cell. Molec. Biol. 2004;50:715–721. [PubMed] [Google Scholar]
- 41.Bourne J.N., Harris K.M. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivy A.S., Rex C.S., Chen Y., Dube C., Maras P.M., Grigoriadis D.E. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyer C.E., Shelton M.A., Sweet R.A. Dendritic spine alterations in schizophrenia. Neurosci. Lett. 2015;601:46–53. doi: 10.1016/j.neulet.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boros B.D., Greathouse K.M., Gentry E.G., Curtis K.A., Birchall E.L., Gearing M., Herskowitz J.H. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann. Neurol. 2017;82:602–614. doi: 10.1002/ana.25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janacsek K., Fiser J., Nemeth D. The best time to acquire new skills: age-related differences in implicit sequence learning across the human lifespan. Dev. Sci. 2012;15:496–505. doi: 10.1111/j.1467-7687.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levone B.R., Cryan J.F., O’Leary O.F. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress. 2015;1:147–155. doi: 10.1016/j.ynstr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder A., Notaras M., Du X., Hill R.A. On the developmental timing of stress: delineating sex-specific effects of stress across development on adult behaviour. Brain Sci. 2018;8(7):121. doi: 10.3390/brainsci8070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patri M., Singh A., Mallick B.N. Protective role of noradrenaline in benzo[a]pyrene-induced learning impairment in developing rat. J. Neurosci. Res. 2013;91:1450–1462. doi: 10.1002/jnr.23265. [DOI] [PubMed] [Google Scholar]
- 49.Crawley J.N. Mouse behavioural assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverman J.L., Yang M., Lord C., Crawley J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forbes N.F., Stewart C.A., Matthews K., Reid I.C. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol. Behav. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- 52.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 53.Pabst M.J., Habig W.H., Jakoby W.B. Glutathione S-transferase A: a novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J. Biol. Chem. 1974;249:7140–7147. [PubMed] [Google Scholar]
- 54.Jones D.P. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 55.Massey, Williams Glutathione reductase: a novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J. Biol. Chem. 1965;249:7140–7148. [PubMed] [Google Scholar]
- 56.Ranjan A., Mallick B.N. A modified method for consistent and reliable golgi-cox staining in significantly reduced time. Front. Neurol. 2010;(1):157. doi: 10.3389/fneur.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enell A., Reichenberg F., Warfvinge P., Ewald G. A column method for determination of leaching of polycyclic aromatic hydrocarbons from aged contaminated soil. Chemosphere. 2002;54:707–715. doi: 10.1016/j.chemosphere.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 58.Jiang C.Q., Xu L., Lam T.H., Lin J.M., Cheng K.K., Thomas G.N. Smoking cessation and carotid atherosclerosis: the Guangzhou biobank cohort Study-CVD. J. Epidemiol. Commun. Health. 2010;64:1004–1009. doi: 10.1136/jech.2009.092718. [DOI] [PubMed] [Google Scholar]
- 59.Xia Y., Cheng S., He J., Liu X., Tang Y., Yuan H. Effects of sub-chronic exposure to benzo[a]pyrene (B[a]P) on learning and memory, and neurotransmitters in male Sprague-Dawley rat. Neurotoxicology. 2011;32(2):188–198. doi: 10.1016/j.neuro.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Townsend D.M., Tew K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7737. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basu S., Saha P.K., Roszkowska M., Magnowska M., Baczynska E., Das N., Plewczynski D., Wlodarczyk J. Quantitative 3-D morphometric analysis of individual dendritic spines. Sci. Rep. 2018;8:3545. doi: 10.1038/s41598-018-21753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calabrese B., Wilson M.S., Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]