Main Text
Intratumoral immunomodulation is emerging as an attractive strategy to deliver immunotherapeutic agents directly into the tumor microenvironment as a means to potentiate systemic anti-tumor immune response. In this issue of Molecular Therapy, two studies have explored such an approach by using viral vectors to deliver anti-programmed death ligand-1 (aPDL1) antibody or a fusion protein of soluble programmed cell death protein 1 (PD-1) linked to CD137 ligand (CD137L).1, 2 These studies demonstrate that the success of such strategies is dependent on the type of vector used for ligand delivery and provide evidence that, within such a context, a fusion ligand encoded by a single vector may be superior to a combination of vectors encoding the individual ligands separately.
Cancer immunotherapy using immunomodulatory antibodies has revolutionized the treatment of many disease types, although responses across tumor types remain limited to a minority of patients and some cancers do not respond at all.3 After the initial wave of anti-PD-1-, aPDL1-, and anti-CTLA-4-targeted antibodies, a number of trials have explored agents targeting additional co-stimulatory and co-inhibitory pathways controlling T cell activation, either as single agents or in combination. Unfortunately, many of the trials have, to date, demonstrated limited efficacy of such approaches or excessive dose-limiting toxicities prevented systemic delivery of effective drug doses. For example, the targeting of CD137 (4-1BB) with agonist antibody urelumab has resulted in unacceptable liver toxicity, preventing further development of the agent at the higher doses that are likely required for its full therapeutic activity.4
Intratumoral immunomodulation is an emerging field of immunotherapeutics that is based on a concept of in situ vaccination,5 which carries a potential to avoid systemic toxicities of some of the immunomodulatory agents. Some studies have demonstrated that injection of immunomodulatory antibodies directly into tumors could induce anti-tumor immune response, which can be effective against distant tumors.6, 7, 8 While several agents have been explored for intratumoral delivery in recent years, engineered therapeutic viruses (including oncolytic viruses and viral vectors) present a particular advantage. Advances in understanding the therapeutic virus-mediated activation of anti-tumor immune response have led to their development as agents that could potentiate the efficacy of systemic immunomodulatory therapy and as vehicles for direct delivery of immunostimulatory ligands into the tumor microenvironment.9 The latter strategies carry the potential to avoid systemic toxicity and maximize local T cell activation. To date, the optimal ligands or antibodies for delivery by viruses, optimal virus vectors, or the role of oncolysis or virus replication in such settings remain unknown.10
In this issue of Molecular Therapy, two separate studies have explored the use of therapeutic viral vectors for delivery of immunomodulatory antibodies or ligands directly into the tumor microenvironment. Ballesteros-Briones et al.2 have developed two different virus vectors: adeno-associated virus (AAV)33 and Semliki Forest virus (SFV) vectors expressing aPDL1 monoclonal antibodies (mAbs), which they evaluated using direct intratumoral injection in syngeneic tumor models. While both virus vectors produced the same levels of aPDL1 antibody, the duration of aPDL1 production was significantly diminished with the SFV vector. Perhaps surprisingly, the SFV vector nevertheless proved to be superior to the AAV vector, with complete elimination of tumors in a number of mice and evidence of abscopal effect in distant tumors. The authors further went on to demonstrate that SFV, but not AAV, induced a potent type I interferon (IFN) response as well as stronger tumor-specific immune responses, with generation of OX40+CD137+ T cells. To capitalize on these findings, the authors performed additional experiments, showing that intratumoral therapy with SFV-aPDL1 in combination with systemic CD137 agonist antibody further improved therapeutic efficacy of this agent.
Zhang et al.1 took a similar approach to assess the efficacy of an immunomodulatory ligand expressed from a replicating adenoviral (Ad5) vector following intratumoral administration. To maximize intratumoral immunostimulatory response, the investigators used a novel strategy by engineering a bispecific fusion protein containing soluble PD-1/CD137L as the immunostimulatory ligand, thus aiming to achieve both an enhancement of T cell co-stimulation and inhibition/prevention of exhaustion. This strategy carries an additional advantage of targeting CD137L only to the sites with PD-L1 expression, thus potentially avoiding hepatotoxicity that can be associated with systemic CD137 targeting. The authors demonstrate that the resultant virus led to potent induction of anti-tumor immune response and complete tumor rejection in the majority of the animals in the H22 hepatocellular carcinoma (HCC) ascites tumor model. Interestingly, the Ad5 virus expressing the PD-1/CD137L fusion protein was superior to the combination of Ad5-PD-1 and Ad-5-CD137L. The authors validated their findings in the solid tumor H22 HCC model as well as a humanized HCC-LM3 model, using the virus expressing the human version of PD-1/CD137L.
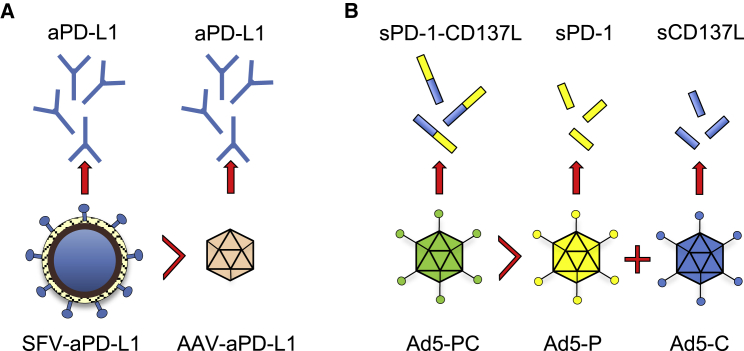
The two studies identify several key novel concepts (Figure 1). First of all, the study by Ballesteros-Briones et al.2 highlights that the efficacy of virus-encoded immunomodulatory antibody/ligand is highly context-dependent. Within the setting of virus therapy using a non-immunogenic vector (AAV), PD-L1 blockade failed to achieve a good therapeutic effect despite the more persistent production and systemic secretion. This finding suggests that appropriate activation of innate immune response (and likely priming) with an immunogenic vector may be required for potentiation of anti-tumor therapy. Second, the study by Zhang et al.1 highlights that a combined agent may be better than the sum of its parts. Indeed, the Ad5 vector expressing the fusion PD-1/CD137L protein was better than the combination of vectors encoding either of the ligands alone. The mechanism responsible for the latter finding, however, remains unknown and is likely a reflection of a direct biologic effect of the fusion ligand, possibly achieved by targeting CD137+ T cells to the site of PD-L1 expression. Lastly, both of the studies demonstrate that intratumoral PD-1/PD-L1 targeting within the context of delivery by an immunogenic viral vector can be an effective strategy to elicit systemic anti-tumor immunity. This finding is somewhat surprising, as, per the current understanding of the mechanisms of anti-PD-1/aPDL1 therapy, blockade of either receptor is required at all tumor sites systemically in order to reverse or prevent chronic antigen stimulation-induced T cell exhaustion.11 Interestingly, a recent study by Garris et al.12 demonstrated that activation of CD8 by PD-1 blockade requires interaction with intratumoral dendritic cells, suggesting that PD-1/PD-L1 interaction may play an additional role in the priming process. This may suggest that, within the context of intratumoral PD-1/PD-L1 blockade and the inflammatory microenvironment induced by the viral vector, the antibody may enhance priming through PD-L1 blockade, although further studies would be needed to validate this theory. Since the tumor-specific T cells generated in response to such therapy would still be subject to PD-L1-mediated inhibition at distant tumor sites, it remains to be seen whether targeting PD-L1 in situ would be sufficient or whether additional systemic targeting with antibodies targeting PD-1 and PD-L1 would be required. Both studies could benefit from bilateral flank tumor models or analogous tumor models where an effect on distant, non-treated tumors could be evaluated within the context of local (expressed from the virus vector) or systemic (via blocking antibody) PD-1/PD-L1 blockade.
Figure 1.
Intratumoral Immunomodulation with Engineered Viruses: Context and Ligand Matters
(A) Ballesteros-Briones et al.2 have demonstrated that PD-L1 expressed from SFV results in superior therapeutic efficacy when compared to PD-L1 expressed from AAV. (B) Expression of sPD-1/CD137L from a single adenoviral vector is superior to the combination of adenoviral vectors expressing either ligand alone.1
In summary, the studies by Ballesteros-Briones et al.2 and Zhang et al.1 have taken a major step toward development of oncolytic virus-based therapeutics targeting co-stimulatory and co-inhibitory components of the adaptive anti-tumor immune response. These studies provide evidence that success of such strategies is dependent on both the type of the vector used for ligand delivery and the ligand itself. It is likely that therapeutic approaches utilizing cocktails of molecules targeting both immune-activating and immune-inhibitory receptors in situ will be required for optimization of anti-tumor immune response while minimizing anti-viral response and toxicity. Optimal combinations of molecules to incorporate into an oncolytic virus are unknown and may depend on the type of oncolytic virus, type of tumor, or, more likely, the underlying mechanisms of immune dysfunction driving tumor resistance to the immune system. In a field where the number of known immunostimulatory and inhibitory mechanisms is growing daily, this creates an opportunity for an almost unlimited number of therapeutic combinations, and careful examination across a number of tumor models will be required to ensure that efficacy of such combinations translates into therapeutic success in clinical trials.
Acknowledgements
D.Z. received funding from the Department of Defense Ovarian Cancer Research Academy (OC150111) and is a member of the Parker Institute for Cancer Immunotherapy, which supports the MSKCC Cancer Immunotherapy Program. MSKCC is supported by the NCI Core grant P30 CA008748.
References
- 1.Zhang Y., Zhang H., Wei M., Mou T., Shi T., Ma Y., Cai X., Li Y., Dong J., Wei J. Recombinant Adenovirus Expressing a Soluble Fusion Protein PD-1/CD137L Subverts the Suppression of CD8+ T Cells in HCC. Mol. Ther. 2019;27:1963–1973. doi: 10.1016/j.ymthe.2019.07.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballesteros-Briones M.C., Martisova E., Casales E., Silva-Pilipich N., Buñuales M., Galindo J., Mancheño U., Gorraiz M., Lasarte J.J., Kochan G. Short-term local expression of a PD-L1 blocking antibody from a self-replicating RNA vector induces potent antitumor responses. Mol. Ther. 2019;27:1892–1905. doi: 10.1016/j.ymthe.2019.09.016. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callahan M.K., Postow M.A., Wolchok J.D. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Segal N.H., Logan T.F., Hodi F.S., McDermott D., Melero I., Hamid O., Schmidt H., Robert C., Chiarion-Sileni V., Ascierto P.A. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017;23:1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 5.Pierce R.H., Campbell J.S., Pai S.I., Brody J.D., Kohrt H.E. In-situ tumor vaccination: Bringing the fight to the tumor. Hum. Vaccin. Immunother. 2015;11:1901–1909. doi: 10.1080/21645515.2015.1049779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fransen M.F., van der Sluis T.C., Ossendorp F., Arens R., Melief C.J. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res. 2013;19:5381–5389. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 7.Marabelle A., Kohrt H., Sagiv-Barfi I., Ajami B., Axtell R.C., Zhou G., Rajapaksa R., Green M.R., Torchia J., Brody J. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammerich L., Binder A., Brody J.D. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol. Oncol. 2015;9:1966–1981. doi: 10.1016/j.molonc.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamarin D., Wolchok J.D. Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer. Mol. Ther. Oncolytics. 2014;1:14004. doi: 10.1038/mto.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington K., Freeman D.J., Kelly B., Harper J., Soria J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 11.Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 12.Garris C.S., Arlauckas S.P., Kohler R.H., Trefny M.P., Garren S., Piot C., Engblom C., Pfirschke C., Siwicki M., Gungabeesoon J. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148–1161.e7. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]