Abstract
Background
Zinc is an essential nutrient that is naturally available in most foods. Deficiency of this micronutrient in particular can cause a number of health complications. Zinc deficiency during infancy is more troublesome as rapid growth and nutrient relied development takes place in this period. Most severe outcomes of zinc deficiency during infancy are considered to be, impaired immunity, growth retardation and impaired neurodevelopment. The aim of this pragmatic study is to determine whether zinc supplementation strategy is feasible and effective for reducing growth retardation at national level.
Methods
A randomized, multicenter, double-blind, parallel group effectiveness trial that evaluated the effect of zinc supplementation in infant development. Children aged 6–24 months were recruited from healthcare centers of Damavand, Pishva and Varamin in the beginning of the study (n = 682). The Subjects were then randomly allocated in two groups of intervention (n = 272), and control (n = 308), where a daily dose of zinc sulfate (5ml) suspension containing 5mg elemental zinc and placebo were administered for the period of 6 month. Investigators, care givers and the parents of the children were blinded to the nature of the intervention. Anthropometric measures were evaluated at the beginning and after the six month intervention period. The primary outcome measured was linear growth and length difference, serum zinc and ferritin concentrations were the secondary outcomes.
Findings
Following the intervention, compared with the placebo, zinc supplementation was associated with significant difference in the average length increment (primary outcome) (placebo 5·23 ± 2·19 vs. intervention 5·79 ± 2·18 cm, p = 0·02). No significant difference was observed in concentrations of serum zinc and ferritin. After the intervention the prevalence of zinc deficiency was significantly lower in the intervention group compared to the placebo group. No complications and adverse effects were reported and the compliance was very good (7 children out of 344 didn't comply with the intake of syrup).
Interpretation
Zinc supplementation for six month among children (6–24 months) had beneficial outcomes on growth and average length increment, therefore we propose it is a feasible strategy for preventing growth retardation.
Keywords: Public health, Physiology, Immunology, Pediatrics, Evidence-based medicine, Health policy, Zinc deficiency, Anthropometry, Child growth
Public Health; Physiology; Immunology; Pediatrics; Evidence-Based Medicine; Health policy; Zinc Deficiency; anthropometry; Child Growth
1. Introduction
Iran is a developing country with a population of approximately 80 million that is experiencing an accelerated nutritional and epidemiologic transition resulting in simultaneous occurrence of disorders related to high food intake and undernutrition in different groups of the population [1, 2]. Zinc deficiency is a common nutritional problem in developing countries, including Iran. Zinc deficiency has been described in the 1960s by Professor Prasad as a local nutritional issue in Iran [3, 4]. Zinc deficiency refers to a state where inadequate amount of metabolic zinc is available to the organism, i.e. serum zinc levels below 70 μg/dl. Zinc deficiency primarily occurs due to insufficient intake, poor quality diets, increased excretion, and increased demand or combination of these factors [5]. According to the National Comprehensive Study on Household Food Consumption, carried out in 2000–2001 [6], meat, dairy products, fruits and nuts accounted for only 11% of energy intake, whereas bread, cereal and sweets accounted for 62% of energy intake, therefore it can be expected that zinc deficiency may be common as grains make up the bulk of the diet. In addition to this, diet based primarily on grains is high in phytate and fiber that actively interfere with iron and zinc absorption.
Inadequate Zinc intake and zinc deficiency is followed by pervasive consequences among children younger than two years of age. Zinc deficiency in children is associated with growth retardation, increased susceptibility to infection and decreased immunocompetence, therefore contributing to a large amount to childhood morbidity and premature mortality [5]. In two national surveys carried out in 2001 and 2012, the prevalence of zinc deficiency (serum zinc below 70 μg/dl)in children younger than two years of age was 19·4% and 19·8%, respectively. The prevalence of decreased linear growth was reported 18·3% in 2001 [7] and 9% in 2012 [8]. This decrease is mainly due to better health care and improved living conditions.
Supplementation is one of the most effective strategies used to address zinc deficiency in this age group. Iran has an expansive national health care infrastructure supporting the rural families and offering free health care services and also in cities a great proportion of the population is covered. Monitoring children's growth rate is one of the routines in such a network discipline [9]. Iron, vitamin A and D supplements are also freely delivered to children less than 2 years. The supplementation has had a great role in reducing iron deficiency anemia and vitamin A and D deficiencies. Fortification of foods, supplementation, nutritional education and diversification of diet are the suggested intervention strategies to improve the situation [10, 11, 12, 13, 14]. Regarding high prevalence of serum zinc deficiency and the growth stunting in the country, implementing a zinc supplementation initiative was considered by the health authorities as an alternative and the authors were assigned to conduct a feasibility study. A number of reports are published indicating the positive effect of zinc supplementation on linear growth of children [11, 13]. A former study in Iran also showed the same positive effect [15].
This pragmatic randomized trial aimed to determine the feasibility and effectiveness of zinc supplementation in the population of children younger than two years of age and also to assess the compliance of families and the rate of probable adverse events.
2. Materials & methods
This trial used a parallel group randomized controlled double blind design, conducted between September 2014 and May 2015 at rural health centers. A random sample of children aged (6–24 month) was recruited from rural health centers of Damavand, Pishva and Varamin. All rural inhabitations in Iran are covered by the national health network, providing primary health care (PHC) and several routines, especially to vulnerable groups. These health centers have a defined population under coverage and the services are delivered for free to all households. Sample selection was on the basis of cluster sampling, i.e. every health center was selected as a cluster. Each cluster (i.e. health center) was randomly assigned to intervention or comparator group in order that the health worker (behvarz) does not get confused about the right intervention for each child. The villages and thus the health centers selected for the study are similar in general characteristics and located in the same geographic area. All 6 to 24-months old children in each health center were identified and recruited unless they declared any serious underlying disease or prescribed contraindication of taking zinc. Hence, the study setting was the normal health centers and the administration of zinc and measurements were carried out by the local health workers to all of the eligible children under coverage of the health center. The health workers were trained in the beginning of the study on a standard procedure of intervention and anthropometric measurements. Three months later a catch up session was held with all the health workers. The health workers had direct access to the investigators by phone to discuss any question or unpredicted situation. The exact birth date was recorded from the Birth certificate of each child.
Randomization & masking. Through a randomized assignment, each health center was assigned to one of the parallel arms of the study. A complete masking scheme of the investigators, health workers (service delivery and outcome assessment), staff of laboratories and the families was carried out (double blinded design). The health network administration of the three counties of Damavand, Pishva and Varamin run in total 66 rural health centers covering a sum of 1952 children aged 6–24 months old with average of 15–75. We recruited the children from the three counties proportional to the number of children of each county. With an average number of less than 30 children per health center we needed about 25 health centers in total, Damavand 8, Pishva 7 and Varamin 10. Three boxes were numbered, each for one county and the names of all health centers of that county were put into its box. We drew the calculated number of the health centers from each box assigning the first draw to group one, the second to group two, the third to group one, the fourth to group two and so on. At the end we had 13 centers in group one and 12 centers in group two. All children from each health center would thus be assigned to the zinc or placebo group. We received two batches of suspensions from the company, completely similar to each other, one batch named group one and the other named group two. The company had attached allocation concealments by sealed letters indicating the real nature of the batches and the content was not revealed until the end of the study. The centers selected as group one received batch one and the centers selected as two received batch two. It was not required to know the real content of either batch throughout the whole study.
Procedures. On the first session, the mothers were informed of the nature of the research and a consent letter including the guidelines and purpose of the research were given to them to be signed by all parents and guardians of the child. So, written consent was obtained from all legal parents of the child. Two bottles of zinc sulphate suspension (Al-Hawi Co.) of 60 ml, 5 ml per day (containing 5 mg of elemental zinc) or placebo that looked quite similar to the syrup were given to the mothers in the intervention and control groups during each visit (once per month) accompanied with guidelines and necessary explanations. The mothers were instructed to administer the suspension each day at the same time in a single dose. If a child would experience any discomfort taking the suspension, the mother was asked to contact the health worker, who was instructed to discuss the issue with their supervisors and the investigators. The treatment (either the zinc or the placebo formula) were given to mothers to carry out for a month and instructed to come back for follow ups. Schedules were announced to mothers by health workers through telephone contacts. In cases the mothers did not refer to heath care center on the due date, on the same day the necessary follow-up was conducted by the health workers.
The anthropometric measurements were carried out at baseline and follow-ups on a monthly basis by the health workers. We provided the 25 health centers by similar calibrated scales and Horizontal length scales to measure the weight and length of the children (Measures included weight to the nearest 0·1 kg, length to the nearest 0·1 cm). Calibrated length boards with fixed headpiece and movable foot pieces are most suitable for measuring the length of infants. One of the routine tasks of the health workers is the measurement of the length and weight of the children under five. They are trained for this task during their formal education. We also trained them using our standard instruments during sessions at the beginning of the study and after three months. Every two weeks a supervisor visited each health center checking the methods administered by the health workers and gathered the forms.
By asking the mother data was collected for each child on the occurrence of respiratory and diarrheal disease during the period between the two visits.
For the serologic assessments 3 ml blood was drawn from the subsample children at the beginning and end of the study in the local laboratories. The transportation expenses from the village to the laboratory were covered by the investigators and each child received a treat in the lab after having breakfast. The time of serologic collection were kept the same for all the participants, it took place at 9:30 to 11 am. The blood samples were immediately centrifuged in the local labs and the serum was divided into 3 mineral free micro tubes and transported to the central lab in a cold box. The specimens were stored in minus 20 degrees Celsius freezers for two weeks until samples were drawn from all eligible children. Zinc concentration was measured by flame atomic absorption, Ferritin concentration by CLIA.
Zinc was measured by flame spectrometry (Hitachi 717, Production serial 201076). We prepared 9 zinc solutions with concentrations ranging from 40 to 521 μg/dl. The standard curve was linear in measured range with a coefficient of 316.590 (p < 0.001) and R2 equal to 0.976. To evaluate the day to day variation during the 20 days that the specimens were analyzed a standard solution of 200 μg/dl (Greiner, UK) was measured every day. The mean of the readings was 202.3 μg/dl with a standard deviation of 9.006 and a CV of 4.452%.
The sample size was determined based on the differences in the rate of length increment in the period of 6 month. Since the length increase from the age of six months to 24 months is about 20 cm on average, 6-month period average length increase would be 6·6 cm. A previous study by Abdollahi et al, [15] reports 0·5 cm difference in length increment after three months of zinc supplementation. It was anticipated to observe an approximately 0·5 to 1 cm difference in length increase between intervention and control groups. According to WHO growth charts, this amount would change the length for age Z score (HAZ) by about 0·2 to 0·3, and the centile would increase by 5–10 percent scores [16]. Considering a type one error of 0·05 and a power of 90% and a design effect of 1·1, a sample size of 313 children was calculated for each group and was defined suitable for maximizing the generalizability of data.
Outcome. The primary outcome relevant to the objective of the study was the linear growth of the children. A follow up period of six month duration was agreed on because this period is enough to show a measurable and clinically significant difference in average length difference between the two groups. In fact if the zinc administration to the children for six months would accelerate their linear growth, the policy makers could make an informed decision. Children were also administered with Iron, Vitamin D and vitamin A supplements as the routine practice. So, the serum concentration of ferritin and zinc were considered as secondary outcomes to assess changes in zinc concentration and to evaluate any probable interaction between zinc and iron. Due to resource constraints these serologic assessments were carried out in a random subsample of 85 children from each group (control and intervention).
3. Analysis
After the data collection period the questionnaires were quality controlled and entered into the computer using MS ACCESS. The exact age of each child was calculated in months. The standard Z scores for length for age (HAZ) and weight for age (WAZ) was calculated by applying the WHO 2006 references, using the ENA software. The difference in physical growth variables was calculated for each child from the start to the end of the study. ANOVA for repeated measurements analysis and simple t-test was used to compare the differences in physical growth between the 2 groups and data was expressed as means ± standard deviation for all children (Values of P < 0.05 were considered significant). We ran an analysis with consideration of the cluster sampling and there was no changes observed compared to the simple analysis. The prevalence of stunting was calculated with a cutoff of -2 for HAZ in the study groups.
Role of the funding source. The funding source of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethics. The study protocol was approved in the 53rd meeting of the research ethics committee of National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences. Tehran, Iran on October 07, 2014. On the first visit to the health center, the mothers were informed of the nature of the research and a consent letter including the description and purpose of the research were given to them to be read and signed by all parents and guardians of the child. So, written consent was obtained from all legal parents of the child.
4. Results
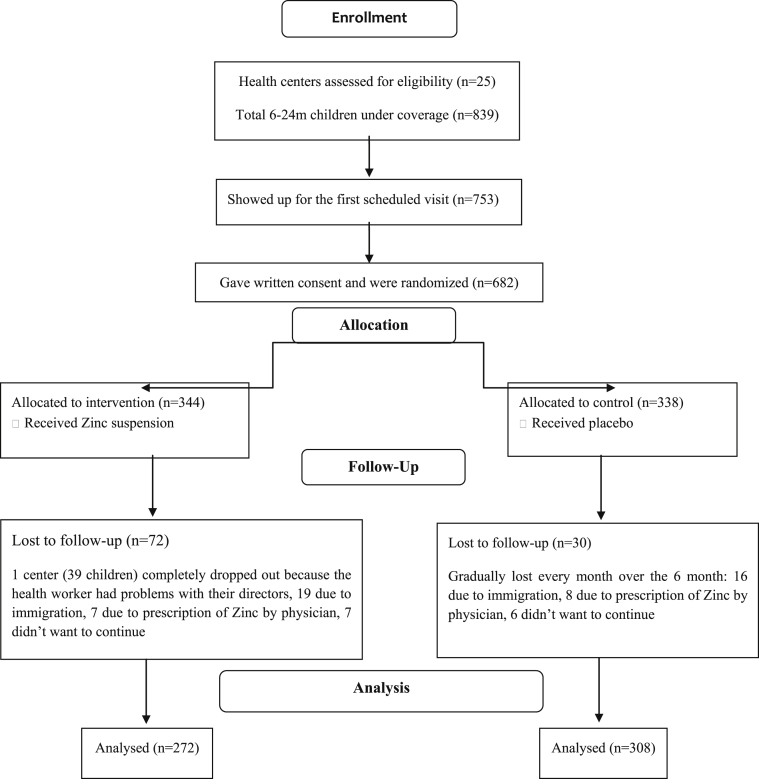
The date of recruitment and start of study was September 2014, and the trial completed and ended by May 2015. Diagram 1 shows the number and flow of the children. The average age of the intervention group was slightly (∼25 days) less than the average age of the control group. This age difference is also reflected in the length and weight averages of the two groups at baseline. The mean follow up period for both groups was 5.9 months. In both groups the number of boys is slightly greater than girls (boys/girls ratio: intervention group = 140/132; control group = 156/152).
Diagram1.
Participant flow.
Table 1 compares physical characteristic of the participants at baseline and after six month intervention period. The data from both placebo and intervention groups are compared, the mean and standard deviation of the Z score is also provided. There was no significant difference between mean ± SD of HAZ and WAZ (p > 0·05), although statistical significance was reached for the length difference variable (placebo 5·23cm ± 2·19 vs. intervention 5·79cm ± 2·18, p = 0·02). Also analysis of variance for repeated measurements were carried out which showed that length and HAZ were significantly different at baseline and after intervention as well as between placebo/intervention groups (p = 0·001).
Table 1.
Comparison of mean quantitative variables at baseline and after the completion of intervention (6 month).
| Variables | Baseline |
After 6 month |
||||
|---|---|---|---|---|---|---|
| Control mean ± SD | Intervention mean ± SD | P value | Control mean ± SD | Intervention mean ± SD | P value | |
| Age (month) | 16.3 ± 5.1 | 15.5 ± 5.3 | 0.1 | 22.2 ± 5.2 | 21.4 ± 5.2 | 0.1 |
| Length (cm) | 79.0 ± 5.52 | 78.4 ± 6.18 | 0.1 | 84.1 ± 5.5 | 84.2 ± 6.1 | 0.8 |
| Weight (kg) | 10.2 ± 1.6 | 9.8 ± 1.7 | 0.002 | 11.6 ± 1.7 | 11.2 ± 1.8 | 0.002 |
| HAZ1 | -0.18 ± 1.1 | -0.03 ± 1.1 | 0.1 | -0.31 ± 1.1 | -0.05 ± 1.2 | 0.008 |
| WAZ2 | -0.09 ± 0.99 | -0.25 ± 0.99 | 0.053 | 0.02 ± 1.0 | -0.17 ± 1.0 | 0.01 |
| Length difference | NA | NA | NA | 5.23 ± 2.19 | 5.79 ± 2.18 | 0.02 |
| Difference in HAZ | NA | NA | NA | -0.13 ± 0.78 | -0.01 ± 0.89 | 0.1 |
| Difference in WAZ | NA | NA | NA | 0.12 ± 0.56 | 0.08 ± 0.53 | 0.5 |
Mean values and standard deviations; difference in mean values and 95 % confidence intervals. NA: Not applicable
Z score of length for age.
Z score of weight for age.
Stunted growth and prevalence of zinc deficiency was also analyzed and data is presented in Tables 2 and 3. The number and frequency of stunted children in both groups increased after 6 months although this increase was very small in the intervention group compared to the control group. Our data show that the growth of these children deteriorates with time elapsing and their age increasing. The zinc supplementation has decreased the growth retardation by a considerable extent. The same happens for the concentration of serum zinc. The prevalence of serum zinc deficiency (i.e. serum zinc concentration less than 70 μg/dl) has increased in the placebo group but remained the same in the zinc group.
Table 2.
The comparison of stunted growth prevalence at baseline and after the intervention.
| Stunted growth | Baseline∗ |
End of intervention∗ |
Difference |
|||
|---|---|---|---|---|---|---|
| Number | Percentage % | Number | Number | Percentage % | ||
| Control (n = 308) | 14 | 4.5 | 26 | 8.4 | 12 | 3.9 |
| Intervention (n = 272) | 10 | 3.7 | 16 | 5.9 | 6 | 2.2 |
No significant difference between groups.
Table 3.
The prevalence of serum zinc concentration below cut-off at baseline and after the intervention.
| Zinc deficiency | Serum zinc concentration <70 μg/dl |
% of prevalence |
||
|---|---|---|---|---|
| Baseline | After 6 month | Baseline | After 6 month | |
| Control | 11 | 17 | 11.8 % | 18.2% |
| Intervention | 19 | 19 | 17.6 % | 17.6% |
From Table 2 it can be speculated that if the entire sample was subject to the placebo, among the 580 children there would be 49 cases (8·4%) of stunted growth overall. On the other hand, if zinc supplementation was administered to the entire sample, then there would be 35 cases (5.9%) of stunted growth overall after the six month period. Considering this, it can be concluded that 6 month of supplementation can prevent stunting in 14 individuals (49–35 = 14: 2.6%) in the overall sample. Relatively speaking, zinc supplementation could have prevented stunting in 30% (14 divided by 49) of the children had these children used the zinc suspension. In other words, 40 children need to adhere to the supplementation program (6 month) in order to prevent stunting in one child (NNT: Number needed to treat). This value is quite reasonable as a preventive measure. For instance, in the case of the influenza vaccination, for prevention of influenza, the NNT value is 71, i.e. to prevent a single person from influenza, 71 individuals need to be vaccinated.
The serum zinc and ferritin concentrations were studied and data is shown in Table 4. No significant difference was detected in the concentration of ferritin between the groups and during the study. The present study also analyzed the association between zinc supplementation and incidence of respiratory diseases and diarrhea (Table 5). According to the findings in regards to these conditions, no difference was detected among the children i.e. zinc supplements did not reduce the risk of respiratory diseases or outbreak of diarrhea.
Table 4.
Serum concentration of zinc and ferritin during the study (mean ± standard deviation).
| Zinc (μg/dl) | Ferritin (mg/ml) | |
|---|---|---|
| Control | ||
| Baseline | 86.4 ± 13.7 | 44.7 ± 45.0 |
| End | 82.6 ± 17.8 | 45.6 ± 41.0 |
| Intervention | ||
| Baseline | 80.5 ± 11.6 | 43.2 ± 34.6 |
| End | 80.0 ± 13.2 | 47.7 ± 43.1 |
Table 5.
The association between zinc supplementation and incidence of respiratory diseases and diarrhea.
| Control (%) | Intervention (%) | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Respiratory diseases | 1.04 | 0.88–1.22 | 0.64 | ||
| Yes | 368 (13.1%) | 335 (12.7%) | |||
| No | 2441 (86.9%) | 2313 (87.3%) | |||
| Diarrhea | 0.82 | 0.067–1.002 | 0.052 | ||
| Yes | 202 (7.2%) | 228 (8.6%) | |||
| No | 2607 (92.8%) | 2420 (91.4%) |
5. Discussion
The present study was designed to determine the feasibility and effectiveness of zinc supplementation on linear growth in children aged 6–24 month. The findings indicate that zinc supplementation given orally for the period of six month does have significant effects on some growth variables and contributes to decreasing the chance of stunting. Average length increment was significantly higher in the intervention group. No difference was found in secondary outcomes, including serum zinc and ferritin concentration. No adverse effects were reported due to supplementation and compliance to the intervention was high.
Findings from previous studies of infant and child supplementation with zinc in developed and developing countries have ranged from no effect to significant reductions in growth retardation and stunting. The results of the current study are consistent with the result of a meta-analysis of RCTs of zinc supplementation carried out by Brown et al [17]. It was demonstrated that zinc supplementation contributes to length increment of infants younger than 6 months. A later meta-analysis by Walker and Black [18] also supports the association between zinc supplementation and clinical growth outcomes among children. This review assessed the evidence from randomized trials of zinc supplementation and correlation studies, confirming that zinc supplementation increases growth and prevents stunting in children younger than five. However their findings indicate a strong association between supplementation and reduced incidence of diarrhea. Additionally these investigators suggest that the greatest impact of zinc supplementation on linear growth is mostly among children with the lowest HAZ scores, regardless of baseline characteristics. More recent meta-analyses are also in agreement with this evidence and the preventive nature of zinc on growth retardation [11, 19]. Imad and Bhutta [19] demonstrated that a daily dose of 10mg of zinc for more than 24 weeks of intervention leads to increases in length by 0·25 ± 0·37 cm.
In 1981, Golden and colleagues [20] reported that inadequate zinc intake in malnourished children leads to stunting. Fourteen years later, a double blind trial by Castillo-Duran et al, reported the beneficial impacts of zinc on length and growth in children born small for gestational age [21]. In accordance with this evidence, Walravens and colleagues in a zinc supplementation trial showed that 3 month of supplementation in 4–9 month infants resulted in significant increases in length to age Z score as well as weight gain among the supplemented group compared to placebo [22]. Ramakrishnan and colleagues [23] in a similar interventional study however found no effect for changes in length except minor increases in the Z score for weight. This effect of Zn supplementation on weight of children does not appear to be the case for other studies assessing linear growth of children in response to zinc supplementation [24, 25, 26]. In addition, Muller et al [27] in an RCT investigating growth response to zinc in West African children found no effect of supplementation in neither length nor weight Z scores. However, it should be pointed out that these studies make no attempt as to specifically assess zinc supplementation on linear growth development in their design. Additionally, non-interpretation or no effect can be due to the fact that presence of infections and parasites can confound the results.
According to research, to estimate zinc deficiency within the populations, measures like length and length for age are selected as the functional growth outcomes. In other words, length and length for age are mostly considered to be the direct response to zinc supplementation or increased zinc intake, while weight gain is likely to be the response to increases in linear growth [28].
Several factors contribute to linear growth development and increased length in children, including genetic background as well as micro and macro nutrient intake. Other factors that should be taken into account are the impact of growth hormone and insulin like growth factor (IGF-1). Nutrition and dietary intake have a key role in regulation of the said hormones. Research suggests that inadequate intake of protein and low energy can cause a drop in IGF-1 activity. The effect of protein on IGF-1 has been well documented in both animal and human studies. In an animal study, zinc deficiency in rats caused major reductions in both plasma IGF-1 concentration and growth hormone receptor expression. Beside the growth hormone implications, IGF-1 can improve the growth process through bone metabolism. Impact of zinc on DNA synthesis should also be considered [29, 30, 31].
To date a number of studies have indicated that other essential micronutrients aside from zinc can have major influences on growth and development. This may be the reason why in our trial and other similar experiments that a single element is studied the results are not quite conclusive. Especially if one considers that poor families usually have diets that do not provide adequate nutrients resulting in multiple deficiencies. For instance vitamin D and calcium which are closely linked to bone development can cause low growth percentiles and growth retardation if infants are deficient [32]. Other nutrient deficiencies that contribute to growth retardation and stunting are iron, magnesium and vitamin A deficiency. Inadequate intake of protein and energy can reduce immune function and alter the bioavailability of trace elements such as zinc, thus affecting growth development. Therefore supplementation with zinc-plus (multivitamin approach) can have greater impact on growth development of children as these nutrients are closely linked together [11, 31, 33, 34].
The present study also assessed the interaction between zinc and iron. Following the 6 month intervention period, supplementation with zinc had increased ferritin concentrations in children; however this finding was not statistically significant. The findings in this trial can be compared to a randomized control trial carried out on 1–5 years old children, investigating the impact of iron-fortified milk and zinc supplementation for four months. The authors suggested that supplemental zinc had positive impacts on hemoglobin response and reduced the prevalence of anemia by 25% among the intervention group [35].
To our knowledge this study is the largest pragmatic trial of effectiveness and feasibility of zinc supplementation intervention in Iran. This trial is novel in the way that assesses the applicability of this strategy in preventive nutritional care. There are several differences in design that is worthy of note. The first point is that this trial was purposely conducted entirely within primary healthcare (PHC), which is a setting where almost everybody within the population is covered. This is not the case with several trials that have recruited their subjects through internet, specialists or single-payer third party systems. It could be argued that this enhances the degree to which the current trial results can be applied to PHC and the positive effect of zinc supplementation combined with other micronutrient supplements may reflect in part to the cost-effective nature and applicability of the design. Secondly, the prospective design and the long intervention period are considered major strengths of this trial which can supposedly ensure sufficient statistical power. The healthcare system has also provided good environment for health workers and the parents which can explain the high compliance to the intervention. Therefore it is highly feasible to implement this supplementation strategy.
We acknowledge that there were also several potential limitations, including measurement error in some self-administrative tools. The effects of zinc supplements on child development were difficult to measure as no standard operating procedures were provided. Measurement of some of the technical and biological factors that influence zinc concentrations was not taken into account, this could be another source of limitation in this design, and thus it could explain non-interpretation or confounding results. However, most important limitation to our design is lack of proper measurements or uncertainty in anthropometric measurements (primary outcome variable). In order to verify the accuracy of measurements, investigators and administers had to monitor and retrain interviewers and health workers in some cases. Since a margin of doubt always exist in measurements i.e. observations from observation varies due to inability to perform exactly the same way, therefore measurement bias or uncertainty (estimations from rulers or calipers) can cause random errors. Small errors of measurements rise from uncertain estimations in a single reading especially when measuring infants. Lastly, although supplementation methods are basic and easy to administer, daily supplementation requires commitment from parents and children.
6. Conclusion
Childhood zinc deficiency is a public health problem in many developing countries where the diets largely consist of plant based and low zinc content items. Zinc is involved in numerous metabolic functions; inadequate intake can cause cell-mediated immune dysfunction, cognitive impairment and most prominently growth retardation. To combat zinc deficiency, supplementation strategies seem to have the potential to prevent the negative consequences of childhood zinc deficiency. The findings of this trial build on previous studies suggesting the effectiveness of zinc supplementation on increasing zinc intake and effectively decreasing growth retardation. For each child, the price of the supplemental solution required for one month was 12440 IRR (estimated 0.3 USD) and the annual fee per child was 150000 IRR (estimated 4.6 USD). Since no serious complications and no negative effect on iron stores were associated with the intervention and the cost-effective nature of this strategy as well as considering the reasonable NNT, it is feasible to acquire this public health intervention to promote zinc intake. Also this preliminary trial has been successful in three counties of the country within the PHC system meaning it can work throughout the country with similar positive outcomes. Given that national data on food consumption for Iran also report that inadequate zinc intake is prevalent in the country, it is fundamental to act at the community level, as our findings reinforce the importance of employing multiple nutrient and population-wide supplementation within the practice.
Declaration
Author contribution statement
Morteza Abdollahi, Marjan Ajami, Zahra Abdollahi, Nasser Kalantari, Anahita Houshiarrad, Foroozan Salehi Mazandarani: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fereshteh Fozouni: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Atieh Fallahrokni: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the The United Nations Children's Fund (UNICEF) office in Tehran, Iran, who provided the field costs of the research and standard scales and length boards for athropometric measurements. The funding sources of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at the Iranian Registry of Clinical Trials (https://www.irct.ir/) under the registration number IRCT2014111519951N1.
Acknowledgements
We would like to thank the following members for their contributions to the success of this trial.
Deputy Minister of health UNICEF office in Tehran and their health and nutrition officers for their valuable contribution Deputy of health of Shahid Beheshti University of Medical Sciences The directors and staff of health networks of Damavand, Pishva and Varamin The health workers of the health centers involved in this trial The staff of Noor Laborarory, Tehran, especially Mr Keyvan Majidi And the parents and guardians of the children involved in this trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2019.e02581.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Ghassemi H., Harrison G., Mohammad K. An accelerated nutrition transition in Iran. Public Health Nutr. 2002;4(1a):149–155. doi: 10.1079/PHN2001287. [DOI] [PubMed] [Google Scholar]
- 2.Motlagh M.E., Kelishadi R., Amirkhani M.A., Ziaoddini H., Dashti M., Aminaee T., Ardalan G., Mirmoghtadaee P., Keshavarz S., Poursafa P. Double burden of nutritional disorders in young Iranian children: findings of a nationwide screening survey. Public Health Nutr. 2011;14(4):605–610. doi: 10.1017/S1368980010002399. [DOI] [PubMed] [Google Scholar]
- 3.Prasad A.S., Oberleas D. Zinc deficiency in man. Lancet. 1974 Mar 16;1(7855):463–464. doi: 10.1016/s0140-6736(74)92430-1. [DOI] [PubMed] [Google Scholar]
- 4.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. : Int. Rev. J. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King J.C., Brown K.H., Gibson R.S., Krebs N.F., Lowe N.M., Siekmann J.H., Raiten D.J. Biomarkers of nutrition for development (BOND)-Zinc Review. J. Nutr. 2016;146(4):858S–885S. doi: 10.3945/jn.115.220079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantari N., Ghaffarpour M., Iran I.R. National Nutrition and Food Technology Research Institute; Tehran, Iran: 2005. National Comprehensive Study on Household Food Consumption Pattern and Nutritional Status. [Google Scholar]
- 7.Ministry of Health and Medical Education . Ministry of Health and Medical Education; Tehran: 2006. National Integrated Micronutrients Survey 2001 (NIMS) in Islamic Republic of Iran. [Google Scholar]
- 8.Siassi F., Mohammad K., Djazayery A., Djalali M., Abdollahi Z., Drorosty A.R., Pouraram H., Heshmat R., Khodaverdian K., Sotoudeh G., Yarparvar A.h. Ministry of Health and Medical Education; Tehran: 2015. National Integrated Micronutrient Survey 2012 (NIMS II) [Google Scholar]
- 9.Mehrdad R. Health system in Iran. JMAJ. 2009;52(1):69–73. [Google Scholar]
- 10.Gibson R.S., Ferguson E.L. Nutrition intervention strategies to combat zinc deficiency in developing countries. Nutr. Res. Rev. 1998;11(01):115–131. doi: 10.1079/NRR19980008. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffari-Khosravi H., Shakiba M., Eftekhari M.-H., Fatehi F. Effects of zinc supplementation on physical growth in 2–5-year-old children. Biol. Trace Elem. Res. 2009;128(2):118–127. doi: 10.1007/s12011-008-8261-1. [DOI] [PubMed] [Google Scholar]
- 12.Griffin I.J., Lynch M.F., Hawthorne K.M., Chen Z., Hamzo M.G., Abrams S.A. Zinc homeostasis in 1–4 year olds consuming diets typical of US children. Br. J. Nutr. 2007;98(02):358–363. doi: 10.1017/S0007114507708796. [DOI] [PubMed] [Google Scholar]
- 13.Silva A.P., Vitolo M.R., Zara L.F., Castro C.F.S. Effects of zinc supplementation on 1-to 5-year old children. Jornal de pediatria. 2006;82(3):227–231. doi: 10.2223/JPED.1480. [DOI] [PubMed] [Google Scholar]
- 14.Shamir R., Makhoul I.R., Etzioni A., Shehadeh N. Evaluation of a diet containing probiotics and zinc for the treatment of mild diarrheal illness in children younger than one year of age. J. Am. Coll. Nutr. 2005;24(5):370–375. doi: 10.1080/07315724.2005.10719487. [DOI] [PubMed] [Google Scholar]
- 15.Abdollahi M., Abdollahi Z., Fozouni F., Bondarianzadeh D. Oral zinc supplementation positively affects linear growth, but not weight, in children 6-24 months of age. Int. J. Prev. Med. IJPM. 2014;5:3. [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . WHO; Geneva: 2009. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. A Joint Statement by the World Health Organization and the United Nations Children’s Fund. [PubMed] [Google Scholar]
- 17.Brown K.H., Peerson J.M., Rivera J., Allen L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002;75(6):1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 18.Walker F.C.L., Black R.E. Functional indicators for assessing zinc deficiency. Food Nutr. Bull. 2007;28:S454–S479. doi: 10.1177/15648265070283s305. [DOI] [PubMed] [Google Scholar]
- 19.Imdad A., Bhutta Z. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health. 2011;11(Suppl 3):S22. doi: 10.1186/1471-2458-11-S3-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden M.H., Golden B.E. Effect of zinc supplementation on the dietary intake, rate of weight gain and energy cost of tissue deposition in children recovering from severe malnutrition. Am. J. Clin. Nutr. 1981;34:900–908. doi: 10.1093/ajcn/34.5.900. [DOI] [PubMed] [Google Scholar]
- 21.Castillo-Duran C., Rodriguez A., Venegas G., Alvarez P., Lcaza G. Zinc supplementation and growth of infants born small for gestational age. J. Pediatr. 1995;127:206–211. doi: 10.1016/s0022-3476(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 22.Walravens P.A., Chakar A., Mokni R., Denise J., Daniel L. Zinc supplements in breastfed infants. Lancet. 1992;340(8821):683–685. doi: 10.1016/0140-6736(92)92229-9. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishnan U., Goldenberg T., Allen L.H. Do multiple micronutrient interventions improve child health, growth, and development? J. Nutr. 2011;141:2066–2075. doi: 10.3945/jn.111.146845. [DOI] [PubMed] [Google Scholar]
- 24.Rosado J.L., López P., Muñoz E., Martinez H., Allen L.H. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am. J. Clin. Nutr. 1997;65:13–19. doi: 10.1093/ajcn/65.1.13. [DOI] [PubMed] [Google Scholar]
- 25.Taneja S., Strand T.A., Sommerfelt H., Bahl R., Bhandari N. Zinc supplementation for four months does not affect growth in young North Indian children. J. Nutr. 2010;140:630–634. doi: 10.3945/jn.109.115766. [DOI] [PubMed] [Google Scholar]
- 26.Dijkhuizen M.A., Winichagoon P., Wieringa F.T., Wasantwisut E., Utomo B., Ninh N.X. Zinc supplementation improved length growth only in anemic infants in a multi-country trial of iron and zinc supplementation in South-East Asia. J. Nutr. 2008;138:1969–1975. doi: 10.1093/jn/138.10.1969. [DOI] [PubMed] [Google Scholar]
- 27.Müller O., Garenne M., Reitmaier P., Van Zweeden A.B., Kouyate B., Becher H. Effect of zinc supplementation on growth in West African children: a randomized double-blind placebo-controlled trial in rural Burkina Faso. Int. J. Epidemiol. 2003;32:1098–1102. doi: 10.1093/ije/dyg190. [DOI] [PubMed] [Google Scholar]
- 28.Gibson R.S., Hess S.Y., Hotz C., Brown K.H. Indicators of zinc status at the population level: a review of the evidence. Br. J. Nutr. 2008;99(S3):S14–S23. doi: 10.1017/S0007114508006818. [DOI] [PubMed] [Google Scholar]
- 29.Dørup I., Clausen T. Effects of magnesium and zinc deficiencies on growth and protein synthesis in skeletal muscle and the heart. Br. J. Nutr. 1991;66:493–504. doi: 10.1079/bjn19910050. [DOI] [PubMed] [Google Scholar]
- 30.Nishi Y. Zinc and growth. J. Am. Coll. Nutr. 1996;15:340–344. doi: 10.1080/07315724.1996.10718608. [DOI] [PubMed] [Google Scholar]
- 31.Abrams S.A. Nutritional rickets: an old disease returns. Nutr. Rev. 2002;60:111–115. doi: 10.1301/00296640260085840. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T., Nishiyama S., Futagoishi-Suginohara Y., Matsuda I., Higashi A. Mild to moderate zinc deficiency in short children: effect of zinc supplementation on linear growth velocity. J. Pediatr. 1993;123:65–69. doi: 10.1016/s0022-3476(05)81538-0. [DOI] [PubMed] [Google Scholar]
- 33.Lawless J.W., Latham M.C., Stephenson L.S., Kinoti S.N., Pertet A.M. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J. Nutr. 1994;124(5):645–654. doi: 10.1093/jn/124.5.645. [DOI] [PubMed] [Google Scholar]
- 34.Clausen T., Dorup I. Micronutrients, minerals and growth control. Bibl. Nutr. Dieta. 1998;54:84–92. doi: 10.1159/000059449. [DOI] [PubMed] [Google Scholar]
- 35.Silva A.P., Vitolo M.R., Zara L.F., Castro C.F.S. Effects of zinc supplementation on 1-to 5-year old children. Jornal de pediatria. 2006;82(3):227–231. doi: 10.2223/JPED.1480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.