Abstract
Purpose
Optimal postoperative radiation therapy (PORT) dose is unclear in penile squamous cell carcinoma (PeSCC). Herein, we characterized the radiosensitivity index (RSI) and genomic-adjusted radiation dose (GARD) profiles in a cohort of patients with PeSCC, and assessed the application of GARD to personalize PORT.
Methods
A total of 25 PeSCC samples were identified for transcriptomic profiling. The RSI score and GARD were derived for each sample. A cohort of 34 patients was reviewed for clinical correlation.
Results
The median RSI for PeSCC was 0.482 (range 0.215–0.682). The majority (n = 21; 84%) of cases were classified as radioresistant. PeSCC GARD ranged from 9.56 to 38.39 (median 18.25), suggesting variable therapeutic effects from PORT. We further determined the optimal GARD-based RT doses to improve locoregional control. We found that therapeutic benefit was only achieved in 52% of PeSCC lesions with PORT of 50 Gy, in contrast to 84% benefit from GARD-modeled PORT of 66 Gy. In the clinical cohort, the majority of patients presented with pathological N2 or N3 disease (n = 31; 91%) and was treated with adjuvant concurrent platinum-based chemoradiotherapy (CRT, n = 30; 88%). Fourteen of the 34 patients (41%) had locoregional recurrence (LRR), of which half had LRR within six months of completion of PORT.
Conclusions
The majority of PeSCC are intrinsically radioresistant with a low GARD-based therapeutic effect from PORT dose of 50 Gy, consistent with the observed high rate of LRR in the clinical cohort. A GARD-based strategy will allow personalizing PORT dose prescription to individual tumor biology and improve outcomes.
Keywords: Radiosensitivity, Penile cancer, Radiation therapy, GARD, Locoregional failure
1. Introduction
Carcinoma of the penis is a rare entity in Western countries.1 It is generally squamous cell carcinoma (PeSCC) developed from penile intraepithelial carcinogenesis.2 PeSCC is a highly aggressive tumor with the potential for early locoregional recurrence (LRR) and distant metastasis (DM) even after multiple treatment modalities.3 Postoperative radiation therapy (PORT) for node-positive PeSCC remains controversial. Currently, there is no high-level evidence to support the benefit of PORT in terms of recurrence and survival, therefore PORT is not recommended in some treatment guidelines.4, 5 Despite this, many institutes continue to apply PORT for PeSCC patients with high-risk features such as pathological N2/N3 disease.6, 7, 8
RT dosing of 50 Gy has been considered adequate for decades to provide >90% clearance of subclinical disease of breast adenocarcinoma and squamous cell carcinoma of the head and neck.20 Such a dose has been routinely used for PeSCC patients in the post-operative adjuvant radiotherapy setting9; however, this frequently is ineffective in the clinic.
Recent advances in our knowledge of the genetic and molecular biology of cancer have shown biological heterogeneity to be central to advances in therapy.10 Preclinical and clinical studies have demonstrated significant differences in sensitivity to RT among different tumor types, and between patients with the same tumor histology. At our institution, we have developed a genome-based assay to estimate the intrinsic radiosensitivity of tumors: the radiosensitivity index (RSI), where low and high RSI scores imply radiosensitive and radioresistant tumors, respectively.12, 13 This model has been validated in multiple clinical cancer cohorts encompassing various disease sites, including head and neck, rectal, prostate, breast, glioblastoma, esophagus, pancreas, lung, and colon metastases.11, 12, 13, 14, 15, 16We have previously developed and validated a clinically actionable metric to model a genomic-adjusted radiation dose (GARD) by integrating RSI into the linear quadratic model11 where a high GARD value predicts for a high therapeutic effect of RT. GARD independently predicts for clinical outcomes in multiple clinical cohorts including glioblastoma, breast, lung, and pancreatic cancers.11 GARD-based clinical trials are under development in multiple disease sites at our institution.
In a rare disease like PeSCC, genome-based intrinsic tumor radiosensitivity and GARD-based RT previously have not been explored. The main objective of this study is to determine the RSI and GARD profiles in PeSCC and their potential clinical correlation.
2. Patients and methods
This study was approved by the appropriate Institutional Review Boards. Surgical samples from 25 patients with PeSCC were obtained from our institution’s tissue biorepository for transcriptomic analysis to assess RSI and GARD modeling.12, 13 We also retrospectively reviewed clinical data of a cohort of 34 PeSCC patients treated with PORT at our center between 2001 and 2018 including ten patients from the tissue cohort. Clinical parameters, including stage, RT and chemotherapy approach, and clinical outcomes were abstracted from the medical chart.
2.1. Genomic-based analysis of PeSCC intrinsic radiosensitivity
Gene transcripts from 25 primary PeSCC tissue samples were analyzed using Affymetrix U133 2.0 microarrays for gene expression profiling.12, 13 The gene microarray data were analyzed using the previously published algorithm and RSI score (range: 0–1.0) was derived from the expression of 10 specific genes including cJun, SUMO1, AR, RelA, STAT1, IRF1, PKC, HDAC1, cABL, and PAK2.12 Radioresistant (high RSI) and radiosensitive (low RSI) groups were divided using a previously published RSI cutpoint (RSI < 0.375 = radiosensitive).14
2.2. Modeling GARD of PeSCC
GARD scores were derived using the linear quadratic model, the individual gene-expression-based RSI, and a 50 Gy radiation dose. The calculation for GARD is similar to the biologically effective dose, except the patient-specific α is derived by substituting the radiosensitivity index for survival (S) in S = e–nd(α + βd), where dose (d) is 2 Gy, n = 1, and β is a constant (0.05/Gy).11 A higher GARD value predicts a higher radiation therapeutic effect. We calculated GARD using a script written into Excel. The final GARD formula is GARD = nd(α + βd). The 50th percentile of GARD was used to model GARD optimization based on previous validation studies.11
2.3. Statistical analysis
Descriptive statistics were used to summarize the clinical cohort. Violin plots were created using MATLAB R2016a (The MathWorks, Natick, MA, USA). Comparison of RSI values between groups was assessed using Kruskal-Wallis tests. Local recurrence (LR) was defined as a failure at the initial gross disease site (i.e. surgical stump). Regional recurrence (RR) was defined as relapse within the pelvic/inguinal RT fields. Distant metastasis (DM) was defined as recurrent disease outside of the RT field. The association between categorical variables and RSI was evaluated using Spearman correlation. p < 0.05 was considered statistically significant. Statistical analyses were performed using R version 3.5.1.
3. Results
3.1. The genomic-based intrinsic radiosensitivity of PeSCC
As shown in Table 1, PeSCC has a wide variation of RSI (range: 0.215–0.682), suggesting biological heterogeneity in intrinsic radiosensitivity among different lesions. The median RSI was 0.48 in the entire cohort. Four patients in bold in Table 1 with RSI scores 0.327, 0.333, 0.248, and 0.215 are categorized as intrinsically radiosensitive using the previously reported RSI cut-point of 0.375. The majority of the lesions (n = 21; 84%) were classified as radioresistant.
Table 1.
RSI in patients with PeSCC.
| PeSCC tumors | RSI |
|---|---|
| 1 | 0.502 |
| 2 | 0.682 |
| 3 | 0.441 |
| 4 | 0.524 |
| 5 | 0.327 |
| 6 | 0.423 |
| 7 | 0.458 |
| 8 | 0.440 |
| 9 | 0.333 |
| 10 | 0.502 |
| 11 | 0.523 |
| 12 | 0.645 |
| 13 | 0.432 |
| 14 | 0.248 |
| 15 | 0.628 |
| 16 | 0.487 |
| 17 | 0.432 |
| 18 | 0.476 |
| 19 | 0.637 |
| 20 | 0.436 |
| 21 | 0.215 |
| 22 | 0.482 |
| 23 | 0.560 |
| 24 | 0.517 |
| 25 | 0.526 |
Abbreviation: SCC, Squamous cell carcinoma; RSI, Radiosensitivity Index. Bold values are intrinsically radiosensitive.
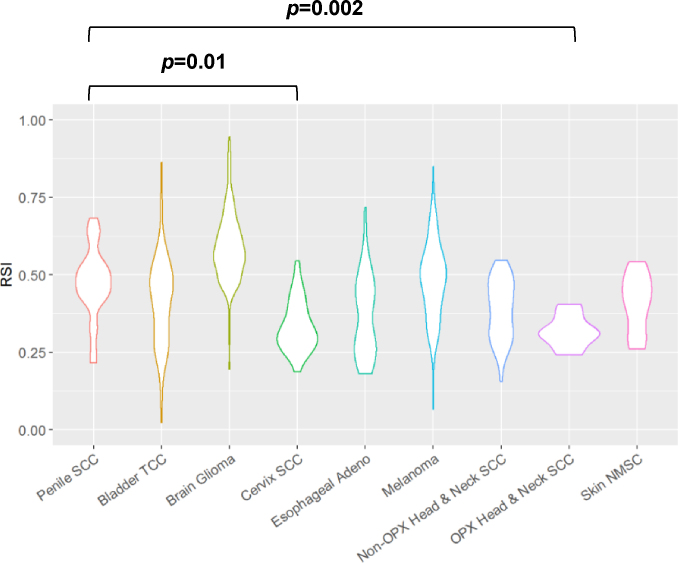
We have previously analyzed the RSIs in multiple lesions such as bladder transitional cell carcinoma, glioma, cervical SCC, esophageal adenocarcinoma, melanoma, non-melanoma skin cancer, non-oropharynx and oropharynx head and neck SCC.11 In comparison to these tumor types (Fig. 1), PeSCC is significantly more radioresistant than oropharynx head and neck SCC (p = 0.01) and cervical SCC (median RSI 0.31, p = 0.002).
Fig. 1.
Comparison of PeSCC RSI with other tumor types. Violin plot was generated for selected cancers including penile SCC, bladder TCC, glioma, cervical SCC, esophageal adenocarcinoma, melanoma, non-oropharynx head and neck SCC, oropharynx head and neck SCC, and non-melanoma skin cancer. Abbreviation: SCC: Squamous cell carcinoma; NMSC: Nonmelanoma skin cancer; TCC: Transitional cell carcinoma; Adeno: Adenocarcinoma.
3.2. Modeling GARD of PeSCC
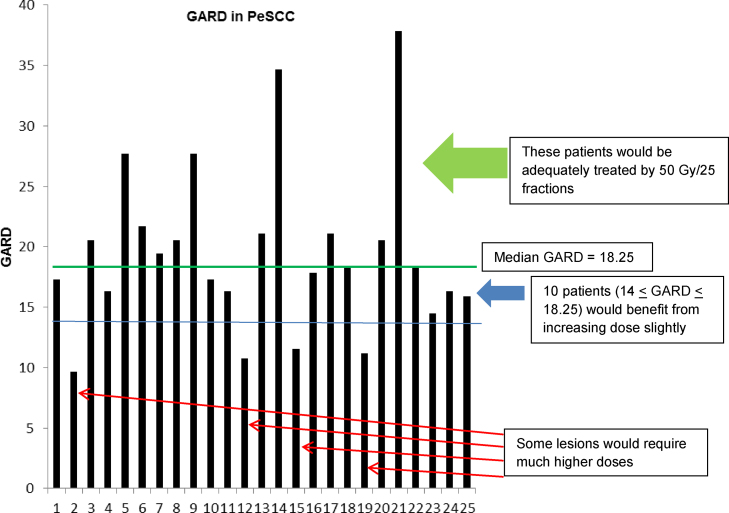
We applied the commonly used PORT dose of 50 Gy to model GARD and assess the genomic-based RT therapeutic effect in the adjuvant setting for PeSCC. GARD values varied from 9.56 to 38.39 with a median score of 18.25 (Fig. 2), suggesting a wide variation of RT therapeutic effects when patients were treated with a uniform dose of 50 Gy.
Fig. 2.
GARD modeling for PeSCC. Individual GARD value was calculated for the 25 penile tumors using commonly prescribed postoperative radiation dose of 50 Gy. The 50th percentile of GARD (18.25, marked with green line) was used to model GARD optimization based on previous validation studies. Green arrow represents lesions with optimal GARD-based therapeutic effects from radiation. Blue arrow represents penile tumors with GARD of 14–18.25, which would benefit from slightly increasing radiation dose prescription to achieve the median GARD of 18.25. Red arrow represents more radioresistant tumors, which would require higher radiation dosing to achieve optimal GARD-based therapeutic effect. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
As described previously, a high GARD value predicts for a high RT therapeutic effect.11 High GARD corresponds with improved outcomes in patients with breast, lung, pancreatic cancers, glioblastoma, and lung metastases.11, 15 When modeling at the median GARD of 18.25 for PeSCC, we identified 12 lesions (48%) in the tissue cohort that were at risk for LRR when treated with the commonly prescribed PORT dose of 50 Gy in 25 fractions. These lesions were identified below the median GARD score (thick green line in Fig. 2). On the other hand, PeSCC patients with high GARD values (> median GARD value of 18.25) would be relatively well treated with 50 Gy (green arrow in Fig. 2).
We then modeled the required RT dose for lesions to achieve a GARD of at least 18.25, the median GARD for PeSCC required for adequate locoregional control (LRC) as predicted in the model. Ten lesions with GARD values of 14–18.25 (blue arrow in Fig. 2) would require a GARD-based dose of 54–62 Gy to be adequately treated (Table 2). Conversely, four lesions (red arrow in Fig. 2) would require a higher GARD-based dose to be well treated (Table 2). As detailed in Table 2, at a GARD-based dose of 30 Gy, 2 of the 25 tumors would be well treated given higher intrinsic radiosensitivity; the modeled dose of 50 Gy would control only 52% of lesions; a dose of 66 Gy controls 84% of lesions; and the 3 most radioresistant lesions would not be controlled by 78 Gy.
Table 2.
Number/percentage of patients in the tissue cohort above the median by total dose (2 Gy fractions).
| GARD-Modeled RT dose (Gy) | Number of Patients Achieving Median GARD | % Patients Achieving Median GARD |
|---|---|---|
| 30 | 2 | 0.08 |
| 34 | 3 | 0.12 |
| 38 | 6 | 0.24 |
| 42 | 6 | 0.24 |
| 46 | 10 | 0.4 |
| 50 | 13 | 0.52 |
| 54 | 16 | 0.64 |
| 58 | 18 | 0.72 |
| 62 | 20 | 0.8 |
| 66 | 21 | 0.84 |
| 70 | 21 | 0.84 |
| 74 | 22 | 0.88 |
| 78 | 22 | 0.88 |
3.3. Clinical cohort patient characteristics and treatment outcomes
We assessed 34 PeSCC patients treated with PORT. With tissues available, ten patients (No.1–10) were assessed for RSI and GARD. Median follow-up from the completion of RT for this entire clinical cohort was 12.1 months (range 0.7–104.7 months). As detailed in Table 3, the majority of patients presented with pathological N2 or N3 disease (n = 31; 91%). Thirty patients (88%) were treated with adjuvant concurrent platinum-based CRT. Two patients were treated with PORT alone. Median PORT dose for the cohort was 50 Gy (range 42.5–64.8 Gy).
Table 3.
Clinical data for penile cancer patients treated with postoperative RT.
| Patient No. | Stage | RT dose (Gy) | Concurrent Chemotherapy | Patterns of failure | Time to failure since completion of RT (Months) | Follow-up (Months) | GARD |
|---|---|---|---|---|---|---|---|
| 1 | pT3 N2 | 50 | N | RR and DM | 1.28 and 1.28, respectively | 5.1 | 19.51 |
| 2 | pT1 N2 | 45 | Y | RR | 14.54 | 15.6 | 20.52 |
| 3 | pT2 N2 | 46 | Y | RR and DM | 0.82 and 1.41, respectively | 4.4 | 16.19 |
| 4 | pT1 N3 | 50.4 | Y | 24.4 | 20.98 | ||
| 5 | pT3 N2 | 45 | Y | 10.3 | 34.90 | ||
| 6 | pT1 N3 | 45 | Y | LR | 2.57 | 30.0 | 18.57 |
| 7 | pT3 N2 | 50 | Y | 12.1 | 20.77 | ||
| 8 | pT3 N2 | 50.4 | Y | 64.5 | 38.39 | ||
| 9 | pT3 N3 | 50 | Y | LR and RR | 37.43 | 84.6 | 14.52 |
| 10 | pT3 N2 | 45 | Y | 16.9 | 16.05 | ||
| 11 | pT1aN2 | 59.4 | Y | RR | 4.51 | 11.3 | N/A |
| 12 | pT2 N2 | 53.75 | N | 18.5 | N/A | ||
| 13 | pT2 N2 | 50 | Y | 43.6 | N/A | ||
| 14 | pT1 N3 | 50 | Y | RR | Recurrence during RT | 19.2 | N/A |
| 15 | pT3 N3 | 46 | Y | LR | Progression during RT | 6.4 | N/A |
| 16 | pT3 N3 | 53.75 | Y | 9.6 | N/A | ||
| 17 | pT2 N2 | 57.4 | Y | LR | 6.12 | 23.3 | N/A |
| 18 | pT1 N2 | 53.75 | Y | 4.2 | N/A | ||
| 19 | pT1 N2 | 54 | Y | RR | 5.69 | 10.8 | N/A |
| 20 | pT1 N3 | 64.8 | Y | 49.7 | N/A | ||
| 21 | pT2 N3 | 50 | Y | 44.7 | N/A | ||
| 22 | pT2 N2 | 45 | Y | LR and DM | 12.01 and 12.01, respectively | 17.1 | N/A |
| 23 | pT2 N0 | 45 | N | 6.7 | N/A | ||
| 24 | pT1 N2 | 60 | Y | DM | 1.02 | 58.8 | N/A |
| 25 | pT3 N1 | 45 | Y | 34.4 | N/A | ||
| 26 | pT4 Nx | 50.4 | Y | 0.7 | N/A | ||
| 27 | pT2 N1 | 45 | Y | 104.7 | N/A | ||
| 28 | pT2 N2 | 50 | Y | 3.5 | N/A | ||
| 29 | pT2 N2 | 59.4 | Y | RR | 3.75 | 9.1 | N/A |
| 30 | pT1 N2 | 50.4 | Y | 5.6 | N/A | ||
| 31 | pT1 N2 | 42.5 | Y | 27.9 | N/A | ||
| 32 | pT2 N2 | 50 | Y | 2.8 | N/A | ||
| 33 | pT2 N2 | 50 | N | RR | 7.99 | 28.8 | N/A |
| 34 | pT3 N2 | 63 | Y | RR | 0.16 | 6.3 | N/A |
Abbreviations: RT = Radiation therapy; LR = Local recurrence; RR = Regional recurrence; DM = Distant metastasis.
Fourteen out of the 34 patients (41%) had locoregional recurrence (LRR) or frank disease progression. Seven of these patients had LRR within six months of completion of PORT. One patient developed regional recurrence and one patient had local disease progression during RT. Four patients (11.8%) had DM within 1 year of completion of treatment.
Among the 10 patients with GARD assessment as listed in Table 3, five patients had LRR. Of whom, two has relatively low GARD values, 16.19 (patient No. 3) and 14.52 (patient No. 9), respectively; three failed locoregionally with a slightly higher GARD, 19.51 (No. 1), 20.52 (No. 2), and 18.57 (No. 6). No failure was observed in the patients with a moderately higher GARD, 20.98 (No. 4), 34.90 (No. 5), and 38.39 (No. 8).
4. Discussion
The development of biology-based treatment algorithms is a major goal of personalized medicine. In this study, we characterize the profiles of RSI and GARD for PeSCC. We found the majority of the PeSCC lesions are radioresistant, which is consistent with our clinical outcomes demonstrating a high risk of early LRR with the commonly used PORT regimen.
This is the first study to explore the intrinsic radiosensitivity of PeSCC on a genome-based level, adding another layer of knowledge that could lead to more personalized strategies in PeSCC. Our prior studies have validated the RSI algorithm in multiple malignancies,13, 14, 15, 16, 18, 19 showing that RSI correlates with clinical outcomes and predicts the response to RT. In addition, we found GARD independently predicted for clinical outcomes in breast cancer, lung cancer, pancreatic cancer, and glioblastoma.11 As reported previously,11 a higher GARD value predicts a higher RT therapeutic effect and GARD can be employed to adjust the RT dose to match an individual tumor’s intrinsic radiosensitivity. In this study, a GARD-modeled dose of 50 Gy would offer LRC benefit to only 52% of PeSCC lesions, implying prescribed dose must, in general, be increased for a higher likelihood of tumor control. This finding has been supported with our data from the clinical cohort showing a high rate of LRR (41%) in patients treated with the current one-size-fits-all approach. Similarly, Tang et al. reported 37.5% (n = 15) of 40 nodal-positive PeSCC patients treated with PORT had LRR at a median follow-up of 9.3 months.17 We are optimistic that GARD-based RT planning will help to facilitate a personalized RT treatment paradigm. Such information (Fig. 2) may also help identify intrinsically radioresistant lesions that the benefit from PORT in any reasonable dose may be so minimal that alternative treatment approaches should be considered. We plan to assess this approach formally in a prospective clinical trial.
PeSCC management remains a challenging paradigm for physicians given its rarity and resultant lack of high-level evidence. Although the findings from our study are hypothesis generating, our data will shine new light on the management of PeSCC in a biology-based approach. Genomic precision RT will potentially transform the clinical outcome of PeSCC. For instance, review of Table 2 informs clinicians that an 84% likelihood of lesion control post-operatively requires 66 Gy, rather than 50 Gy as we had previously thought.
In conclusion, our study demonstrates the majority of PeSCC tumors are intrinsically radioresistant with a high risk of recurrence after PORT. Genome-based adjustment of RT dose will help personalize the management of this rare and devastating disease.
Funding sources
NCI K05 mentee award and internal funding from the Department of Radiation Oncology, Moffitt Cancer Center.
Conflicts of interest
Dr. Anna R. Giuliano is on a member of Merck’s Scientific and Global Advisory Boards and receives funding from Merck for investigator-initiated studies related to HPV. Dr. Javier F. Torres-Roca is named inventors in a patent pending for systems for providing personalized radiation therapy, inventors in patent number 8,660,801, patent number 8,665,598 and patent number 7,879,545 which are related to radiosensitivity index, reports stock in Cvergenx and has a patent issued for radiation sensitivity index with royalties paid to Cvergenx, and a patent pending for GARD.
Financial disclosure
None declared.
IRB approval status
Reviewed and approved by Moffitt Cancer Center IRB.
References
- 1.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Mannweiler S., Sygulla S., Winter E., Regauer S. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol. 2013;69(1):73–81. doi: 10.1016/j.jaad.2012.12.973. [DOI] [PubMed] [Google Scholar]
- 3.Sonpavde G., Pagliaro L.C., Buonerba C., Dorff T.B., Lee R.J., Di Lorenzo G. Penile cancer: current therapy and future directions. Ann Oncol. 2013;24(5):1179–1189. doi: 10.1093/annonc/mds635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakenberg O.W., Comperat E.M., Minhas S., Necchi A., Protzel C., Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67(1):142–150. doi: 10.1016/j.eururo.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Robinson R., Marconi L., MacPepple E. Risks and Benefits of Adjuvant Radiotherapy After Inguinal Lymphadenectomy in Node-positive Penile Cancer: A Systematic Review by the European Association of Urology Penile Cancer Guidelines Panel. Eur Urol. 2018;74(1):76–83. doi: 10.1016/j.eururo.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Z., Naghavi A.O., Tang D. The relationship between HPV status and chemoradiotherapy in the locoregional control of penile cancer. World J Urol. 2018 doi: 10.1007/s00345-018-2280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zouhair A., Coucke P.A., Jeanneret W. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur J Cancer. 2001;37(2):198–203. doi: 10.1016/s0959-8049(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen M.F., Chen W.C., Wu C.T., Chuang C.K., Ng K.F., Chang J.T. Contemporary management of penile cancer including surgery and adjuvant radiotherapy: an experience in Taiwan. World J Urol. 2004;22(1):60–66. doi: 10.1007/s00345-003-0383-7. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Penile Cancer, version 2.2018. 2018; Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf.
- 10.Kayes O., Ahmed H.U., Arya M., Minhas S. Molecular and genetic pathways in penile cancer. Lancet Oncol. 2007;8(5):420–429. doi: 10.1016/S1470-2045(07)70137-7. [DOI] [PubMed] [Google Scholar]
- 11.Scott J.G., Berglund A., Schell M.J. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211. doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschrich S.A., Pramana J., Zhang H. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75(2):489–496. doi: 10.1016/j.ijrobp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschrich S.A., Fulp W.J., Pawitan Y. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18(18):5134–5143. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed K.A., Fulp W.J., Berglund A.E. Differences Between Colon Cancer Primaries and Metastases Using a Molecular Assay for Tumor Radiation Sensitivity Suggest Implications for Potential Oligometastatic SBRT Patient Selection. Int J Radiat Oncol Biol Phys. 2015;92(4):837–842. doi: 10.1016/j.ijrobp.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed K.A., Scott J.G., Arrington J.A. Radiosensitivity of Lung Metastases by Primary Histology and Implications for Stereotactic Body Radiation Therapy Using the Genomically Adjusted Radiation Dose. J Thorac Oncol. 2018;13(8):1121–1127. doi: 10.1016/j.jtho.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed K.A., Caudell J.J., El-Haddad G. Radiosensitivity Differences Between Liver Metastases Based on Primary Histology Suggest Implications for Clinical Outcomes After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016;95(5):1399–1404. doi: 10.1016/j.ijrobp.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang D.H., Djajadiningrat R., Diorio G. Adjuvant pelvic radiation is associated with improved survival and decreased disease recurrence in pelvic node-positive penile cancer after lymph node dissection: A multi-institutional study. Urol Oncol. 2017;35(10):605.e17–605.e23. doi: 10.1016/j.urolonc.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed K.A., Chinnaiyan P., Fulp W.J., Eschrich S., Torres-Roca J.F., Caudell J.J. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget. 2015;6(33):34414–34422. doi: 10.18632/oncotarget.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strom T., Hoffe S.E., Fulp W. Radiosensitivity index predicts for survival with adjuvant radiation in resectable pancreatic cancer. Radiother Oncol. 2015;117(1):159–164. doi: 10.1016/j.radonc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher G.H. 3rd ed. Lea & Febiger; Philadelphia: 1978. Textbook of Radiotherapy; p. 194. [Google Scholar]