Abstract
Purpose
Existing studies evaluating patient adherence to oral targeted therapies such as tyrosine kinase inhibitors focus on small populations with single malignancies. This study evaluated patterns of use of oral agents in a larger population across multiple hematologic malignancies.
Methods
Adult patients diagnosed with a hematologic malignancy and prescribed oral targeted therapy between 2011 and 2016 (N = 18,976) were identified from the MarketScan Commercial Claims and Encounters, and Medicare Supplemental databases. Eligible patients were enrolled in monthly prescription plans 6 months before and 12 months after the index date (date of first prescription claim; n = 2442). Multivariable logistic regressions were used to determine predictors of adherence using the medication possession ratio (MPR) and persistence through prescription refill gaps.
Results
The overall median adherence was 0.9 (MPR ≥ 80%) and was comparable between once-daily (QD) and twice-daily (BID) groups. Overall, 59% of patients were persistent at 12 months. Patients on QD and BID products did not have any significant differences in adherence (fixed-interval MPR, odds ratio 0.94; 95% confidence interval (CI), 0.75–1.18) or persistence (odds ratio 0.93; 95% CI, 0.75–1.17) 12 months from index. Significant predictors of adherence and persistence included patient age, total inpatient admissions, number of adverse events, and total hospital visits.
Conclusion
Patient-specific clinical factors, rather than regimen-specific factors, were the main predictors of oral targeted therapy adherence and persistence. Adherence to oral targeted therapies appears to be similar for patients on QD and BID regimens in the real-world setting.
Keywords: Dosing, Leukemia, lymphoma, predictors, real-world
Introduction
Following their introduction over a decade ago, oral tyrosine kinase inhibitors (TKIs) have taken on an increasingly important role in the treatment of malignancies. These agents have been shown to play a critical role in the inhibition of growth factor signaling, which is critical for tumor cell proliferation and metastasis.1 Some of the first molecules in this category, such as imatinib, targeted the BCR-ABL tyrosine kinase for therapy of chronic myeloid leukemia (CML). These were followed by molecules that targeted other tyrosine kinases, including epidermal growth factor receptor (gefitinib), vascular endothelial growth factor receptor (sorafenib), and Bruton's tyrosine kinase (ibrutinib).1,2
Despite the promise of these oral agents, it is estimated that 20%–50% of patients with chronic malignancies may not be adherent to their therapy.3 Physicians assume that patients may be better adherent to their prescribed oral therapy, primarily owing to the perceived convenience of self-administration of oral medications. These therapies have commonly been prescribed to be taken continuously until disease progression or intolerable adverse effects. Importantly, several studies evaluating adherence to oral targeted therapies have reported a mean adherence in the range of 77%–90%.4–8 For example, in an analysis of the adherence to daily use of imatinib, it was suggested that despite clear improved clinical benefits and the known risk of relapse associated with treatment interruptions, adherence was still not optimal.9
It is recognized that treatment adherence can have a direct bearing on clinical outcome. In a subanalysis of the phase III RESONATE trial comparing the use of once-daily (QD) ibrutinib therapy to anti-CD20 antibody intravenous therapy, progression-free survival was found to be significantly longer in patients with chronic lymphocytic leukemia (CLL) who adhered to the recommended dose of ibrutinib than in those who did not.10 In addition, nonadherence to an oral regimen has also been suggested to increase the overall economic burden of disease, as demonstrated by a retrospective claims database study that showed an increase of over 280% in medical costs, primarily driven by increased inpatient services, among a low-adherence group of commercially insured patients with CML.11
Several studies have observed that a range of factors may contribute to poor adherence to a continuous oral regimen in patients who have hematologic malignancies. Adherence to an oral TKI in patients with CML was shown to be negatively impacted by the duration of therapy, whereas factors such as participation in clinical trials and better patient socioeconomic status such as age, sex, and ethnicity were associated with higher rates of compliance.5,12 Moreover, adherence to oral TKI therapy in patients with CML was shown to be positively influenced by the concomitant pill burden and long duration of treatment, while toxicity to therapy appears to have had no impact on adherence behavior.13 Additional factors, such as trust of prescribing provider, impact of medication on lifestyle, cost of medication, and social support, have also been identified as being associated with adherence to oral therapy.14–17
The effect of dosing frequency on patient adherence to oral targeted therapies remains unclear. Although Claxton et al.18 attempted to define the association between dosing frequency and adherence, their review included cancer studies conducted before the widespread adoption of oral TKIs into clinical practice. Therefore, it is important to understand the effect of dosing frequency on adherence in the modern era, recognizing that most oral TKIs require either a QD or a twice-daily (BID) dosing regimen. Therefore, in this study, we assessed claim-based adherence and persistence between QD and BID dosing of a TKI or other oral targeted therapies among patients with hematologic malignancies.
Materials and methods
Study design
This was a retrospective observational study focusing on patients in the USA who were diagnosed with a hematologic malignancy and who initiated an oral targeted therapy. The analysis utilized the MarketScan Commercial Claims and Encounters Database, as well as the MarketScan Medicare Supplemental Database, maintained by Truven Health Analytics,19 for patient-level data. These databases cover 86 million commercially insured and 8 million Medicare-covered unique patients. This study was non-interventional and utilized a secondary data source; therefore, there was no requirement for patient informed consent.
Patients
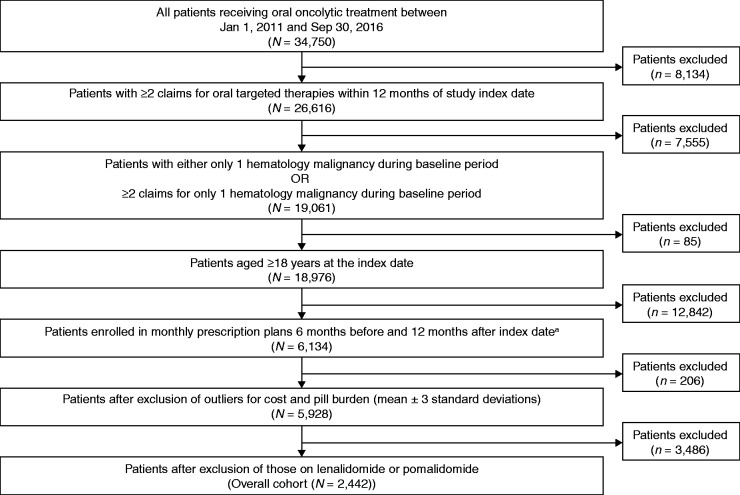
Between 1 January 2011 and 30 September 2016, patients diagnosed with a hematologic malignancy and on continuous oral targeted therapies were included in this study. Hematologic malignancy was defined as acute lymphoid leukemia, acute myeloid leukemia, CLL, small lymphocytic lymphoma (SLL), CML, essential thrombocythemia, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, multiple myeloma, chronic eosinophilic leukemia, chronic neutrophilic leukemia, polycythemia vera, primary myelofibrosis, and Waldenström's macroglobulinemia. Continuous oral targeted therapies included bosutinib, nilotinib, ruxolitinib, imatinib, dasatinib, ponatinib, ibrutinib, idelalisib, and thalidomide. Patients on lenalidomide and pomalidomide were not included because their associated dosing regimens are not continuous QD or BID. Patients were 18 years or older at the index date (date of first prescription claim), or the date of first oral targeted therapy prescription with at least one diagnosis of hematologic malignancy during the six months before the index date. Eligible patients had at least two prescription claims for oral targeted therapies following diagnosis of a hematologic malignancy and had continuous enrollment in the medical and drug plans during the baseline period and at least 12 months after the study index date. Patients who had diagnoses of multiple hematologic malignancies during the baseline period, and in whom the primary malignancy could not be defined, were excluded from the analysis (Figure 1). Patients with outlier cost and pill burden values were excluded from the analysis. Adverse events (AEs) related to TKI therapy were selected based on a literature review for patients receiving TKIs and on consultation with clinical experts. AEs were identified based on ICD-9/10 diagnosis codes in any position of the claims data.
Figure 1.
Sample attribution flow chart for patients with hematologic malignancy. Hematologic malignancy includes chronic myeloid leukemia, chronic lymphocytic leukemia, mantle cell lymphoma, acute myeloid leukemia, acute lymphocytic leukemia, myeloproliferative neoplasms, follicular lymphoma, marginal zone lymphoma, Waldenström's macroglobulinemia, and multiple myeloma.
ICD-9/10 CM: International Classification of Diseases, Ninth Revision, Clinical Modification.
aIndex date represents the date of first oral oncolytic treatment observed between 1 January 2011 and 30 September 2016, inclusive. Patients with any other solid tumor are not excluded from the analysis.
Adherence and persistence monitoring
Multiple prescription claims for each patient were concatenated to provide a longitudinal view into patient adherence and persistence behavior. The medication possession ratio (MPR) was used to assess adherence to oral targeted treatment. MPR was investigated in two ways: refill MPR and fixed-interval MPR. Refill MPR was calculated as the total number of days' medication supply divided by the sum of the last prescription fill date and the number of days' supply remaining at last prescription, minus the index date. Fixed-interval MPR was calculated as the total number of days' supply within the fixed interval divided by the fixed interval in days. Patients who did not adhere to their treatment regimen, either temporarily or by permanently discontinuing (that is, there was no other refill of the medication during the study period), had a lower MPR. As defined in previous studies,20 patients with an MPR lower than 80% were categorized as nonadherent, and those with an MPR of at least 80% were categorized as adherent. Patients were categorized on the basis of their prescription refill gaps as persistent (i.e. remaining on oral targeted therapy and having a gap of <60 days between prescription refills) or nonpersistent (i.e. patients with refill gaps of ≥60 days, with or without subsequent re-initiation of the same oral targeted therapy). If a patient was hospitalized during the analysis period, the duration of hospitalization was removed from the calculation (denominator), as the data did not provide visibility into prescriptions dispensed within the hospital setting. This was conducted with the assumption that the patient was fully adherent to the prescribed treatment while hospitalized. Sensitivity analyses were conducted using therapy gaps of 30 days and 90 days in assessing treatment persistence.
Statistical analysis
Descriptive statistics (mean, medium, minimum, maximum, quartile) were generated for patient characteristics (demographics, baseline comorbidities, baseline pill burden), treatment-related variables (oral targeted therapy treatment pattern, concomitant drug usage), and study measures (adherence, persistence). Multivariable logistic regression analyses were used to assess whether predictors such as age, gender, pill burden, daily dosing, and Charlson comorbidity index (CCI)21 were associated with treatment adherence and persistence. Stepwise selection in combination with multivariable logistic regression was used to determine the best models. To determine the significance of the variables, 95% confidence intervals (CIs) were used. The best model was determined on the basis of the model performance on the validation data set. In addition, Mann–Whitney U test and Chi-square test were used to determine the statistical significance of the results. The following subsets were compared: patients with MPR ≥ 80% versus MPR < 80% and persistent versus nonpersistent patients. Data processing and metric calculations were conducted in RStudio version 3.4.0.
Results
Patient characteristics
The MarketScan Commercial Claims and Encounters database included 34,750 patients who were receiving an oral targeted therapy during the study period of 1 January 2011 to 30 September 2016. There were 2442 eligible patients (Figure 1) who met the inclusion/exclusion criteria for the final analysis. The overall patient baseline characteristics are summarized in Table 1. In the cohort, 1757 patients (72%) were on QD regimens and 685 patients (28%) were on BID regimens. Forty-three percent of patients were female, and the mean age was 61 years. Fifty-one percent of patients were on a preferred provider organization healthcare plan. Patients had a mean CCI score of 2.1 (range 0.0–14.0). The most common malignancies at index were CML (45%), CLL/SLL (21%), and myeloproliferative neoplasm (16%). Only 14% of the patients were already on cancer-directed therapy.
Table 1.
Baseline and study period characteristics of patients with hematologic malignancy.
| Overall cohort |
All QD products |
All BID products |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Baseline characteristics | ||||||
| All patients | 2442 | 100 | 1757 | 100 | 685 | 100 |
| Age at index date, years (range) | ||||||
| Median (Q1, Q3) | 61 (52, 72) | 61 (52, 72) | 61 (52, 72) | |||
| Age group, years | ||||||
| 18–50 | 599 | 25 | 433 | 25 | 166 | 24 |
| 51–64 | 915 | 37 | 668 | 38 | 247 | 36 |
| 65+ | 928 | 38 | 656 | 37 | 272 | 40 |
| Health plan type | ||||||
| CDHP | 121 | 5 | 89 | 5 | 32 | 5 |
| COMP | 444 | 18 | 303 | 17 | 141 | 21 |
| EPO | 20 | 1 | 14 | 1 | 6 | 1 |
| HDHP | 64 | 3 | 43 | 2 | 21 | 3 |
| HMO | 260 | 11 | 194 | 11 | 66 | 10 |
| PPO | 1257 | 51 | 897 | 51 | 360 | 53 |
| POS | 182 | 7 | 139 | 8 | 43 | 6 |
| Unknowna | 94 | 4 | 78 | 4 | 16 | 2 |
| Malignancy at index dateb | ||||||
| Multiple myeloma | 208 | 9 | 204 | 12 | 4 | 1 |
| CML | 1093 | 45 | 812 | 46 | 281 | 41 |
| CLL/SLL | 513 | 21 | 497 | 28 | 16 | 2 |
| MCL | 61 | 2 | 61 | 3 | 0 | 0 |
| AML | 32 | 1 | 24 | 1 | 8 | 1 |
| ALL | 50 | 2 | 45 | 3 | 5 | 1 |
| MPN | 386 | 16 | 41 | 2 | 345 | 50 |
| FL | 40 | 2 | 16 | 1 | 24 | 4 |
| MZL | 10 | 0 | 8 | 0 | 2 | 0 |
| WM | 49 | 2 | 49 | 3 | 0 | 0 |
| With prior cancer-directed therapy | ||||||
| Chemotherapy | 107 | 4 | 96 | 5 | 11 | 2 |
| Immunomodulatorsc | 106 | 4 | 85 | 5 | 21 | 3 |
| Immunotherapyd | 144 | 6 | 128 | 7 | 16 | 2 |
| Year in which treatment initiated (study index date) | ||||||
| 2011 | 273 | 11 | 215 | 12 | 58 | 8 |
| 2012 | 443 | 18 | 276 | 16 | 167 | 24 |
| 2013 | 464 | 19 | 301 | 17 | 163 | 24 |
| 2014 | 689 | 28 | 547 | 31 | 142 | 21 |
| 2015 | 573 | 23 | 418 | 24 | 155 | 23 |
| Time from preindex diagnosis to index date (days) | ||||||
| Median (Q1, Q3) | 5 (1, 13) | 4 (1, 11) | 7 (2, 21) | |||
| Daily pill burdene (number of pills) | ||||||
| Median (Q1, Q3) | 1.4 (1.0, 2.0) | 1.4 (1.0, 2.0) | 1.4 (1.0, 2.0) | |||
| Study-period characteristics | ||||||
| Hematologic AEs (any grade) | ||||||
| Anemia | 1153 | 47 | 819 | 47 | 334 | 49 |
| Thrombocytopenia | 453 | 19 | 323 | 18 | 130 | 19 |
| Neutropenia | 312 | 13 | 245 | 14 | 47 | 7 |
| Nonhematologic AEs (any grade) | ||||||
| Nausea | 337 | 14 | 270 | 15 | 67 | 10 |
| Hyperglycemia | 724 | 30 | 533 | 30 | 191 | 28 |
| Fatigue | 704 | 29 | 502 | 29 | 202 | 29 |
| Fluid retention | 61 | 2 | 50 | 3 | 11 | 2 |
| Edema | 418 | 17 | 315 | 18 | 103 | 15 |
AE: adverse event; ALL: acute lymphoid leukemia; AML: acute myeloid leukemia; BID: twice daily; CDHP: consumer-driven health plan; CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; COMP: comprehensive; EPO: exclusive provider organization; FL: follicular lymphoma; HDHP: high deductible health plan; HMO: health maintenance organization; MCL: mantle cell lymphoma; MPN: myeloproliferative neoplasm; MZL: marginal zone lymphoma; POS: point of service; PPO: preferred provider organization; Q: quarter; QD: once daily; SLL: small lymphocytic lymphoma; WM: Waldenström's macroglobulinemia.
Health plan type: unknown, plan type not available.
Malignancy at index, malignant condition with the maximum number of diagnosis claims during baseline.
Immunomodulators, nonbiologic disease-modifying antirheumatic drugs.
Immunotherapy, biologic therapy.
Daily pill burden is the average pill burden assessed during a 30-day preindex period.
AEs related to the TKI therapy were also analyzed. Common reported hematologic AEs were anemia (47%), thrombocytopenia (19%), and neutropenia (13%); common nonhematologic AEs were hyperglycemia (30%), fatigue (29%), and edema (17%). The observed AE frequencies were comparable between QD and BID groups; however, patients on BID regimens had significantly less neutropenia (7% vs. 14%; p < 0.001) and nausea-related AEs (10% vs. 15%; p < 0.001) than patients on QD regimens.
Effect of dosing regimen on adherence and persistence
The overall patient adherence to and persistence with oral targeted treatments are summarized in Table 2. The median 12-month fixed MPR for all patients was 90% (interquartile range 50%–100%). At 12 months from the index date with 30-, 60-, and 90-day gaps, the patient persistence was 49%, 59%, and 63%, respectively. Patients on QD regimens had a median 12-month fixed MPR of 90% (interquartile range 50%–100%). The patient persistence for patients on QD regimens at 12 months from the index date at 30-, 60-, and 90-day gaps was 50%, 59%, and 63%, respectively. Patients on BID regimens had a median 12-month fixed MPR of 90% (interquartile range, 50%–100%). The patient persistence for BID regimens at 12 months from the index date at 30-, 60-, and 90- day gaps was 47%, 59%, and 65%, respectively. Patients on QD and BID products did not have any significant differences in adherence (fixed-interval MPR, 12 months from index, odds ratio [OR] 0.94; 95% CI, 0.75–1.18) or persistence (12 months from index, OR 0.93; 95% CI, 0.75–1.17).
Table 2.
Adherence to and persistence with oral targeted therapy.
| Overall cohort |
All QD products |
All BID products |
Mann–Whitney U test | ||||
|---|---|---|---|---|---|---|---|
| Adherence (MPR)a | N | % | N | % | N | % | P value |
| All patients | 2442 | 100 | 1757 | 100 | 685 | 100 | |
| Fixed-interval MPR (primary) 12 months from index | |||||||
| Median (Q1, Q3) | 0.9 (0.5, 1.0) | 0.9 (0.5, 1.0) | 0.9 (0.5, 1.0) | ||||
| Adherent total (MPR ≥ 0.85) | 1320 | 54 | 945 | 54 | 375 | 55 | .171 |
| Adherent total (MPR ≥ 0.80) | 1446 | 59 | 1044 | 59 | 402 | 59 | .742 |
| Fixed-interval MPR (primary) 24 months from index | |||||||
| Median (Q1, Q3) | 0.8 (0.3, 0.9) | 0.8 (0.3, 0.9) | 0.9 (0.3, 0.9) | ||||
| Adherent total (MPR ≥ 0.85) | 545 | 47 | 382 | 45 | 163 | 52 | .308 |
| Adherent total (MPR ≥ 0.80) | 597 | 51 | 417 | 49 | 180 | 57 | .543 |
| Persistenceb |
N
|
% |
N
|
% |
N
|
% |
Chi-square test |
| P value | |||||||
| 12 months from index | |||||||
| Gapc = 30 days | 1196 | 49 | 871 | 50 | 325 | 47 | .3682 |
| Gapc = 60 days | 1431 | 59 | 1030 | 59 | 401 | 59 | 1.0000 |
| Gapc = 90 days | 1547 | 63 | 1102 | 63 | 445 | 65 | .3238 |
| 24 months from index | |||||||
| Gapc = 30 days | 390 | 34 | 277 | 33 | 113 | 36 | .3323 |
| Gapc = 60 days | 531 | 46 | 369 | 44 | 162 | 52 | .01847 |
| Gapc = 90 days | 592 | 51 | 403 | 48 | 189 | (60 | .00002 |
Note: BID: twice daily; MPR: medication possession ratio; Q: quarter; QD: once daily.
MPR = total days of targeted therapy / (days post index date – days hospitalized).
Persist through treatment where respective refill gap between prescriptions.
Therapy gaps between prescription refills.
Factors affecting adherence and persistence
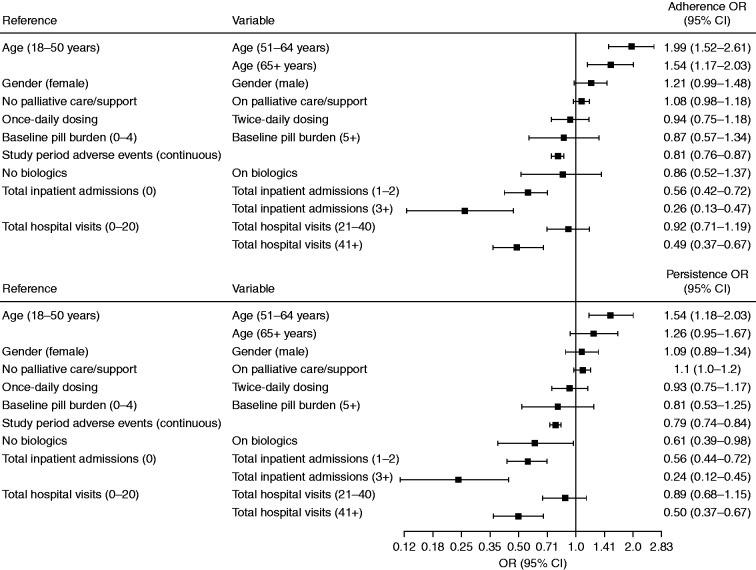
Patient age, total inpatient admissions, and total hospital visits were found to have a strong association with patient adherence and persistence to oral targeted therapy (Figure 2). Patients aged ≥ 51 years were more likely to adhere (OR 1.99; 95% CI, 1.52–2.61; and OR 1.54; 95% CI, 1.17–2.03, respectively) and persist through treatment (OR 1.54; 95% CI, 1.18–2.03) than patients aged 18–50 years. Inpatient admissions (all cause) had an inverse relationship with adherence and persistence, whereas patients with inpatient admissions of 1–2, or 3 or more were associated with less adherence and persistence than patients with no hospital admissions (OR 0.56; 95% CI, 0.42–0.72; and OR 0.26; 95% CI, 0.13–0.47, respectively) and persistence (OR = 0.56; 95% CI, 0.44–0.72; and OR = 0.24; 95% CI, 0.12–0.45, respectively).
Figure 2.
Predictors of adherence and persistence. CI: confidence interval; OR: odds ratio.
Compared with patients with up to 20 outpatient visits per year, patients with more than 41 total outpatient visits were less likely to be adherent (OR = 0.49; 95% CI, 0.37–0.67) and persistent through treatment (OR = 0.50; 95% CI, 0.37–0.67). Patients with a larger number of AEs were also less likely to be adherent (OR = 0.81; 95% CI, 0.76–0.87) or persistent (OR = 0.79; 95% CI, 0.74–0.84). During the study period, 10% of patients (N = 244) were on more than one oral targeted therapy.
Discussion
Patient nonadherence has likely been a major barrier to the effectiveness of oral targeted therapy.22 While some studies have attempted to assess the impact of various patient and drug-formulation factors on adherence and persistence,5,6,13 these analyses have been conducted in small cohorts and were specific for only one disease. The claims data utilized in this study attempted to provide a transparent view of a patient's therapeutic oral intervention through the healthcare system in the USA. Within the limits of this study, this retrospective investigation used a large patient pool across 15 hematologic malignancies to try to develop a clearer understanding of the association between patient factors and therapy characteristics on patient adherence and persistence to oral targeted therapies.
Dosing regimen, specifically QD versus BID dosing, was not associated with differences in adherence or persistence of oral targeted therapy in our study. The prominent factors in our study that influenced adherence and persistence were patient age, number of AEs, total number of inpatient admissions, and total number of hospital and office visits. Previous research exploring the influence of patient age and gender on adherence provided mixed results.8,9,13,17 Since this was a claims-based study, we were not able to capture the personal factors that may have been associated with age and gender that have been shown to be predictors of increased adherence. These predictive factors include higher education, understanding of potential side effects, knowledge of the treated disease, benefits of therapy, social support, and psychological well-being.9,15–17
Intuitively, QD dosing regimens may be an appealing choice over more frequent daily dosing for physicians and patients owing to the perception of better adherence associated with ease of therapy administration. A systematic review of 76 clinical trials across a variety of medical disorders and prescribed regimens, where adherence was measured by microelectronic monitoring systems, observed that the mean dose-taking compliance declined significantly as the number of daily doses increased; however, there was no difference in compliance in the pairwise comparison between QD and BID regimens.18 While the review highlighted the broad trends of dosing regimens, the findings were across multiple diseases, and the oncology therapies did not include TKIs. Our data suggest that the dosing regimen of either QD or BID oral targeted therapies does not appear to affect patient adherence to or persistence with oral treatment for hematologic malignancies.
Perhaps surprisingly, we found that patients on BID regimens experienced significantly fewer hematologic AEs related to neutropenia and nonhematologic AEs such as nausea than patients on QD dosing regimens. Further study will be needed to determine why this association could exist. One possible explanation for these differences might be that the pharmacokinetic properties of the specific single-daily dosing oral targeted compounds used in this study may result in peak drug concentrations that would be associated with the development of significant AEs. For example, concentrations of imatinib greater than 3180 ng/mL have been associated with an increased frequency of all-grade neutropenia, anemia, and leukopenia observed within the first three months of therapy.23 The peak concentrations (Cmax) for imatinib were dose-proportional, and the Cmax of imatinib 600 mg QD was 3395 ng/mL compared with a Cmax of 1907 ng/mL for 400 mg delivered QD, suggesting a narrow therapeutic index at higher single-daily doses.24 Previous studies have also observed that patients taking high single doses of imatinib (>400 mg compared with those who received <400 mg daily) were more likely not to adhere to treatment owing to intolerance.8,25 Pharmacokinetic properties of specific oral targeted therapies may therefore contribute, at least in part, to the differences in AEs seen in QD versus BID oral therapies in this study.
Earlier studies observed that a high initial pill burden of 1–4 pills per day was a positive predictor for patient adherence to therapy.13,26 In our analysis, five or more pills per day were not found to have an impact on treatment adherence and persistence compared with 0–4 pills per day. Healthcare resource utilization, as indicated by the number of inpatient admissions or outpatient clinic visits, had a strong association with adherence and persistence. Patients with more than three inpatient admissions had a mean CCI of 3.4 and a higher pill burden (2.7 times greater than the overall cohort) and were older than 50 years of age. Wu et al.11 observed in their cohort study with 592 patients with CML that nonadherent patients had a higher number of frequent all-cause inpatient admissions than adherent patients. In our study, patients with more than 40 hospital and office visits had pill burdens comparable with those with up to 40 (2.7 vs. 2.0, respectively), but were older and had higher-than-average CCI scores (2.6 vs. 1.9, respectively), AEs (3.5 vs. 2.7, respectively), and emergency-room visits than the rest of the cohort (0.56 vs. 0.39, respectively).
The results of this study were subject to certain limitations relating to claims data and the retrospective study design. Claims data may contain coding errors and missing data as a result of variable reimbursement coding practices of physician offices, outpatient pharmacies, and hospitals. Additionally, claims data do not include information such as dose interruption, dose holds, and dose reduction, which may affect adherence measured by MPR. The MPR was based on filled pharmacy prescriptions and did not ensure the patient adhered to the prescribed dosing regimen. The gold standard for measuring compliance is plasmatic dosage and pharmacokinetics analysis. Administrative data were also collected for financial and administrative rather than research purposes and, therefore, may not provide insights into clinical variables of interest such as phases of malignancy, response to treatment, grade of AEs, or reasons for nonadherence, which could be patient-, treatment-, or physician-driven. The cut-off for hospitalizations of three or more admissions was specific to this study and may not be generalizable to other observational studies. It is possible that the lower adherence observed in this study was due to AEs, although this was not specifically evaluated. The study population included patients with commercial and Medicare supplemental insurance. Thus, we recognize that the results might not be generalizable to people with other types of insurance, or for individuals with no insurance. Due to the differing methods used to report adherence and persistence, it is often difficult to compare studies. It would be interesting to investigate how the experience of clinicians could be utilized to identify patients who are non-adherent for either voluntary or involuntary reasons, and to measure non-adherence based on its impact on clinical effectiveness.27
Conclusion
In summary, oral targeted therapies have provided significant clinical benefit to patients with hematologic malignancies. Poor adherence to and persistence with therapy have been known barriers to efficacy of oral targeted therapies and may be influenced by numerous patient-related factors. The data presented in this report suggest that adherence to and persistence with an oral targeted therapy may be similar for patients on QD and BID dosing regimens in the real-world setting.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by AstraZeneca LP, Gaithersburg, MD. Editorial support with styling and submission, sponsored by AstraZeneca LP, was provided by Oxford PharmaGenesis, Oxford, UK.
References
- 1.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 2005; 315: 971–979. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med 2007; 167: 540–550. [DOI] [PubMed] [Google Scholar]
- 4.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics 2007; 25: 481–496. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida MH, Pagnano KB, Vigorito AC, et al. Adherence to tyrosine kinase inhibitor therapy for chronic myeloid leukemia: a Brazilian single-center cohort. Acta Haematol 2013; 130: 16–22. [DOI] [PubMed] [Google Scholar]
- 6.Henk HJ, Woloj M, Shapiro M, et al. Real-world analysis of tyrosine kinase inhibitor treatment patterns among patients with chronic myeloid leukemia in the United States. Clin Ther 2015; 37: 124–133. [DOI] [PubMed] [Google Scholar]
- 7.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood 2009; 113: 5401–5411. [DOI] [PubMed] [Google Scholar]
- 9.Al-Barrak J, Cheung WY. Adherence to imatinib therapy in gastrointestinal stromal tumors and chronic myeloid leukemia. Support Care Cancer 2013; 21: 2351–2357. [DOI] [PubMed] [Google Scholar]
- 10.Morgan L. Adherence to ibrutinib therapy improves outcomes in patients with CLL. Am Health Drug Benefits 2015; 8: 39. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu EQ, Johnson S, Beaulieu N, et al. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin 2010; 26: 61–69. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez Rosa V, Gutierrez Nicolas F, Gavira Moreno R, et al. [Adherence and toxicity to tyrosine kinase inhibitor therapy in chronic myeloid leukemia]. Farm Hosp 2013; 37: 434–440. [DOI] [PubMed] [Google Scholar]
- 13.Efficace F, Baccarani M, Rosti G, et al. Investigating factors associated with adherence behaviour in patients with chronic myeloid leukemia: an observational patient-centered outcome study. Br J Cancer 2012; 107: 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LA. Factors influencing oral adherence: qualitative metasummary and triangulation with quantitative evidence. Clin J Oncol Nurs 2015; 19: 6–30. [DOI] [PubMed] [Google Scholar]
- 15.Efficace F, Breccia M, Cottone F, et al. Psychological well-being and social support in chronic myeloid leukemia patients receiving lifelong targeted therapies. Support Care Cancer 2016; 24: 4887–4894. [DOI] [PubMed] [Google Scholar]
- 16.Okumura LM, Antunes VD, Aguiar KS, et al. Tyrosine kinase inhibitors in patients with chronic myelogeneous leukemia: defining the role of social risk factors and non-adherence to treatment. Pharm Pract 2015; 13: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gater A, Heron L, Abetz-Webb L, et al. Adherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemia. Leukemia Res 2012; 36: 817–825. [DOI] [PubMed] [Google Scholar]
- 18.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296–1310. [DOI] [PubMed] [Google Scholar]
- 19.Truven Health Analytics, https://truvenhealth.com/your-healthcare-focus/analytic-research/marketscan-research-databases (accessed 14 November 2017).
- 20.Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin 2009; 25: 2303–2310. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008; 61: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 22.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 2002; 94: 652–661. [DOI] [PubMed] [Google Scholar]
- 23.Guilhot F, Hughes TP, Cortes J, et al. Plasma exposure of imatinib and its correlation with clinical response in the tyrosine kinase inhibitor optimization and selectivity trial. Haematologica 2012; 97: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 2004; 22: 935–942. [DOI] [PubMed] [Google Scholar]
- 25.StCharles M, Bollu VK, Hornyak E, et al. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia. Blood 2009; 114: 2209, (abstract).19745073 [Google Scholar]
- 26.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288: 462–467. [DOI] [PubMed] [Google Scholar]
- 27.Okumura LM. How to report adherence to treatment as clinically relevant data-making a case of CML and TKI. Support Care Cancer 2018; 26: 323–324. [DOI] [PubMed] [Google Scholar]