Abstract
Background
Tripartite motif‑containing 27 (TRIM27) belongs to the TRIM protein family, which is closely related to the progression of some certain human cancers. Nevertheless, the biological function of TRIM27 in esophageal squamous cell carcinoma (ESCC) is still not clear. The aim of present research is to examine the function of TRIM27 in ESCC cells.
Methods
In the present study, RNA interference (RNAi) and lentiviral vector were used to knockdown and overexpression of TRIM27 in ESCC cells respectively. qRT-PCR and western blot were used to examine the expression of TRIM27 in ESCC cells. Cell counting kit-8 (CCK-8) assay was performed to determine the proliferation of cells.
Results
Our analyses indicated that TRIM27 was a pro-proliferation factor in ESCC cells. Moreover, overexpression of TRIM27 deeply suppressed the apoptosis of ESCC cells and accelerated its glucose uptake. In addition, an AKT inhibitor LY294002 was used to determine the connection between TRIM27 and AKT in ESCC cells. Our results demonstrated that TRIM27 has involved in the PI3/AKT signaling pathway. Moreover, TRIM27 interacted with PTEN and mediated its poly-ubiquitination in ESCC cells. Importantly, the glycolysis inhibitor 3-BrPA also inhibited the effect of TRIM27 on ESCC cells. Hence, TRIM27 also participated in the regulation of energy metabolism in ESCC cells.
Conclusions
This research not only gained a deep insight into the biological function of TRIM27 but also elucidated its potential target and signaling pathway in human ESCC cells.
Keywords: Esophagus cancer, TRIM27, PI3/AKT, PTEN
Background
Esophageal squamous cell carcinoma (ESCC) is one of the common death related cancers worldwide, which ranks the sixth place in cancer mortality [1]. Although the traditional treatments (radiation, chemotherapy or esophagogastric resection) contribute to the treatment of ESCC patients, the overall 5-year survival rate is still less than 20% [2]. Previous report has demonstrated that ESCC can be possibly cured at its early phase [3]. Therefore, the effective biomarkers for diagnosing ESCC are urgently needed.
Tripartite motif‑containing 27 (TRIM27) is one of the family members of TRIM, which inherits the basic structure of TRIM family [4]. As a DNA binding protein, TRIM27 consists of a RING finger protein domain, a B‑box type I domain, and a B‑box type II domain and exhibits a RBCC motif at the N-terminus [5]. Previous report has demonstrated that TRIM27 accelerates the progression of colorectal cancer [5]. Moreover, it have been confirmed that inhibiting the expression of TRIM27 suppresses the proliferation of ovarian and nasopharyngeal cancer cells respectively [6, 7]. In addition, TRIM27 positively regulates the TNF-α-induced apoptosis [8]. Importantly, some single nucleotide polymorphisms (SNPs) of TRIM27 is associated with human ESCC [9]. However, the underlying molecule network of TRIM27 is still less identified in human ESCC.
PI3K/AKT signaling pathway plays a key role in the progression of human cancers, which is identified as a promising target for anti-cancer therapy [10]. Previous reports have confirmed that TRIM59 and TRIM27 promote the proliferation of CRC cells through regulating PI3K/AKT signaling pathway [5, 11]. Moreover, TRIM14 promotes the activity of PI3K/AKT signaling pathway in osteosarcoma cells [12]. Further, suppressing the PI3K/AKT signaling pathway contributes to inhibiting the proliferation of ESCC cells [13, 14]. However, the detailed relationship between TRIM27 and PI3/AKT pathway remains unclear in human ESCC cells.
The aim of present study is to explore the biological function of TRIM27 in ESCC cells. RNA interference (RNAi) and lentiviral vector were used to silencing and overexpression of TRIM27 in human ESCC cells. Our research not only provided novel evidences to understand the biological function of TRIM27 in human ESCC cells but also elucidated its potential target and signaling pathway in ESCC cells.
Materials and methods
ESCC tissue specimens
Esophageal squamous cell carcinoma tissues (n = 25) and adjacent-matched noncancerous samples (n = 18) were collected from The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China. All tissues were snap-frozen in liquid nitrogen, stored at − 80 °C.
Cell culture
The cell lines used in this research were TE-1, TE-11, ECA-109, KYSE150 and HEEC, which were purchased from Shanghai biology institute (Shanghai, China). 10% fetal bovine serum (GIBCO, USA) was added to culture media that containing 2 mM l-glutamine and 1% penicillin/streptomycin (Solarbio, China). Cells were grown in DMEM Medium (Trueline, USA) and maintained in a 5% CO2, at 37 °C incubator. This study was in agreement with the Declaration of Helsinki. The AKT inhibitor LY294002 (25 μmol/L, S1105, Selleck, USA) and glycolytic inhibitor 3-BrPA (20 μmol/L, 1113-59-3, Sigma, USA) were dissolved in DMSO (D2650, Sigma, USA) and used to culture cells.
RNA isolation and real-time PCR
Total RNA from different samples were isolated by using TRIzol Reagent (Invitrogen, USA). Then, cDNA synthesis kit (Fermentas, Canada) was used to reverse transcribe RNA into complementary DNA (cDNA). The program of the real-time PCR reaction was listed as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 45 s. GAPDH was used to normalize the gene expression. 2−ΔΔCt method was applied to calculate the relative gene expression. All data represented the mean of three replicates. Primer sequences are provided in Additional file 1.
Knockdown and overexpression of TRIM27
For silencing human TRIM27 (NM_006510.4), three shot interference RNAs (siRNA) that targeting TRIM27 were synthesized (Major, Shanghai, China) and subsequently transfected into the KYSE150 and TE-11 cells respectively by using Lipofectamine 2000 (Invitrogen, USA). Meanwhile, a nonspecific scrambled siRNA sequence was transfected into KYSE150 and TE-11 cells as negative control (siNC). The targeting locus and sequence of TRIM27 siRNA is provided in Additional file 1: Table S1.
As for overexpression of TRIM27, a lentiviral plasmid (pLVX-puro) containing the full-length human TRIM27 cDNA sequence and a mock plasmid (oeNC) were transiently transfected into TE-1 cells respectively.
Western blot
RIPA lysis buffer (JRDUN, Shanghai, China) was used to extract protein as indicated. An enhanced BCA protein assay kit (Thermo Fisher, USA) was utilized to estimate the protein content. Total protein (25 μg) was fractionated by using 10% SDS-PAGE and transferred to a nitrocellulose membrane (Millipore, USA) for 2 h, which were probed at 4 °C for 12 h with the primary antibodies followed by incubation for 1 h at 37 °C with the secondary antibody (HRP-labeled goat anti rabbit IgG antibody; 1:1000, Beyotime, China). An enhanced chemiluminescence system (Tanon, China) was utilized to quantify the protein content. Each analysis was detected in triplicate. GAPDH was used as the internal reference. The crucial information of the primary antibodies was listed in Additional file 1: Table S2.
Cell proliferation assay
Cell counting kit-8 (CCK-8) assay kits (SAB, USA) was used to examine cell proliferation. All procedures were performed according to the instruction of the manufacture. In brief, cells were seeded in 96-well plates and incubated with CCK-8 solution (1:10) for 1 h. Then, OD450 value was examined by microplate reader (Pulangxin, China) at 12, 24 and 48 h after seeding. The experiment was independently repeated thrice at each time point.
Cell apoptosis
Briefly, Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Beyotime, China) was used to examine the apoptosis rate of cells according to the instructions of the manufacturer. After 48 h of viral infection, flow cytometer (BD, USA) were utilized to determine cells. Three replications were needed for each sample.
Glucose transport
In brief, the glucose analog 2-NBDG kits (Biovision, San Francisco, USA) were used as a fluorescent probe for determining the activity of Glucose transport. In order to examine the uptake of 2-NBDG, a total of 5 × 105 cells from different groups were seeded in 6-well plates. Then, all cells were pre-incubated in Krebs–Ringer bicarbonate (KRB) buffer (glucose free) for 15 min after maintaining in a 5% CO2 atmosphere at 37 °C for 24 h. After that, cells were incubated in fresh KRB buffer supplemented with 2-NBDG for 45 min at 37 °C, 5% CO2. Flow cytometry using a GloMax®-Multi + flow cytometer (Promega, USA) was used to quantitatively analyze the stained cells.
Co-immunoprecipitation (Co-IP)
For IP, whole-cell extracts were prepared after transfection or stimulation with appropriate ligands, followed by incubation overnight with the appropriate antibodies plus Protein A/G beads (Santa Cruz Biotechnology, USA). Beads were washed five times and separated by SDS-PAGE. Western blot was performed by using the antibodies as indicated above.
Ubiquitination assay
KYSE150 cells were transfected with siNC or siTRIM27, the cells were lysed in 1% SDS-containing radio immunoprecipitation assay (RIPA) buffer by sonication on ice. Then, Lysates were treated by Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, USA) for 1 h. After that, each samples were incubated with the IgG (Proteintech, USA) overnight at 4 °C. Then, the nuclear pellet was collected by centrifugation at 3000 rpm for 5 min at 4 °C and subsequently washed by Protein A/G Plus-Agarose beads for four times. The purified proteins were separated by 4–20% gradient SDS-PAGE. Anti-TRIM27 antibody (12205-1-AP, Proteintech, USA), Anti-PTEN (ab32199, Abcam, UK) and anti-Ubiquitin antibody (ab7780, Abcam, UK) antibody were used for immunoblotting.
Statistical analysis
Statistical analyses were performed by using the software of GraphPad Prism Version 7.0 (CA, USA). Data were presented by mean ± SD. ANOVA for multiple comparisons was used to determine statistical significance and p-value < 0.05 was accepted.
Results
TRIM27 was upregulated in ESCC tissues and cells
A total of 25 ESCC tissues and 18 adjacent-matched noncancerous samples were used to examine the mRNA level of TRIM27. Moreover, six pairs of ESCC tissues and matched noncancerous samples were utilized to quantify the protein content of TRIM27. Both the mRNA and protein level of TRIM27 were significantly increased in ESCC tissues compared with that of adjacent samples (Fig. 1a, b). Therefore, our results demonstrated that TRIM27 was upregulated in ESCC tissues.
Fig. 1.
TRIM27 was upregulated in ESCC tissues and cells. N normal, T tumor. a The relative mRNA level of TRIM27 detected in ESCC tissues (n = 25) and adjacent noncancerous samples (n = 18), ***p < 0.001 vs normal. b The protein content of TRIM27 examined in six pairs of ESCC tissues and matched adjacent samples, ***p < 0.001 vs normal. c, d Stands for the mRNA and protein level of TRIM27 in HECC, TE-1, TE-11, EAC-109 and KYSE150 cells respectively, ***p < 0.001 vs HECC
Moreover, qRT-PCR and western blot were used to determine the mRNA and protein level of TRIM27 in four ESCC cell lines, including TE-1, TE-11, EAC-109 and KYSE150. The esophageal epithelial cells HECC were used as control. In the present study, the level of TRIM27 was also upregulated in ESCC cells compared with that of HECC, especially in TE-11 and KYSE150 cells (Fig. 1c, d). Therefore, knockdown of TRIM27 was induced in TE-11 and KYSE150 cells respectively. Meanwhile, TE-1 cells were chosen for overexpression of TRIM27.
Knockdown and overexpression of TRIM27 in ESCC cells
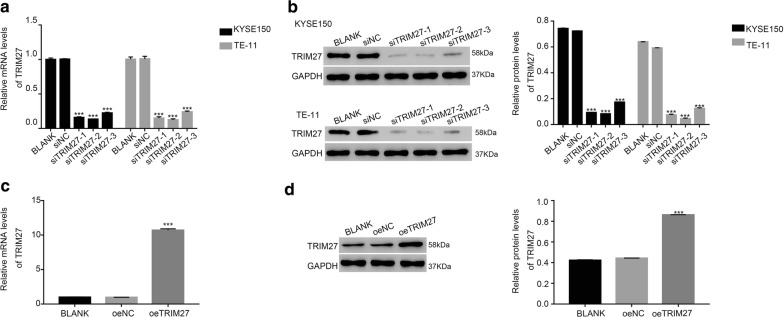
To further examine the function of TRIM27 in ESCC cells, we synthesized three short interference RNAs (siRNAs) targeting human TRIM27 (siTRIM27-1, siTRIM27-2 and siTRIM27-3) and a nonspecific scrambled siRNA (siNC) and subsequently transfected into KYSE150 and TR-11 cells. The unprocessed cells were served as blank control (BLANK). All TRIM27-siRNAs significantly suppressed the expression of endogenous TRIM27. Cells transfected with siTRIM27-1 and siTRIM27-2 showed a stronger effect than that of siTRIM27-3 in ESCC cells. Therefore, siTRIM27-1 and siTRIM27-2 transfected cells were used for the following analyses (Fig. 2a, b).
Fig. 2.
Knockdown and overexpression of TRIM27 in ESCC cells. a, b The mRNA and protein level of TRIM27 was deeply suppressed by siTRIM27 in KYSE150 and TE-11 cells, ***p < 0.001 vs siNC. c, d The TRIM27 was significantly upregulated by oeTRIM27 in TE-1 cells. ***p < 0.001 vs oeNC
Meanwhile, TE-1 cells were transfected with a plasmid for overexpressing TRIM27 (oeTRIM27). Both the mRNA and protein content of TRIM27 was significantly overexpressed in oeTRIM27-transfected cells (Fig. 2c, d). Therefore, the oeTRIM27 transfected cells were utilized for the following analyses.
Knockdown of TRIM27 suppressed the growth of ESCC cells
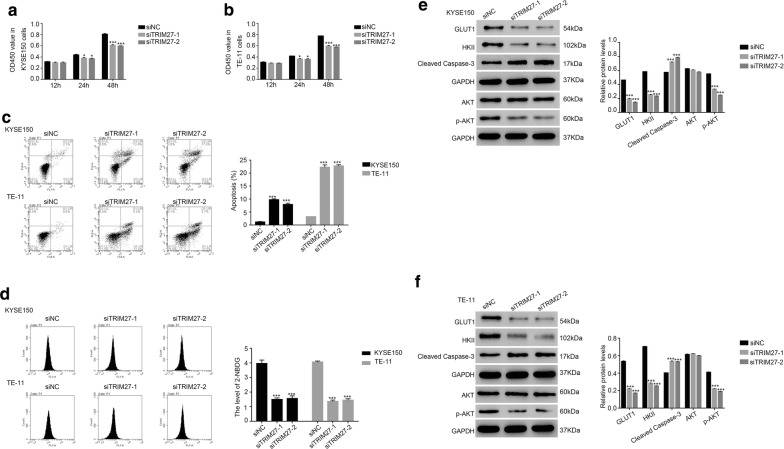
Cell proliferation rate was examined by cell counting kit-8 (CCK-8). As shown in Fig. 3a, b, siTRIM27-1 and siTRIM27-2 were deeply suppressed the proliferation rate of ESCC cells in two cell lines. Therefore, TRIM27 presented the pro-proliferation property in ESCC cells. Moreover, we also analyzed the apoptosis profile of siTRIM27 transfected cells. Obviously, the apoptosis profile of siTRIM27-1 or siTRIM27-2 transfected cells was much higher than that of siNC transfected cells (Fig. 3c). These results elucidated the anti-apoptosis function of TRIM27 in ESCC cells.
Fig. 3.
Knockdown of TRIM27 inhibited the growth of ESCC cells. a, b The cell proliferation rate detected at 12 h, 24 h and 48 h after transfection with siNC, siTRIM27-1 and siTRIM27-2 in KYSE150 and TE-11 cells respectively, *p < 0.05 vs siNC, ***p < 0.001 vs siNC. c Cell apoptosis profile was upregulated in siTRIM27 transfected cells as indicated, ***p < 0.001 vs siNC. d The level of 2-NBDG was suppressed in siTRIM27 transfected cells, ***p < 0.001 vs siNC. e, f The protein level of GLUT1, HKII, cleaved caspase-3, AKT and p-AKT examined in different cells as indicated, ***p < 0.001 vs siNC
Glucose transport activity was deeply suppressed by siTRIM27 in ESCC cells
The abnormal of energy metabolism is one of the biochemical characters for cancer cells, which leads to the acceleration of glycolysis activity [15]. In this study, we analyzed the activity of glycolysis by using the glucose analog 2-NBDG. Interestingly, the level of 2-NBDG was deeply suppressed in ESCC cells transfected with siTRIM27-1 or siTRIM27-2 (Fig. 3d). Therefore, TRIM27 might promote the proliferation of human ESCC cells through enhancing the glycolysis activity.
Previous reports have demonstrated that GLUT1 and HKII are positive correlative with the activity of glycolysis [16–19]. To further examine the function of TRIM27 in glycolysis, we quantified the protein content of GLUT1 and HKII in different cells as indicated. The protein content of GLUT1 and HKII were significantly decreased in siTRIM27 transfected cells (Fig. 3e, f). Moreover, the protein level of cleaved caspased-3 was remarkably upregulated in siTRIM27-1 or siTRIM27-2 transfected cells. In addition, the phosphorylation of AKT (p-AKT) was also inhibited in siTRIM27-1 or siTRIM27-2 transfected cells.
The AKT inhibitor LY29400 and glycolytic inhibitor 3-BrPA abolished the function of TRIM27 in ESCC cells
In order to further explore the correlation between TRIM27 and AKT, the AKT inhibitor LY294002 (25 μmol/L) was used to culture cells transfected with oeTRIM27. Meanwhile, the oeTRIM27 transfected cells were also cultured in the present of the glycolytic inhibitor 3-BrPA (20 μmol/L).
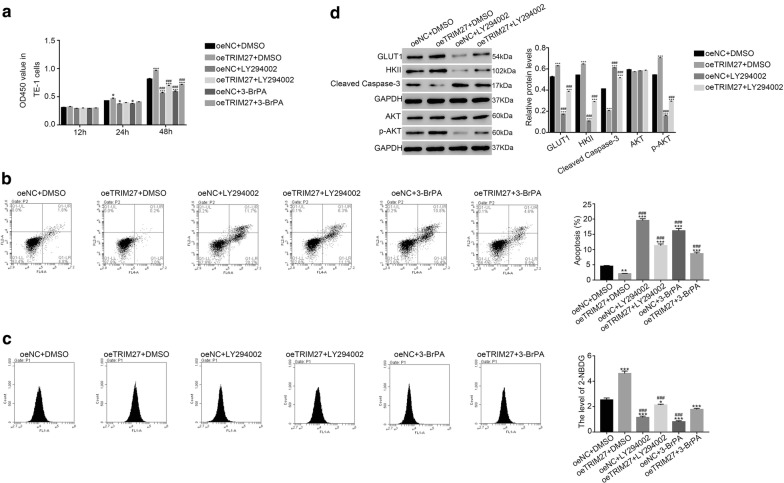
The cell proliferation rate was significantly promoted in oeTRIM27 transfected cells (Fig. 4a). However, the AKT inhibitor LY294002 deeply suppressed the cell proliferation rate of oeNC and oeTRIM27 transfected cells. Meanwhile, the similar result was also obtained in 3-BrPA cultured cells. Moreover, the apoptosis rate was deeply inhibited in oeTRIM27 transfected cells. Interestingly, the inhibitor LY294002 and 3-BrPA remarkably promoted the cell apoptosis rate in oeNC or oeTRIM27 transfected cells (Fig. 4b).
Fig. 4.
The inhibitor LY294002 and 3-BrPA inhibited the effect of TRIM27 in ESCC cells. a The cell proliferation rate detected at 12 h, 24 h and 48 h examined in different cells as indicated. *p < 0.05 vs oeNC, ***p < 0.001 vs oeNC. b Cell apoptosis profile was suppressed in oeTRIM27 transfected cells. **p < 0.01 vs oeNC, ***p < 0.001 vs oeNC; ###p < 0.001 vs oeTRIM27. c The level of 2-NBDG was upregulated in oeTRIM27 transfected cells. *p < 0.05 vs oeNC, ***p < 0.001 vs oeNC; ###p < 0.001 vs oeTRIM27. d The protein level of GLUT1, HKII, cleaved caspase-3, AKT and p-AKT examined in different cells as indicated, ***p < 0.001 vs oeNC; ###p < 0.001 vs oeTRIM27
In addition, we also analyzed the activity of glucose transport in different cells as indicated. It was clearly identified that overexpression of TRIM27 promoted the transport of glucose in cells transfected with oeTRIM27. Moreover, the inhibitor LY294002 and 3-BrPA deeply inhibited the glucose transportation in oeTRIM27 transfected cells (Fig. 4c). As shown in Fig. 4d, the protein content of GLUT1 and HKII were significantly increased in oeTRIM27 transfected cells. However, the AKT inhibitor LY294002 deeply inhibited the expression of GLUT1 and HKII in oeNC or oeTRIM27 transfected cells. Moreover, the protein level of cleaved caspase-3 showed the opposite results as that of GLUT1 and HKII in oeTRIM27 transfected cells. Importantly, the phosphorylation of AKT was positively correlated with TRIM27, which was deeply suppressed by the inhibitor LY294002 and 3-BrPA. Taken together, all these results demonstrated that the effect of TRIM27 was deeply abolished by the AKT inhibitor LY294002 and 3-BrPA on ESCC cells.
TRIM27 interacted with PTEN and promoted its poly-ubiquitination in ESCC cells
PTEN is identified as a critical component in PI3/AKT pathway [20]. PTEN/AKT pathway is the common abnormal signaling pathway in human cancers [21]. In order to further examine the function of TRIM27 in PI3/AKT, we analyzed the interaction between TRIM27 and PTEN by co-immunoprecipitation (Co-IP). As shown in Fig. 5a, it was easily identified that there was a stronger interaction between TRIM27 and PTEN. These results indicated that TRIM27 directly interacted with PTEN in ESCC cells.
Fig. 5.
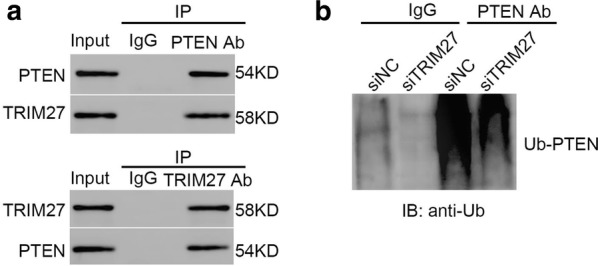
TRIM27 interacted with PTEN and promoted its poly-ubiquitination in ESCC cells. a CoIP assay was performed to examine the interaction between TRIM27 and PTEN in ESCC cells. b siTRIM27 inhibited the poly-ubiquitination of PTEN in ESCC cells
Previous report has demonstrated that the ubiquitination of PTEN is critical for its stability and nuclear localization [22]. Enhancing the ubiquitination of PTEN has improved the activity of PI3/AKT pathway [23]. In this study, intracellular ubiquitination assays was established to examine whether TRIM27 affected the poly-ubiquitination of PTEN. As shown in Fig. 5b, knockdown of TRIM27 deeply reduced the level of PTEN poly-ubiquitination in ESCC cells. Therefore, TRIM27 not only interacted with PTEN but also promoted its poly-ubiquitination in ESCC cells.
Discussion
Esophageal squamous cell carcinoma is one of the common tumors of digestive tract. Numerous deaths are caused by ESCC all over the world [24]. Worse still, the outcome of traditional therapy is far from satisfactory. Therefore, enhancing the understanding into the molecule pathogeny of ESCC is a critical step for developing novel strategies for its treatment.
At present study, we systemically explored the function of TRIM27 in ESCC cells. Knockdown and overexpression of TRIM27 were induced in ESCC cells respectively. Analyses from those two experimental sections were consistent. Therefore, our conclusions were more credible.
TRIM protein family are closely related to the development of human tumors [25]. In this study, we found TRIM27 was upregulated in ESCC tissue and cells. Moreover, our results indicated that TRIM27 was a pro-proliferation factor in ESCC cells. Meanwhile, overexpression of TRIM27 deeply inhibited the apoptosis of ESCC cells. Taken together, all these results demonstrated that TRIM27 was a positive oncogene for ESCC.
Growing evidences have indicated that the dysfunction of PI3/AKT signaling pathway is one of the hallmarks for human cancers [26, 27]. In the present study, our results demonstrated that the AKT inhibitor LY294002 disrupted the function of TRIM27 in ESCC cells. These results illuminated that TRIM27 was involved in PI3/AKT signaling pathway.
Previous report has demonstrated that PTEN accelerates cell apoptosis via PI3/AKT pathway [28]. Moreover, TRIM27 has enhanced the phosphorylation of AKT pathway in colorectal cancer [5]. In the present research, TRIM27 was identified to interact with PTEN and promoted its poly-ubiquitination. Therefore, our results further confirmed that TRIM27 involved in the p-AKT pathway. Moreover, TRIM27 might inhibit the apoptosis of ESCC cells via enhancing the ubiquitination of PTEN, which subsequently promoted the activity of PI3/AKT signaling pathway in ESCC cells.
Previous report has demonstrated that the glycolysis modulation is the potential anti-cancer therapy [29]. Moreover, some evidences have demonstrated that PTEN/AKT signaling enhances glycolysis in refractory acute myeloid leukemia [30]. PTEN/AKT pathway is reported as a target in suppressing glycolysis activity in cancer cells under hypoxia [31]. In this research, overexpression of TRIM27 promotes the activity of glycolysis metabolism in ESCC cells. Moreover, the protein content of GLUT1 and HKII were positively correlated with the expression of TRIM27. Moreover, the glycolysis inhibitor 3-BrPA deeply suppressed the function of TRIM27 in ESCC cells. Therefore, TRIM27 might promote the activity of glycolysis through upregulating GLUT1 and HKII in ESCC cells. It has been demonstrated that the deficiency of PTEN increases the activity of glycolysis [32]. Hence, the TRIM27/PTEN signaling pathway might also involve in the regulation of glycolysis metabolism in ESCC cells.
Conclusion
In this study, we induced knockdown and overexpression of TRIM27 in ESCC cells. This research not only gained a deep understanding of TRIM27 but also indicated its possible target and molecule network in ESCC cells.
Supplementary information
Additional file 1. Primer sequence information. Table S1. Human gene TRIM27 (NM_006510.4) RNAi targeting locus information. Table S2. The primary antibodies information.
Acknowledgements
We acknowledged the support given by The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, 224001, China.
Abbreviations
- TRIM27
tripartite motif-containing protein 27
- ESCC
esophageal squamous cell carcinoma
- SNPs
single nucleotide polymorphisms
- Co-IP
co-immunoprecipitation
- RNAi
RNA interference
- cDNA
complementary DNA
- CCK-8
cell counting kit-8
- IHC
immunohistochemistry
- H & E
hematoxylin and eosin
- IACUC
Institutional Animal Care and Use Committee
Authors’ contributions
The concept of the paper was devised and the first draft was written by ZZ and PC. The experiment was done by LM. NY analyzed the data. All authors read and approved the final manuscript.
Funding
This work was funded by the Natural and Science Foundation of Nantong (MS12017017-5 and GJZ16075).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This research was approved by the independent ethics committee of The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China and all patients provided written informed consent, in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ping Chen, Phone: +86 0515 88592872, Email: doc_pchen@126.com.
Zhixiang Zhuang, Phone: 86+ 0512 68282030, Email: zzx22066@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12935-019-0998-4.
References
- 1.Cristina B, Fabio L, Jacques F, Werner G, Franca L, Paola B, Eva N, Carlo LV. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2010;122(5):1118–1129. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Fedewa SA, Miller KD, Ann GS, Pinheiro PS, Dinorah MT, Ahmedin J. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2016;65(6):457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 3.di Pietro M, Canto MI, Fitzgerald RC. Endoscopic management of early adenocarcinoma and squamous cell carcinoma of the esophagus: screening, diagnosis, and therapy. Gastroenterology. 2018;154(2):421–436. doi: 10.1053/j.gastro.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micale L, Chaignat E, Fusco C, Reymond A, Merla G. The tripartite motif: structure and function. Adv Exp Med Biol. 2012;770:11–25. [PubMed] [Google Scholar]
- 5.Zhang Y, Feng Y, Ji D, Wang Q, Qian W, Wang S, Zhang Z, Ji B, Zhang C, Sun Y, et al. TRIM27 functions as an oncogene by activating epithelial–mesenchymal transition and p-AKT in colorectal cancer. Int J Oncol. 2018;53:620–632. doi: 10.3892/ijo.2018.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Wei Z, Bast RC, Jr, Wang Z, Li Y, Gao M, Liu Y, Wang X, Guo C, Zhang L, et al. Downregulation of TRIM27 expression inhibits the proliferation of ovarian cancer cells in vitro and in vivo. Lab Invest. 2016;96(1):37–48. doi: 10.1038/labinvest.2015.132. [DOI] [PubMed] [Google Scholar]
- 7.Meng YY, Wang X, Huang DD, Jin QZ, Chen WB, Cai ZY. Knockdown of TRIM27 expression regulates cell proliferation, invasion and migration in human nasopharyngeal 5-8F carcinoma cells. Chin J Pathophysiol. 2017;33(01):53–59. [Google Scholar]
- 8.Mohammad Mahabub-Uz Z, Teruaki N, Tsuyoshi T, Tomoo O, Wanzhu J, Toshie S, Yasunori T, Shunsuke I. Ubiquitination-deubiquitination by the TRIM27–USP7 complex regulates tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2013;33(24):4971–4984. doi: 10.1128/MCB.00465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang-Fang S, Wen-Bin Y, Fu-You Z, Ying P, Xue-Ke Z, Yan J, Xin S, Bei L, Xue-Na H, Sa T. Variations in the MHC region confer risk to esophageal squamous cell carcinoma on the subjects from high-incidence area in northern China. PLoS ONE. 2014;9(3):e90438. doi: 10.1371/journal.pone.0090438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Chen X, Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice) Br J Cancer. 2017;117(2):159–163. doi: 10.1038/bjc.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Ji B, Feng Y, Zhang Y, Ji D, Zhu C, Wang S, Zhang C, Zhang D, Sun Y. TRIM59 facilitates the proliferation of colorectal cancer and promotes metastasis via the PI3K/AKT pathway. Oncol Rep. 2017;38(1):43–52. doi: 10.3892/or.2017.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu G, Guo Y, Xu D, Wang Y, Shen Y, Wang F, Lv Y, Song F, Jiang D, Zhang Y. TRIM14 regulates cell proliferation and invasion in osteosarcoma via promotion of the AKT signaling pathway. Sci Rep. 2017;7:42411. doi: 10.1038/srep42411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He D, Zhang S. UNBS5162 inhibits the proliferation of esophageal cancer squamous cells via the PI3K/AKT signaling pathway. Mol Med Rep. 2017;17(1):549–555. doi: 10.3892/mmr.2017.7893. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Wen WX, Lam AKY, Yang W, Hu HF, Xin YG, Yan RQ, Saremi N, Tsao SW, He QY. Significance of PI3K/AKT signaling pathway in metastasis of esophageal squamous cell carcinoma and its potential as a target for anti-metastasis therapy. Oncotarget. 2017;8(24):38755–38766. doi: 10.18632/oncotarget.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganapathy-Kanniappan S, Geschwind JFH. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12(1):152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu YX, Wu QN, Chen DL, Chen LZ, Wang ZX, Ren C, Mo HY, Chen Y, Sheng H, Wang YN, et al. Pharmacological ascorbate suppresses growth of gastric cancer cells with GLUT1 overexpression and enhances the efficacy of oxaliplatin through redox modulation. Theranostics. 2018;8(5):1312–1326. doi: 10.7150/thno.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riskin A, Mond Y. Prolactin-induced subcellular targeting of GLUT1 glucose transporter in living mammary epithelial cells. Rambam Maimonides Med J. 2015 doi: 10.5041/RMMJ.10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Murray-Stewart T, Casero RA, Jr, Kagiampakis I, Jin L, Zhang J, Wang H, Che Q, Tong H, Ke J, et al. Targeting hexokinase 2 inhibition promotes radiosensitization in HPV16 E7-induced cervical cancer and suppresses tumor growth. Int J Oncol. 2017;50(6):2011–2023. doi: 10.3892/ijo.2017.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Zhang L. MicroRNA-143 suppresses oral squamous cell carcinoma cell growth, invasion and glucose metabolism through targeting hexokinase 2. Biosci Rep. 2017 doi: 10.1042/BSR20160404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi A, Ellenson LH. PI3K/PTEN/AKT genetic mouse models of endometrial carcinoma. Adv Exp Med Biol. 2017;943:261–273. doi: 10.1007/978-3-319-43139-0_9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Zhang Y, Qu J, Che X, Fan Y, Hou K, Guo T, Deng G, Song N, Li C. Exosomes promote cetuximab resistance via the PTEN/Akt pathway in colon cancer cells. Braz J Med Biol Res. 2018;51(1):e6472. doi: 10.1590/1414-431X20176472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao C, Li Z, Ahmad N, Liu X. Regulation of PTEN degradation and NEDD4–1 E3 ligase activity by Numb. Cell Cycle. 2017;16(10):957. doi: 10.1080/15384101.2017.1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JT, Jing S, Jiayun Z, Muyang L, Brenda Z, Amanda Z, Ramon P, Wei G. RFP-mediated ubiquitination of PTEN modulates its effect on AKT activation. Cell Res. 2013;23(4):552–564. doi: 10.1038/cr.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, He J. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26(1):48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42(4):297. doi: 10.1016/j.tibs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol Ther. 2016;172:101. doi: 10.1016/j.pharmthera.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miriam M, Maria Chiara DS, Laura B, Federico G, Emilio H. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 28.Jia M, Chen X, Liu J, Chen J. PTEN promotes apoptosis of H2O2-injured rat nasal epithelial cells through PI3K/Akt and other pathways. Mol Med Rep. 2017;17(1):571. doi: 10.3892/mmr.2017.7912. [DOI] [PubMed] [Google Scholar]
- 29.Gill KS, Fernandes P, Donovan TR, Mckenna SL, Doddakula KK, Power DG, Soden DM, Forde PF. Glycolysis inhibition as a cancer treatment and its role in an anti-tumour immune response. Biochim Biophys Acta. 2016;1866(1):87–105. doi: 10.1016/j.bbcan.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Ryu MJ, Han J, Kim SJ, Lee MJ, Ju X, Lee YL, Son JH, Cui J, Jang Y, Chung W, Song IC, Kweon GR, Heo JY. PTEN/AKT signaling mediates chemoresistance in refractory acute myeloid leukemia through enhanced glycolysis. Oncol Rep. 2019;42(5):2149–2158. doi: 10.3892/or.2019.7308. [DOI] [PubMed] [Google Scholar]
- 31.Fenglin C, Mingkai Z, Canmei Z, Jun P, Xiaozhong W, Jianying L, Zhixin C, Yuehong H. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol Rep. 2015;33(1):457–463. doi: 10.3892/or.2014.3550. [DOI] [PubMed] [Google Scholar]
- 32.Blouin MJ, Zhao Y, Zakikhani M, Algire C, Piura E, Pollak M. Loss of function of PTEN alters the relationship between glucose concentration and cell proliferation, increases glycolysis, and sensitizes cells to 2-deoxyglucose. Cancer Lett. 2010;289(2):246–253. doi: 10.1016/j.canlet.2009.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Primer sequence information. Table S1. Human gene TRIM27 (NM_006510.4) RNAi targeting locus information. Table S2. The primary antibodies information.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.