Abstract
Objective:
To determine whether maternal Epstein-Barr virus (EBV) IgG antibody levels are associated with risk of multiple sclerosis (MS) in the offspring.
Methods:
We conducted a prospective nested case-control study in the Finnish Maternity Cohort (FMC) with serum samples from over 800,000 women collected during pregnancy since 1983. Cases of MS among offspring born between 1983 and 1991 were identified via hospital and prescription registries; 176 cases were matched to up to 3 controls (n=326) on region and dates of birth, sample collection, and mother’s birth. We used conditional logistic regression to estimate relative risks (RR) and adjusted models for sex of the child, gestational age at sample collection, and maternal serum 25-hydroxyvitamin D and cotinine levels. Similar analyses were conducted among 1,049 women with MS and 1,867 matched controls in the FMC.
Results:
Maternal viral capsid antigen IgG levels during pregnancy were associated with an increased MS risk among offspring (RRtop vs. bottom quintile=2.44, 95%CI: 1.20–5.00, p trend=0.004); no associations were found between maternal EBNA-1, EA-D, or cytomegalovirus IgG levels and offspring MS risk. Among women in the FMC, those in the highest versus lowest quintile of EBNA-1 IgG levels had a 3-fold higher risk of MS (RR=3.21, 95%CI: 2.37–4.35, p trend <1.11e-16). These associations were not confounded or modified by 25-hydroxyvitamin D.
Interpretation:
Offspring of mothers with high VCA IgG during pregnancy appear to have an increased risk of MS. The increase in MS risk among women with elevated pre-diagnostic EBNA-1 IgG levels is consistent with previous results.
Introduction
There is strong epidemiologic evidence that multiple sclerosis (MS) is likely a rare complication of Epstein-Barr virus (EBV) infection. Primary infection with EBV is a strong risk factor for MS1 and increasing IgG antibody titers against the EBV nuclear antigen (EBNA) have been associated with an increased MS risk in healthy adults.2 Here we expand on these observations by examining whether serum levels of IgG antibodies against EBV or cytomegalovirus (CMV) measured during the first trimester of pregnancy in a large cohort of Finnish women—the Finnish Maternity Cohort (FMC)—contribute to predict MS risk among the offspring. Viral antibodies, including against EBV, are known to cross the placenta during pregnancy3 and by conferring temporary protection may delay the age at primary EBV infection in the child, possibly altering the child’s immune response to EBV infection and thus MS risk.
We also assessed the association between pre-diagnostic anti-EBV IgG antibody and MS risk among women in the FMC and explored potential interactions between anti-EBV IgG levels with vitamin D deficiency, which is highly prevalent in the FMC and associated with increased MS risk in both the offspring4 and women5 in the cohort.
Methods
Study population
The Finnish Maternity Cohort (FMC) began in 1983 and is a nationwide serum bank with over 1.9 million samples collected during the first and early second trimester of pregnancy (5th to 95th percentile: months 2–4 of pregnancy). The FMC covers approximately 98% of pregnancies in Finland. The samples were collected at local maternity care units for routine screening for congenital infections (HIV, Hepatitis B and syphilis). Following informed consent, the remaining serum samples (one sample of 1–3 mL for each pregnancy) are stored at −25°C in a protected biorepository at the Northern Finland Biobank Borealis in Oulu and are available for scientific research.6 This study was approved by the data protection authorities at the National Institute for Health and Welfare and by the Regional Ethics Committee of the Northern Ostrobothnia Hospital District and by the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health.
Case and control identification
MS cases among the offspring born to women in the FMC between January 1, 1983 and December 31, 1991 (when offspring would be 18 to 27 years old by December 31, 2009) and MS cases among women in the FMC occurring between 1983 and 2009 were identified by searching the Finnish Hospital Discharge Register, which includes both inpatient and outpatient visits, for diagnostic codes for MS and related diseases (ICD-10 code G35, G36, H46, ICD-9 and ICD-8 codes 340, 341, 367, 377) indicating an MS diagnosis. Follow-up ended in 2009 as this was the most recent year through which all registers were updated at the time of data collection. We also searched the records of the Social Insurance Institution as they track prescription medication reimbursements, including those for MS disease modifying therapies. A prescription for any of the disease modifying therapies approved for MS requires that patients have a certificate from their neurologist confirming the MS diagnosis. When available, medical records of the cases were reviewed and confirmed by the study neurologists (KH, JÅ, MS-H). For cases that were identified through the Social Insurance Institution registry, an abstract of the medical record was available. For patients diagnosed before 2001 the Poser criteria were used and for patients diagnosed thereafter McDonald criteria were used to confirm definite MS diagnosis. Patients in whom the date of the first symptom was before the sample collection were excluded.
MS cases among offspring and control selection
The mothers of the offspring with MS were identified using an overgeneration linkage step via the Population Census Register. This list was then linked to the FMC to identify the mothers who had a serum sample stored from the pregnancy with the affected offspring. There were 193 offspring with confirmed MS (138 confirmed by medical record review and 55 by the prescription/reimbursement of MS disease modifying therapy) whose mother had a serum sample available. The cases were individually matched to up to 2 controls on region of birth in Finland (south, southwest, southeast, middle, and north); date of maternal sample collection (±60 days); date of mother’s birth (±6 months); and date of offspring birth (±2 months). There were 17 cases for which a suitable control could not be identified, leaving 176 cases and 326 matched controls.
MS cases among the women in the FMC and control selection
Identification of MS cases among women in the FMC has been described in detail.5 Briefly, linking the cases of MS occurring among women in Finland between 1983 and 2009 to the FMC, we identified 1,264 women with MS and at least one stored sample. For 1,252 cases, there was medical record (n=612) or Social Insurance Institution confirmation of the diagnosis (n=640). For the cases with medical records, all but 5 were clinically confirmed. The date of MS diagnosis was set as the earliest date recorded in the registries. Date of MS onset was only available for women with a medical record and was used as the index date. Date of diagnosis was used at the index date for cases without a medical record. Cases were matched to up to 3 controls on date of birth (±2 years) and area of residence (postal code: <10000, 10000 to <30000, 30000-<50000, 50000-<91000, >90000). One serum sample was available for 1,092 women from a pregnancy occurring prior to their MS onset/diagnosis and from 2,123 controls.
EBV and CMV IgG measurement
For the offspring analysis, maternal IgG antibodies to the EBV antigens viral capsid antigen (VCA), EBV nuclear antigen-1 (EBNA-1) and diffuse early antigen (EA-D), and to cytomegalovirus (CMV) were measured using an enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions (DiaMedix Corp., Miami, FL). For the analysis among the FMC women, only serum EBNA-1 IgG antibodies were measured because these antibodies were the strongest predictors of MS risk in previous prospective studies.7–10 Samples were positive for EBV IgG antibodies if the ELISA index value was ≥1.10 and negative if the index value was <0.90. Index values in between were equivocal. For CMV, index values <8.0 were negative, ≥10 positive, and 8.0–9.99 equivocal. Quality control samples were included amongst the study samples and coefficients of variation ranged from 3.1% (VCA) to 8.2% (EA-D).
25-hydroxyvitamin D and cotinine
25(OH)D was measured in both maternal samples for offspring and in pre-onset samples for women with MS and controls in the FMC using a chemiluminescence microparticle immunoassay and an Architect i2000SR automatic analyzer (AbbottDiagnostics). as previously described.4, 5 Cotinine, a by-product of nicotine metabolism and thus a biomarker for cigarette smoking, was also measured in these serum samples using a commercially available quantitative immunoassay kit (OraSure Technologies, Bethlehem, PA, USA) (sensitivity=96–97%, specificity=99–100%). Intra- and interassay variation are 3.5–6.2%, and 6.0–9.6%, respectively. The limit of detection was 0.08 ng/ml. Women were considered non-smokers if their cotinine levels were < 10 ng/ml and smokers if their cotinine levels were >25 ng/ml. Cotinine levels >=10 ng/ml and <=25ng/ml were considered equivocal and were categorized separately.
Statistical analysis
The statistical analysis approach was similar in both the offspring and women. Analyses of EBV IgG antibodies were restricted to the offspring of mothers or women who were EBV positive and had at least one matched EBV positive control (offspring: 170 MS case mothers and 311 control mothers; FMC women: 1,049 MS cases and 1,867 controls) and analyses of CMV IgG antibodies were restricted to those matched case/control groups who were CMV positive (offspring: 116 MS case mothers and 177 control mothers). The index values for IgG antibody against the EBV and CMV antigens were standardized to control values, and were modeled as continuous variables, as well as quintiles that were determined based on the distribution of the standardized IgG index value for each antigen among the controls. We used conditional logistic regression, consistent with the matched design, to estimate the relative risks (RR) and 95% confidence intervals (CI). In the offspring analysis we also adjusted models for sex of the offspring, gestational age at sample collection, and maternal 25-hydroxyvitamin D (25(OH)D) levels (<30 nmol/L, 30–50, >50) and cotinine levels previously measured in these samples.4 In the analysis among women, models were adjusted for parity (0, 1, ≥2), gravidity (1, 2, ≥3), and previously measured 25(OH)D and cotinine levels.5 Trend p values for the quintile analyses were calculated by modeling the median IgG antibody index value for each quintile as a continuous variable. SAS v9.4 was used to conduct the analysis. Sensitivity analyses were conducted restricting to EBV positive cases with medical record confirmation of MS (offspring n=138; FMC women n=588) and their matched controls.
Results
Offspring
The majority (70%) of maternal samples were collected during the first trimester of pregnancy and 98.3% of case mothers and 97.8% of control mothers were positive for EBV infection, and 79.6% of case mothers and 73.4% of control mothers were positive for CMV infection. Other characteristics are shown in table 1. Maternal seropositivity to EBV or CMV was not associated with MS risk in the offspring (EBV: RR=1.28, 95%CI: 0.32–5.21, p=0.73; CMV: 1.39, 95%CI: 0.87–2.22, p=0.17); after adjusting for sex of the offspring, gestational age at sample collection, cotinine and 25(OH)D levels there were no statistically significant associations observed (EBV: RR=0.74, 95%CI: 0.17–3.19, p=0.68; CMV: RR=1.23, 95%CI: 0.74–2.03, p=0.43).
Table 1.
Characteristics of MS cases among offspring and matched controls with EBV seropositive mothers during pregnancy—FMC
| MS cases | Controls | ||
|---|---|---|---|
| N | 170 | 311 | |
| Age at dx, yrs, mean (SD) | 19.5 (3.1) | NA | |
| Female, % | 85 | 68 | |
| Gestational age*, weeks, mean (SD) | 11.4 (3.6) | 10.8 (3.4) | |
| Maternal age*, weeks, mean (SD) | 27.7 (5.4) | 27.6 (5.4) | |
| Maternal EBNA-1 IgG index value, mean (SD) | 5.3 (0.97) | 5.3 (0.91) | |
| Maternal VCA IgG index value, mean (SD) | 4.5 (0.91) | 4.2 (1.1) | |
| Maternal 25(OH)D, nmol/L, mean (SD) | 34.4 (13.7) | 37.5 (16.1) | |
| Maternal vitamin D deficiency (<30 nmol/L), % | 48.2 | 36.3 |
at sample collection
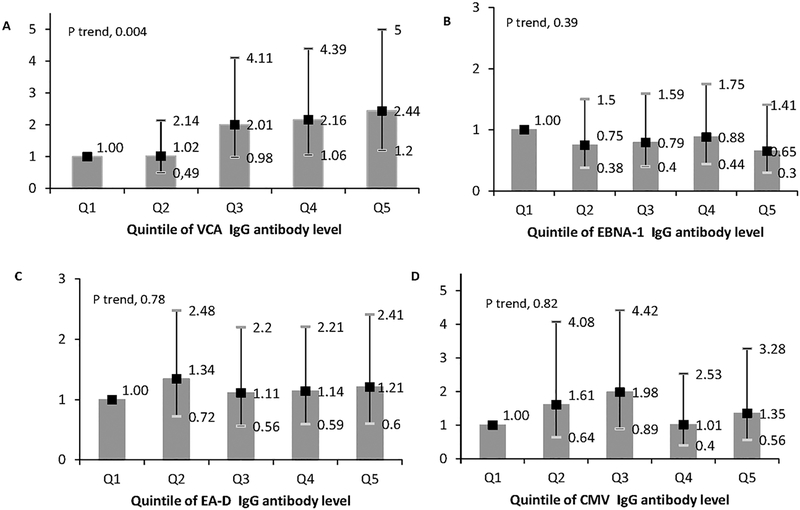
A one standard deviation increase in maternal VCA IgG index value was associated with a 34% increased risk of MS in the offspring (RR=1.34, 95%CI: 1.07–1.67, p=0.01). Adjustment for sex of the offspring, gestational age at sample collection, and maternal 25(OH)D and cotinine levels did not materially change these results (RR=1.41, 95%CI: 1.11–1.80, p=0.006). In quintile analyses, the offspring of mothers in the highest quintile of VCA IgG index value had a 2-fold increased risk of developing MS (RR=2.01, 95%CI: 1.05–3.86, p trend=0.01);this strong association remained after adjusting for sex of the offspring, gestational age at sample collection, and maternal 25(OH)D and cotinine levels (RR=2.44, 95%CI: 1.20–5.00, p trend=0.004) (Figure 1A). In contrast, no associations were seen with a one standard deviation increase in maternal EBNA-1 IgG, EA-D IgG or CMV IgG index values and MS risk in the offspring either without (EBNA-1: RR=0.95, 95%CI: 0.78–1.15; EA-D: RR=0.95, 95% CI: 0.76–1.17; CMV: RR=0.96, 95%CI: 0.75–1.23) or with adjustment for sex of the offspring, gestational age at sample collection and maternal 25(OH)D and cotinine levels (EBNA-1 RR=0.88, 95%CI: 0.71–1.08; EA-D RR=0.98, 95% CI: 0.78–1.23; CMV RR=1.04, 95% CI: 0.79–1.37) or in quintile analyses (Figure 1B, C & D).
Figure 1.
Relative risks of MS in the offspring by quintile of maternal EBV and CMV IgG antibody level. A) Viral capsid antigen; B) Epstein-Barr nuclear antigen-1; C) Epstein-Barr early antigen; D) Cytomegalovirus. Adjusted for sex of offspring, gestational age (in weeks) of sample collection, and maternal 25(OH)D level (<30 nmol/L, 30-<50, >=50) and cotinine levels during pregnancy with affected offspring.
The Pearson correlation coefficients between the maternal EBV IgG and 25(OH)D levels were: VCA: 0.01; EBNA-1 −0.04; EA-D: 0.05; CMV: 0.08. From the same adjusted models shown in figure 1A–D, after adjustment for EBV IgG levels and cotinine, maternal deficient 25(OH)D levels <30 nmol/L, as compared to levels of 30–50 nmol/L, remained statistically significantly associated with a nearly 2-fold increased MS risk in the offspring (adjusted for VCA IgG: RR=1.95, 95%CI: 1.20–3.17, p=0.007; adjusted for EBNA-1 IgG: RR=1.98, 95%CI: 1.23–3.19, p=0.005; adjusted for EA-D: RR=1.97, 95%CI: 1.23–3.16, p=0.005). There was no statistical interaction between the maternal EBV IgG and 25(OH)D levels or cotinine levels on MS risk in the offspring (data not shown). Restricting these analyses to 124 cases with medical record confirmation and 225 matched controls did not materially change these results. (data not shown)
Women in the FMC
Characteristics of the MS cases among women in the FMC and controls are shown in table 2. Serum samples were collected on average 9.4 years (SD: 6) prior to MS diagnosis. For clinically confirmed cases for whom date of MS onset was available, samples were collected on average 10.4 years (SD: 6.3) prior to onset. Notably, women who developed MS had higher mean pre-onset serum cotinine levels than control women (35.8 ng/ml vs. 28.0 ng/ml, and 24.5% of cases versus 19.7% of controls had cotinine levels indicative of recent smoking. Women with levels of cotinine >25 ng/ml (“smokers”) had a 45% increased risk of MS as compared to women with cotinine levels <10 ng/ml (“non-smokers”) (RR=1.45; 95%CI: 1.19–1.75. p=0.0002); these results were virtually unchanged after adjusting for EBNA-1 index values, parity, gravidity, and 25(OH)D levels. (RR=1.50; 95% CI:1.21–1.85, p=0.0002)
Table 2.
Characteristics of MS cases and controls among women EBV seropositive prior to MS diagnosis—FMC
| MS Cases | Matched controls | |||
|---|---|---|---|---|
| N | 1,049 | 1,867 | ||
| Age at MS diagnosis, y, mean (SD) | 37 (7.1) | NA | ||
| Age sample collection, y, mean (SD) | 27.6 (5.1) | 27.6 (5.1) | ||
| EBNA-1 IgG Index, mean (SD) | 4.60 (0.50) | 4.37 (0.70) | ||
| 25(OH)D, nmol/L, mean (SD) | 29.6 (12.2) | 31.0 (13.3) | ||
| Cotinine, ng/ml, mean (SD) | 35.8 (79.8) | 28.0 (69.6) | ||
| Gravidity, n (%)* | ||||
| 1 | 540 (51.5) | 557 (29.8) | ||
| 2 | 206 (19.6) | 512 (27.4) | ||
| 3+ | 176 (16.8) | 569 (30.5) | ||
| Parity, n (%)* | ||||
| 0 | 628 (59.9) | 704 (37.7) | ||
| 1 | 176 (16.8) | 564 (30.2) | ||
| 2+ | 126 (12.0) | 394 (21.1) |
does not sum to total due to missings
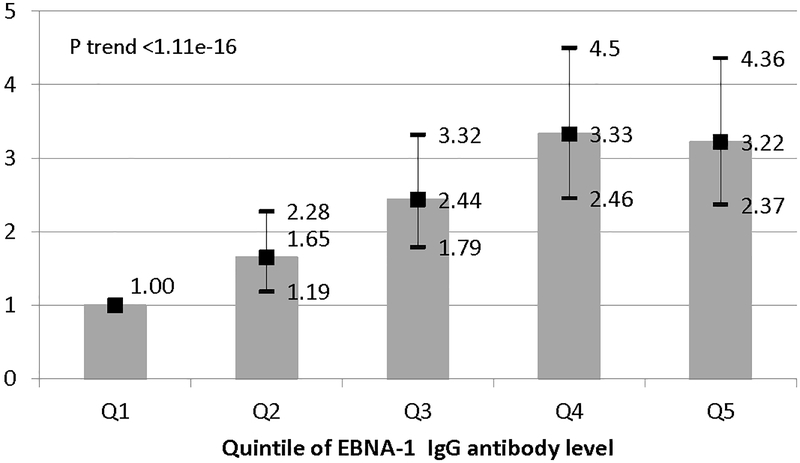
In the pre-onset samples available, 99.3% of women who developed MS (n=1053) and 97.7% of controls (n=1892) were seropositive for EBV, yielding a nearly 3.5-fold increased MS risk associated with EBV seropositivity (RR=3.44, 95%CI:1.55–7.63), p=0.003.; The RR adjusted for parity, gravidity, 25(OH)D and cotinine was similar (RR=3.54, 95%CI: 1.56–8.02, p=0.0024). A one standard deviation increase in pre-diagnostic IgG EBNA-1 index value levels was associated with an increased MS risk (RR=1.61, 95%CI: 1.44–1.80, p=1.11e-16). Adjusting for parity, gravidity, 25(OH)D and cotinine levels did not change these results (RR=1.57, 95%CI:1.40–1.76, p=2.22e-15). In quintile analyses, there was a monotonic increase in MS risk as IgG index levels of EBNA-1 increased (RR top vs. bottom quintile=3.42, 95%CI: 2.56–4.56, p trend <1.11e-16), and this association persisted after adjusting for parity, gravidity, 25(OH)D and cotinine. (Figure 2) Serum EBNA-1 IgG index value levels and 25(OH)D levels were not correlated (r=−0.003) and the previously observed increased risk of MS among women with deficient levels of 25(OH)D(<30 nmol/L) as compared to women with sufficient levels (>50 nmol/L)5, persisted after adjusting for EBNA-1 IgG and cotinine (RR=1.41, 95% CI: 0.99–2.01. p=0.06). Restricting these analyses to women with medical record confirmation of MS did not change these results.
Figure 2.
Relative risks and 95% CI of MS in women by quintile of EBNA-1 IgG index. Adjusted for gravidity, parity, and 25(OH)D levels (<30 nmol/L, 30-<50, >=50) and cotinine levels
Discussion
In this prospective nested case-control study, we found a 2.5-fold increase in MS risk among offspring of women with the highest levels (top quintile) of EBV VCA IgG antibodies during pregnancy. In addition to this novel finding, we confirmed previous reports that elevated EBNA-1 IgG levels are an independent risk factor for MS, and not confounded or modified by 25(OH)D7–11 or cotinine levels.
Maternal antibodies against a variety of pathogens, including EBV, are known to cross the placenta during pregnancy.3 Given the immature immune system of the fetus and neonate, these maternal antibodies are crucial in providing protection against infection during this time of life. However, there are few studies examining whether maternal EBV antibodies are associated with longer-term chronic disease in the offspring. In prior studies in the FMC, higher maternal VCA IgG antibody levels were associated with a 2.5-fold increased risk of testicular cancer among male offspring12, whereas maternal EBV antibodies were not associated with acute lymphoblastic leukemia in the offspring.13
The biological mechanism behind why elevated maternal VCA IgG antibodies during pregnancy may increase risk of MS in the offspring is uncertain. One possibility is that offspring of mothers with high VCA IgG are exposed to higher maternal VCA IgG during pregnancy, which may delay the child’s own immune response to a primary EBV infection. One study found that of 66 infants with maternal EBV IgG antibodies at birth, 8 continued to have detectable maternal EBV IgG antibodies at 4 months old, and of these 8, 4 became infected between 20–24 months old while 4 remained free from EBV infection through their second year.14 When the maternal protection wanes, the “older” child may be prone to a more aberrant immune response to a primary EBV infection than if the infection occurred when the child was “younger”, possibly predisposing them to MS. Another possibility is shared genetics between the mother and child that influence their immune responses to EBV (e.g. human leukocyte antigen haplotypes) such that the mother’s elevated VCA IgG levels may be an indicator of an elevated antibody response to EBV in the offspring. Unfortunately, little is known about how maternal EBV antibodies may influence the immune response to EBV in the offspring and in turn, how this affects risk of chronic diseases in the offspring.
Several studies in populations that differ by sex and race/ethnicity have consistently found that healthy young adults with higher EBV IgG antibody titers—in particular EBNA IgG—or a history of infectious mononucleosis (typically a manifestation of primary EBV infection during adolescence or young adulthood) have an increased risk of MS.2, 11, 15 The observed association here of an increased risk of MS among women in the FMC with higher EBNA-1 IgG antibody levels on average 9 years prior to MS is thus consistent with the previous literature.7–10 In the only prospective study of EBV seronegative young adults, all the incident MS cases occurred after EBV seroconversion, a result that strongly supports a causal role of EBV in MS etiology.1, 2 Plausible biological mechanisms for the role of EBV in MS pathogenesis have been extensively reviewed.16
In our previous work in the FMC, we have shown that maternal vitamin D deficiency during pregnancy increases risk of MS in the offspring,4 and that vitamin D deficiency on average 9 years prior to an MS diagnosis increases risk of MS among women in the cohort.5 We found no evidence of confounding of the anti-EBV IgG associations in the offspring or women by 25(OH)D levels or of the association between vitamin D deficiency and MS risk by the EBV IgG antibodies measured. Further, there was no statistical interaction between EBV IgG and 25(OH)D levels on MS risk. These findings are consistent with a nested case-control study of MS in the US military, which found that while elevated EBV IgG titers were associated with an increased risk of MS and elevated 25(OH)D levels a decreased risk of MS, there was no confounding or statistical interaction between them.11
Cotinine is a biomarker of nicotine exposure and levels above 25 ng/ml indicate recent smoking. We used cotinine as a surrogate to classify women as “non-smokers” or “smokers”. Consistent with the vast literature on cigarette smoking and risk of MS,2 women with elevated cotinine levels had a 50% increased risk of MS, and there was evidence of a dose-response trend. Adjusting for cotinine levels did not change the observed associations between EBNA-1 IgG index values nor 25(OH)D levels and risk of MS. As cotinine is a short-term biomarker of smoking, we cannot rule out residual confounding or misclassification of women who were past smokers or light smokers as non-smokers. Further, in the analysis in the offspring, adjusting for maternal levels of cotinine during pregnancy with the affected child also did not change the associations between maternal VCA IgG levels or 25(OH)D deficiency and MS risk; however, smoking history for the affected child is not available.
The strengths of our study include the utilization of a population-based cohort; the FMC includes approximately 98% of all pregnancies in Finland occurring since 1983. Identifying MS cases via the nationwide hospital and prescription registries also minimized selection bias. The prospective nature of the study is also a considerable strength as exposure to maternal EBV IgG antibodies necessarily occurred prior to the offspring developing MS and among the women, the average time between serum sample collection and MS symptom onset/diagnosis was almost 10 years, greatly reducing the probability of reverse causation. However, there are some limitations to consider. First, EBV IgG antibody levels were measured in serum samples collected while most women were in their first trimester of pregnancy. Second, information on other MS risk factors including body size in early life, HLA status and on a variety of demographic variables including race/ethnicity and education levels were not available for either the offspring or women, though previous studies indicate none of these are confounders of the EBV-MS association.8, 17 We also did not have information whether the offspring was breastfed, which has been associated with a decreased risk of MS in the child in some studies,18, 19Breast feeding, however, is unlikely to be associated with maternal antibody levels early in pregnancy, and thus differences in breast feeding behavior do not provide a plausible explanation for the higher MS risk among children born to mothers with higher VCA levels. While the FMC is largely composed of Caucasian women, results of previous studies in more diverse populations suggest that these findings can be extrapolated to Finnish men8, 10 and possibly other race groups.11, 15
The association between maternal levels of VCA IgG and risk of MS in the offspring is a novel finding and requires cautious interpretation and confirmation in other populations. The study among women in the FMC is the largest prospective study of pre-diagnostic EBNA-1 IgG levels and MS risk to date and thus strongly supports previous findings suggesting EBV plays a causal role in the etiology of MS.
Acknowledgements
The authors thank Dr. Marja-Liisa Sumelahti, MD, PhD and Professor Anne Remes, MD, PhD for assistance with MS case ascertainment. This study was funded by the National Institute of Neurological Disorders and Stroke (NIH R01 NS073633, PI: Ascherio). The funding agency had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Levin LI, Munger KL, O’Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 2010;67:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascherio A, Munger KL. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin Neurol 2016;36:103–114. [DOI] [PubMed] [Google Scholar]
- 3.Gotlieb-Stematsky T, Meron I, Modan M, et al. Viral antibodies in maternal and cord sera. Med Microbiol Immunol 1983;172:67–74. [DOI] [PubMed] [Google Scholar]
- 4.Munger KL, Aivo J, Hongell K, Soilu-Hanninen M, Surcel HM, Ascherio A. Vitamin D Status During Pregnancy and Risk of Multiple Sclerosis in Offspring of Women in the Finnish Maternity Cohort. JAMA Neurol 2016;73:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger KL, Hongell K, Aivo J, Soilu-Hanninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology 2017;89:1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gissler M, Surcel H. Combining health register data and biobank data. Statistical Journal of the LAOS 2012;28:53–58. [Google Scholar]
- 7.Ascherio A, Munger KL, Lennette ET, et al. Epstein-barr virus antibodies and risk of multiple sclerosis: A prospective study. JAMA 2001;286:3083–3088. [DOI] [PubMed] [Google Scholar]
- 8.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of Epstein Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 2005;293:2496–2500. [DOI] [PubMed] [Google Scholar]
- 9.Sundstrom P, Juto P, Wadell G, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology 2004;62:2277–2282. [DOI] [PubMed] [Google Scholar]
- 10.DeLorenze GN, Munger KL, Lennette E, Orentreich N, Vogelman J, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol 2006;63:839–844. [DOI] [PubMed] [Google Scholar]
- 11.Munger KL, Levin LI, O’Reilly EJ, Falk KI, Ascherio A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel Mult Scler 2011;17:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holl K, Surcel HM, Koskela P, et al. Maternal Epstein-Barr virus and cytomegalovirus infections and risk of testicular cancer in the offspring: a nested case-control study. APMIS 2008;116:816–822. [DOI] [PubMed] [Google Scholar]
- 13.Tedeschi R, Luostarinen T, Marus A, et al. No Risk of Maternal EBV Infection for Childhood Leukemia. Cancer Epidemiol Biomarkers Prev 2009;18:2790–2792. [DOI] [PubMed] [Google Scholar]
- 14.Chan KH, Tam JS, Peiris JS, Seto WH, Ng MH. Epstein-Barr virus (EBV) infection in infancy. J Clin Virol 2001;21:57–62. [DOI] [PubMed] [Google Scholar]
- 15.Langer-Gould A, Wu J, Lucas R, et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: A multiethnic study. Neurology 2017;89:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012;8:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianfrancesco MA, Acuna B, Shen L, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract 2014;8:e435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conradi S, Malzahn U, Paul F, et al. Breastfeeding is associated with lower risk for multiple sclerosis. Mult Scler 2013;19:553–558. [DOI] [PubMed] [Google Scholar]
- 19.Ragnedda G, Leoni S, Parpinel M, et al. Reduced duration of breastfeeding is associated with a higher risk of multiple sclerosis in both Italian and Norwegian adult males: the EnvIMS study. J Neurol 2015;262:1271–1277. [DOI] [PubMed] [Google Scholar]