Abstract
Background and Aims:
Neuromyelitis optica spectrum disorder (NMOSD) is a demyelinating disorder of central nervous system with deleterious effects. At present Intravenous corticosteroids are used for the relapse as the first line of treatment, but with only a class evidence III-IV. Having an underlying humoral immune mechanism in the pathogenesis of NMOSD and as it is rightly said that “Time is Cord and Eyes”, delaying the time to start plasma exchange (PLEX) awaiting favorable outcome in response to corticosteroids is detrimental for the patient. Hence, PLEX may be a promising first line therapeutic approach in the management of severe attacks of NMOSD. The aim of this study is to evaluate the efficacy of PLEX as the first line of treatment for the acute attacks in patients with NMOSD that is being largely used as an add-on therapy for more than 10 years, and also to define the time opportunity window for the starting of PLEX.
Methods:
The study analysed the therapeutic efficacy and safety profile of PLEX as a first line therapy in 30 patients diagnosed with NMOSD over a period of 30 months. PLEX was performed using a Hemonetics Mobile Collection System plus machine with due written consent including the risks and benefits of the treatment that is being proposed to the patient/relative in their own language.
Results:
A total of 30 patients were analysed, out of which 16 were females and rest males. 85% of the patients were in the age group of 25-35 years. All the patients had severe Expanded Disability Status Scale (EDSS) scores at the baseline, and 73.33% showed significant improvement following PLEX. The only predictor of good outcome was the time to PLEX i.e shorter delay betters the outcome.
Conclusion:
The study ascertained the importance of early PLEX as a therapeutic intervention in severe attacks of NMOSD irrespective of their Anti-Aquaporin 4 (AQP4) antibody status.
Keywords: Corticosteroids, first line therapy, NMOSD, PLEX, time window
INTRODUCTION
Neuromyelitis optica spectrum disorders (NMOSD) comprises of a spectrum of inflammatory demyelinating disorders involving the brain, spinal cord and optic nerves.[1,2] Contrary to multiple sclerosis (MS), relapses of NMOSD are often very severe and without complete improvement resulting in prominent residual deficits with subsequent relapses.[3] Most NMOSD patients present with stepwise neurological impairment. Maintenance therapy for NMOSD are aimed to prevent the relapses with the administration of different immunosuppressive drugs.[4,5,6] Due to the low incidence and prevalence of NMO, interventional studies with level I or II evidence are not currently available. Therefore, treatment strategies are mostly based on small case series and reports. However, treatment for relapses is still a matter of debate and is the need of hour.
Intravenous methylprednisolone (IVMPS) have been used since long for the treatment of acute attacks of many demyelinating disorders including multiple sclerosis, NMOSD and isolated optic neuritis.[4,5,7] This widely used steroid treatment usually fails to control severe attacks of NMOSD, and so generally the next step is the use of PLEX as an add-on or a rescue therapy in order to halt the stepwise progression of residual impairment.[8] In the indications given by American Society for Apheresis (ASFA), PLEX for NMOSD comes under category II i.e disorders for which apheresis is accepted as second-line therapy, either as a standalone treatment or in conjunction with other modes of treatment. PLEX has been used since long in many other neurological disorders including various demyelinating diseases like Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy with conduction block and other neuroinflammatory disorders.[9,10] In view of the pathophysiology [Figure 1] of NMOSD that a strong humoral response underlies the disease process, PLEX seems to be the most appropriate therapy in severe NMO relapses.[1,10] Studies and case series have reported significant improvement in around 44-75% of NMOSD patients treated with PLEX.[11,12,13] Weinshenker et al. considered a transition from corticosteroids to PLEX in patients with myelitis. They randomized NMO patients who were unresponsive to steroid therapy to active or sham plasma treatment in a double-blind study, the patients experienced marked therapeutic benefit with plasmapheresis.[14] In the present study, we are trying to propose the rationale of the PLEX as a first line of treatment based on pathophysiological grounds, and summarize the relevant data of PLEX studies in the setting of NMOSD, documenting and assessing the results obtained in all the attacks.
Figure 1.
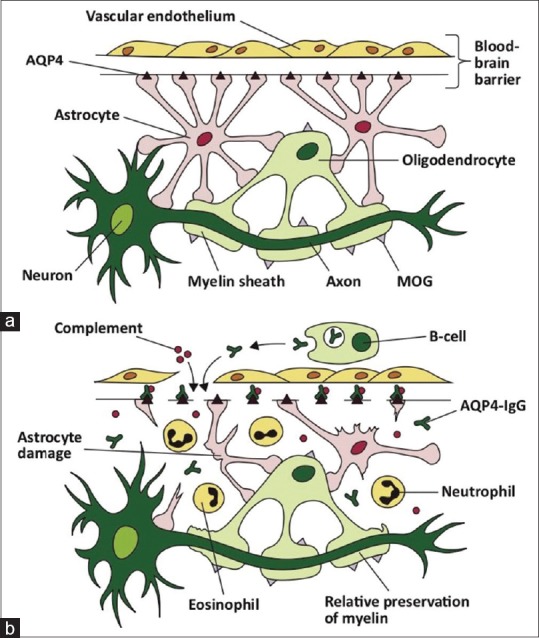
(a) This figure illustrates the sites of expression of aquaporin-4 (AQP4) in the central nervous system (CNS). AQP4 is expressed on astrocyte ‘foot-like’ processes at the blood-brain barrier. (b) AQP4-Abs (IgG) are produced systemically by mature B-cells, and upon crossing the blood-brain barrier, activate complement-mediated astrocyte damage. There is relative preservation of myelin initially. The inflammatory milieu consists of neutrophils and eosinophils. Reprinted with permission from Whittam D, Wilson M, Hamid S, et al. What's new in neuromyelitisoptica? A short review for the clinical neurologist. J Neurol 2017;264:2330-44[15]
METHODS
This prospective study analyzed the outcome in response to PLEX in thirty patients diagnosed with NMOSD fulfilling the criteria given by Wingerchuk et al.[2] over a period of 30 months from November 2016 to April 2019. It was approved by the institutional ethical committee. A total number of 53 longitudinally extensive myelitis (LETM) were screened, out of which 48 fulfilled the criteria for Neuromyelitis optica spectrum disorders (NMOSD). However, we decided to exclude those patients who did not fulfill the Wingerchuk criteria, even though they suffered from LETM. The patients with isolated optic neuritis were also excluded despite of having Anti aquaporin 4 antibody positivity. Also, the patients who met Wingerchuk et al. criteria for NMO, but were hemodynamically unstable and had deranged coagulation profile were excluded. The patients who did not give consent for plasma exchange (PLEX) procedure were excluded too.
PLEX was offered as a first line therapy to all the patients having severe acute attack of NMOSD and was never initiated as a delayed rescue treatment after a standard steroid treatment failure, although we included the patients who had received IVMPS elsewhere. All the patients under went detailed structured physical, opthalmological examination and functional scoring (FS) on admission, after completion of PLEX (three or five cycles), at one month and at three months. For the better judgment and quantified assessment of response to treatment, Kurtzke Expanded Disability Status Scale (EDSS) score was calculated.[16] The Δ EDSS score was calculated as difference between EDSS score at three months and EDSS at presentation, further percentage improvement in EDSS was calculated {(Δ EDSS/EDSS at presentation) × 100}. The outcome was also evaluated based on the criteria given by Keegan et al. “no improvement” (no improvement in neurological symptoms or function), “mild improvement” (improvement in symptoms or examination, but with residual impairments in daily function), “moderate improvement” (improvement in primary symptoms but not completely resolved; no impairments in daily function), and “marked improvement” (complete resolution of symptoms).[11] PLEX was done using Hemonetics Mobile Collection System plus. The formula for plasma volume is 0.07× hematocrit (HCT) × body weight, a total of three or five exchanges depending on the severity were done on alternate days, with prematurely stopping the exchange in two patients having adverse reactions. The study analyzed the relationship of “Time to PLEX” with the final outcome i.e the percentage improvement in EDSS scores at three months post PLEX. Other disease characteristics and their significance with respect to outcome were also studied, like anti-AQP4 antibody status, total duration of illness, whether the patient was previously on immunosuppressant or not, already been administered IVMPS or not. The analysis was done using SPSS 16 software.
THERAPEUTIC PLEX PROTOCOL
Therapeutic PLEX is based on the extracorporeal blood separation technique designed to remove either plasma or its constituents from the blood's cellular elements.[17] Centrifugation devices or highly permeable filters are used to separate the plasma filtrate with molecules up to 1,000 kD, including immunoglobulins, complement factors, and albumin from blood cells. The plasma filtrate is discarded, and then before reinfusing the filtered blood, 5% albumin solution or fresh-frozen plasma is added to it. According to the revised 2016 ASFA guidelines, the recommended standard volume treatment in NMO is 1-1.5-times the plasma volume per session or 39-55 mL of plasma per kg of body weight.[18] Daily or alternate day treatment, with a duration of 10-14 days and consisting of 5-7 sessions, is recommended for cases of acute exacerbation of NMOSD.
RESULTS
Demographic characteristics of the study population
A total of 30 patients, with a cumulative sum of one hundred eight PLEXs were analyzed. Out of all these patients, 16 were females, and 14 were males. Median age of patients was 32 years.
Clinical characteristics of the study population
In the present study, 19 patients (63.3%) patients had their first attack, 9 patients (30%) had 2-4 attacks and 2 patients had more than 7 attacks. Median disease duration 12.5 days. Symptoms at presentation/attack included Optic Neuritis in 16 patients, Acute Transverse Myelitis in 24 patients, Area Postrema Syndrome in 5 patients, Acute Brainstem Syndrome in 10 patients, and cerebral syndrome in 5 patients.
Correlation between “Time to PLEX and outcome at three months” (Percentage improvement in EDSS score)
Median time to PLEX was 7 days.
Keegan scoring: In this study, 4 patients had no improvement, 2 patients had mild improvement, 16 patients had moderate and 8 patients had marked improvement in Keegan scoring.
Delta EDSS score: Majority of the patients had EDSS score improvement. Mean EDSS score at different time points for all patients are given in Figure 2.
Percentage EDSS score improvement: Association between time to PLEX and Percentage Improvement in EDSS score was tested by Pearson correlation. Correlation coefficient value was -0.437, and it showed moderate negative correlation and P value was 0.016 i.e “Earlier the PLEX, Better is the Outcome”.
Figure 2.
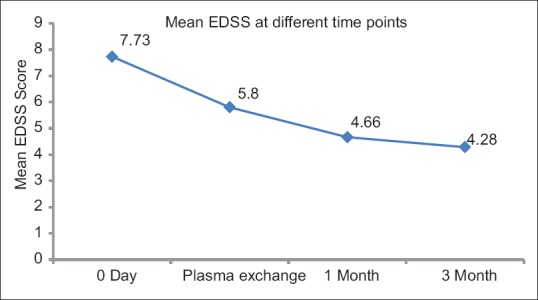
Mean EDSS score at different time points
Difference in outcome between “Patients who received IVMPS + PLEX” and “Patients who underwent PLEX alone”
Out of the total 30 patients [Figure 3], 9 patients had already received intravenous methylprednisolone elsewhere. When a comparison was done between the two groups, significant difference was found in time to PLEX (P value = 0.012), and no significant difference in percentage improvement (P = 0.266) [Table 1].
Figure 3.
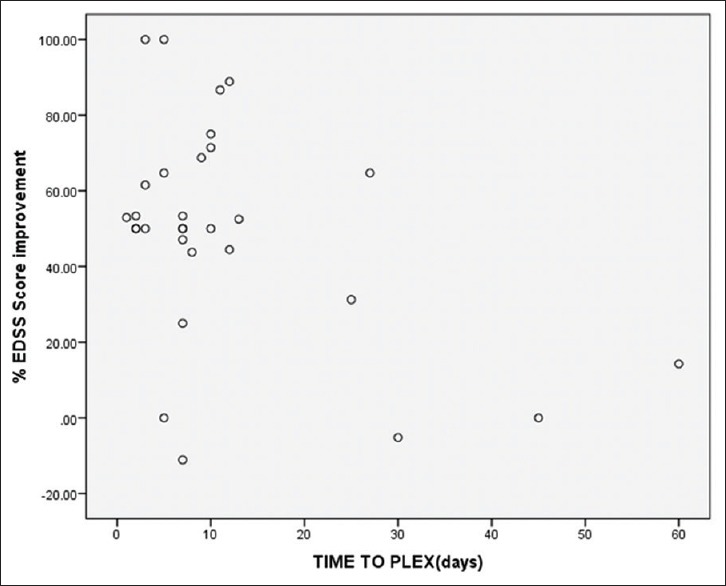
Correlation between time to PLEX and EDSS score Percentage Improvement
Table 1.
Comparison of Time to PLEX and % improvement with Previous MPS
| Variable | MPS Received | MPS Not received | P |
|---|---|---|---|
| Time to PLEX (Days) | 0.012 | ||
| Mean | 25.33 | 6.05 | |
| SD | 17.81 | 3.3 | |
| EDSS score % Improvement | 0.266 | ||
| Mean | 40.3 | 53.3 | |
| SD | 33.0 | 26.6 |
Correlation between Anti-AQP4 antibody positivity and outcome
Anti AQP4 antibody was present in 19 patients. There was no association between presence of Anti AQP4 antibody and outcome (P value = 0.552) [Table 2].
Table 2.
Association between presence of Anti AQP4 antibody and outcome
| Anti AQP4 antibody | No Improvement | Improvement Present | Total | P |
|---|---|---|---|---|
| Present | 2 (10.5%) | 17 (89.5%) | 19 | 0.552 |
| Absent | 2 (18.2%) | 9 (81.8%) | 11 |
Correlation between patients already on immunosuppressant and outcome
Four patients were already on immunosuppressant and there was no significant difference noted in the EDSS score percentage improvement (P = 0.475) between the two groups i.e patients who received long-term immunosuppressant prior to the relapse versus the patients who did not receive any long-term therapy.
Adverse effects
In this study, one patient had one plasma exchange, 19 patients had three plasma exchanges and 10 patients had five plasma exchanges. Plasma exchange was stopped prematurely in 2 patients because of minor adverse reactions. In the study, 18 patients (60%) did not have complications. The remaining 40% had any one of the complications. Rash was the most common adverse event reported in 5 patients. Less common adverse reactions were itching, breathlessness and hypocalcemia. Two patients died due to severity of disease itself, not because of adverse reactions. None of the patients had infections, pneumothorax and hypomagnesemia [Table 3].
Table 3.
Adverse reactions after plasma exchange
| Complications | Number | Percentage |
|---|---|---|
| Rash | 5 | 16.7 |
| Paresthesia | 3 | 10.0 |
| Cramps | 2 | 6.7 |
| Itching | 1 | 3.3 |
| Breathlessness | 1 | 3.3 |
| Hypocalcemia | 1 | 3.3 |
DISCUSSION
Effectiveness of PLEX
In the present study, PLEX proved to be efficient in managing severe acute attacks of NMOSD. The clinical data from 30 patients, with most of the patients receiving PLEX as first line therapy for NMOSD showed a moderate or marked improvement (73.3%). In fact, the improvement in EDSS scores were documented immediately after the first or second exchange in many patients, similar to the study by Watanabe S, et al.[13] The classical Lazarus effect, which is defined as a very short-term dramatic improvement, was rather unusual in this study group as our study was not designed to analyze short-term improvement.[14] The patients who experienced this effect have all received a very early treatment (less than 5 days). However, in Magaña et al. study, patients who exhibited functional improvement did so within a median of 4 days (third PLEX), although a minority (6%) exhibited a delayed response (more than 2 months).[19] The final outcome taken into consideration was at three months post PLEX which was more or less same as that at the end of first month. Moreover, the natural history of single spinal relapse in NMOSD has never been addressed, so any improvement bias after PLEX cannot be appreciated in the absence of a control group.
Time to PLEX
Besides knowing PLEX is effective and safe, the central dilemma remains: Is PLEX necessary as soon as and as often as possible?
The answer is yes, because after an attack by anantibody the tissue undergoes various stages of destruction ranging from reversible damage to an irreversible one, so “hitting the iron when hot” should be the main aim. Bonnan and Kimbrough proposed a link between the staging of NMOSD lesion and the PLEX effect on clinical and radiological outcome; a fairly good outcome was seen when PLEX was performed at either Stage 1 or 2.[12,20] In another study by Batra et al., it was found that the non-responder subjects were enrolled late for active treatment i.e around Stage 3, due to severe and irreversible axonal injury PLEX was not found to be useful.[21] Also evident from the results of the present study; the time to PLEX has significantly influenced the outcome, ranging from immediate dramatic improvement (the Lazarus effect) to no effect according to whether they are given early or very late, with shorter delay leading to better outcomes similar to other studies.[14,22,23] However, further prospective, randomized, multicentre clinical trials would be required to definitively answer this question in a better way. For example, PLEX was delayed from onset by a median of 30 days (6-90 days) in Llufriu et al.; in their study, early initiation of PLEX [Odds Ratio (OR) 6.29, 95% Confidence Interval (CI) 1.18-52.96] and improvement at discharge [OR 7.32, 95% CI 1.21-44.38] were significantly associated at 6 months.[24]
IVMPS with PLEX vs PLEX alone
Most authors till date consider PLEX to be an add-on rescue treatment after steroid failure, which should not be the case.[8,25] Although a synergistic effect of steroids and PLEX was long expected due to their complementary action, very few studies compared conventional “IVMPS monotherapy” with “ IVMPS and add on PLEX”.[25,26] A study done by Deschamps R, et al. analyzing the therapeutic outcome to PLEX as an add on treatment following failure of improvement to corticosteroids showed that the High-contrast visual acuity, visual fields, and temporal retinal nerve fiber layer thickness improved significantly with the add on PLEX treatment.[26] The other two studies by Bonnan et al. and Ruprecht et al. observed lower residual and mean difference in EDSS score in the add on PLEX-treated group compared to the IVMPS-only group.[23,27] In a study by Abboud Hesham et al., 65% of patients treated with IVMPS plus PLEX attained an EDSS score almost same or lower than their baseline at follow-up while only 35% of the IVMPS-only patients achieved their baseline EDSS on follow-up (odds ratio = 3.36, 95% CI 1.0657 to 10.6004, P = 0.0386).[25] In a study by Kumar et al. too the patients who were severely disabled, with bad EDSS scores (6-9.5) at baseline and no improvement with IVMPS responded well to PLEX.[28] In the study by Srisupa et al. although IVMP-responders showed faster improvement since the time of discharge but seemed to have less treatment benefit over time. However, IVMP non-responders/PLEX responders showed continuous and maximum improvement at 6 months (ΔEDSS from nadir: 1 for IVMP-responders vs 0.5 for IVMP non-responders without PLEX vs 2.75 IVMP non-responders/PLEX-responders vs 0.5 IVMP/PLEX non-responders; P = 0.49).[29] However, to the best of our knowledge, no studies are available on comparison between “IVMPS with add on PLEX” and “PLEX alone”, the analysis of the data between these two groups in the present study showed no significant difference in terms of better outcome in the previously steroid treated group. Further larger studies are needed for comparison between these two groups.
Anti AQP4 antibody correlation to outcome
The final outcome in the present study was independent of the Anti AQP4 antibody status (P value = 0.689). As the Anti AQP4 antibody status does not affect the response rate of PLEX, Bonnan et al. and Magna et al. also came across similar correlation and hence anti AQP4 antibody status is not required to start treatment in a severe relapse patient.[12,19]
Correlation with duration of illness
Various other factors which can possibly influence the outcome like the total duration of illness were analyzed, it was found that the patients who present with longer duration of illness showed less improvement in the previously existing deficits. Also, the patient who underwent PLEX for their first attack had a dramatic improvement as compared to the ones who were into the illness for past many months or years.
Correlation with previously on immunosuppressant
No statistically significant difference in terms of outcome was found between the patients already on long term immunosuppressive agents and the ones who were not (P value 0.903), similar to the study by Abboud Hesham et al. where among patients in the IVMPS + PLEX group, PLEX significantly reduced disability from presentation to discharge regardless of whether patients were on preventive medication at the time of relapse.[25]
Adverse events
Minor side effects like rashes, paresthesias were present in 40% patients, but only two serious reactions resulted in premature PLEX interruption.
FUTURE OF PLEX IN MANAGEMENT OF NMOSD
PLEX in isolated optic neuritis
The present study dealt with only severe attacks which disabled the patient functionally in terms of mobility in NMOSD; with the favorable outcome in this study we try to propose PLEX as a first line treatment for isolated optic neuritis too, rather than awaiting a response to corticosteroids. It was evident form ONTT trial that the final outcome at 6 months following administration of steroids in patients of isolated optic neuritis was same or worse than that of the patients who did not receive corticosteroids.[27,30] Various studies have shown visual impairment in NMOSD is very severe. One such study demonstrated that an immediate unilateral blindness occurred in a third of patients after the first optic neuritis (ON), and generally two attacks are sufficient to cause a definitive loss of vision.[31,32] Bonnan et al. gathered data from various studies which showed a clear effect of PLEX delay since success rate was 8/8 (100%) during the first 11 days, then 4/7 (57%) from days 12 to 22, and 7/13 (53%) from days 23 to 73.[12] Furthermore, even when patients recovered, the mean residual visual acuity tended to be lower in delayed PLEX patients. In conclusion, strong clues support that PLEX change the outcome of severe ON only when they are given early. However, broader studies using carefully chosen patients are still lacking to confirm this hypothesis.
PLEX as a maintenance therapy
As Anti AQP4 antibody positivity is both predictive of number of further attacks and severity, achieving a low concentration of plasmatic antibodies remains a goal to achieve. Kim SH and colleagues demonstrated that Anti-AQP4 antibody serum levels declined significantly following plasmapheresis, to a mean of 15% of the pre-plasmapheresis levels.[33] Besides immunosuppressive drugs, weekly PLEX have been used at various centers to achieve a sustained depletion of Anti AQP4 antibody and complement, making PLEX as part of maintenance therapy too. Favorable cases have been reported but large studies are lacking. A case series by Miyamoto and Kusunoki analyzed the efficacy of concurrent PLEX treatment in NMOSD relapse prevention and proposed to use PLEX as preventive treatment as an add-on therapy after immunosuppressive drugs failure.[34]
LIMITATIONS OF THE PRESENT STUDY
Firstly, the basal EDSS scores of the patients already a known case of NMOSD were not available in the present study, as it would have led to a better assessment of the outcome in these patients. Secondly, the EDSS score, although universally used in clinical trials, has a number of limitations. Even with special training and examiner blinding, interrater and intrarater variations in scoring are common as EDSS scores of 4 and higher depend almost entirely on the ability to walk; developing dementia, vision loss, and weakness of hands may pass undetected by the scoring once one reaches these levels. Lastly, though the study analyzed the difference in outcome between IV PLEX and IVMPS + PLEX, the number of patients in these groups was not equal. Hence larger multicentre studies are required to assess the difference.
CONCLUSION
To summarize, PLEX-treated patients achieved a better outcome, especially if PLEX was given during the first attack and as early as possible. The exact effect of PLEX should be validated in larger multicentre studies. The only good outcome predictor in the present study was a shorter PLEX delay similar to the previous studies.[19,23] The same PLEX response rate was obtained irrespective of Anti-AQP4 antibody status in the present study, which was similar to the Mayo Clinic cohort and in the study by Bonnan et al.[28,12] As a practical consequence, patient suffering from a severe relapse, the status of Anti-AQP4 antibody should not influence the decision of starting PLEX as promptly as possible.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen T, You Y, Arunachalam S, Fontes A, Liu S, Gupta V, et al. Differing structural and functional patterns of optic nerve damage in multiple sclerosis and neuromyelitis optica spectrum disorder. Ophthalmology. 2019;126:445–53. doi: 10.1016/j.ophtha.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: Acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016;18:2. doi: 10.1007/s11940-015-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman E, Han MH. Acute and chronic management of neuromyelitis optica spectrum disorder. Curr Treat Options Neurol. 2015;17:48. doi: 10.1007/s11940-015-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collongues N, de Seze J. Current and future treatment approaches for neuromyelitis optica. Ther Adv Neurol Disord. 2011;4:111–121. doi: 10.1177/1756285611398939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowarik M, Soltys J, Bennett J. The treatment of neuromyelitis optica. J Neuroophthalmol. 2014;34:70–82. doi: 10.1097/WNO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baharnoori M, Hohol M, Pavenski K, O’Connor P. Therapeutic effect of plasma exchange (PLEX) in neuromyelitis optica (NMO): Immediate and long term response. Neurology. 2014;82:10. [Google Scholar]
- 9.Gwathmey K, Balogun RA, Burns T. Neurologic indications for therapeutic plasma exchange: 2013 update. J. Clin. Apheresis. 2014;29:211–9. doi: 10.1002/jca.21331. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein R. Therapeutic apheresis in neurological disorders. J Clin Apher. 2000;15:74–128. doi: 10.1002/(sici)1098-1101(2000)15:1/2<74::aid-jca6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG, et al. Plasma exchange for severe attacks of CNS demyelination: Predictors of response. Neurology. 2002;58:143–6. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 12.Bonnan M, Cabre P. Plasma exchange in severe attacks of neuromyelitis optica. Mult Scler Int. 2012;2012:787630. doi: 10.1155/2012/787630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, Füjihara K, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128–32. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 14.Weinshenker BG. Plasma exchange for severe attacks of inflammatory demyelinating diseases of the central nervous system. J Clin Apher. 2001;16:39–42. doi: 10.1002/jca.1010. [DOI] [PubMed] [Google Scholar]
- 15.Whittam D, Wilson M, Hamid S, Keir G, Bhojak M, Jacob A, et al. What's new in neuromyelitisoptica? A short review for the clinical neurologist. J Neurol. 2017;264:2330–44. doi: 10.1007/s00415-017-8445-8. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairement in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1144–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 17.Bronzlik P, Toto S, Kielstein J, Schmidt B, Stangel M, Trebst C, et al. Therapeutic plasma exchange and immunoadsorption therapy in neurological diseases. Eur Neu J. 2011;3:56–61. [Google Scholar]
- 18.Schwartz J, Winters J, Padmanabhan A, Balogun R, Delaney M, Linenberger M, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the american society for apheresis: The sixth special issue. J Clin Apheresis. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 19.Magaña S, Keegan BM, Weinshenker BG, Erickson BJ, Pittock SJ, Lennon VA, et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68:870–8. doi: 10.1001/archneurol.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimbrough DJ, Fujihra K, Jacob A, Lana-Peixoto MA, Leite MI, Levy M, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord. 2012;1:180–7. doi: 10.1016/j.msard.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batra A, Periyavan S. Role of low plasma volume treatment on clinical efficacy of plasmapheresis in neuromyelitisoptica. Asian J Transfus Sci. 2017;11:102–7. doi: 10.4103/ajts.AJTS_111_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aungsumart S, Apiwattanakul M. Clinical outcomes and predictive factors related to good outcomes in plasma exchange in severe attack of NMOSD and long extensive transverse myelitis: Case series and review of the literature. Mult Scler Relat Disord. 2017;13:93–7. doi: 10.1016/j.msard.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Bonnan M, Valentino R, Debeugny S, Merle H, Fergé JL, Mehdaoui H, et al. Short delay to initiate plasma exchange predicts outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89:346–51. doi: 10.1136/jnnp-2017-316286. [DOI] [PubMed] [Google Scholar]
- 24.Llufriu S, Castillo J, Blanco Y, Ramio-Torrenta L, Rio J, Valles M, et al. Plasma exchange for acute attacks of CNS demyelination: Predictors of improvement at 6 months. Neurology. 2009;73:949–53. doi: 10.1212/WNL.0b013e3181b879be. [DOI] [PubMed] [Google Scholar]
- 25.Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M, et al. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22:185–92. doi: 10.1177/1352458515581438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deschamps R, Gueguen A, Parquet N, Saheb S, Driss F, Mesnil M, et al. Plasma exchange response in 34 patients with severe optic neuritis.J. Neurol. 2016;263:883–7. doi: 10.1007/s00415-016-8073-8. [DOI] [PubMed] [Google Scholar]
- 27.Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R. Plasma exchange for severe optic neuritis—treatment of 10 patients. Neurology. 2004;63:1081–3. doi: 10.1212/01.wnl.0000138437.99046.6b. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Paul BS, Singh G, Kaur A. Therapeutic efficacy of plasma exchange in neuromyelitis optica. Ann Indian Acad Neurol. 2018;21:140–3. doi: 10.4103/aian.AIAN_330_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srisupa-Olan T, Siritho S, Kittisares K, Jitprapaikulsan J, Sathukitchai C, Prayoonwiwat N, et al. Beneficial effect of plasma exchange in acute attack of neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2018;20:115–21. doi: 10.1016/j.msard.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Beck R. The optic neuritis treatment trial: Three-year follow-up results. Arch Ophthalmol. 1995;113:136–7. doi: 10.1001/archopht.1995.01100020014004. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt F, Zimmermann H, Mikolajczak J, Oertel FC, Pache F, Weinhold M, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2017;11:45–50. doi: 10.1016/j.msard.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Merle H, Olindo S, Bonnan M, Donnio A, Richer R, Smadja D, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology. 2007;114:810–5. doi: 10.1016/j.ophtha.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Kim W, Huh S, Lee K, Jung I, Kim H, et al. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti-aquaporin-4 antibody levels. J Clin Neurol. 2013;9:36–42. doi: 10.3988/jcn.2013.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto K, Kusunoki S. Intermittent plasmapheresis prevents recurrence in neuromyelitis optica. Ther Apher Dial. 2009;13:505–8. doi: 10.1111/j.1744-9987.2009.00780.x. [DOI] [PubMed] [Google Scholar]


