Sir,
Loss-of-function mutations in the calcium/calmodulin-dependent serine protein kinase (CASK) gene were reported to contribute toward severe intellectual disability in females and reduced viability or in utero lethality in males.[1] CASK mutations in males are classified into three categories: i) microcephaly with pontine and cerebellar hypoplasia (MICPCH) with severe epileptic encephalopathy, associated with loss-of-function mutation; ii) MICPCH associated with inactivating alterations in the mosaic state; and iii) syndromic/nonsyndromic mild to severe intellectual disability, with or without nystagmus caused by CASK mis-sense/splicing mutations that leave the CASK protein intact but likely to alter its function.[2] Here, we report the first case of male infant hemizygous for a novel CASK mutation, i.e., NM_003688.3: c.2546T>C (p.V849A) from India. The elder male sib died at the age of 11 months with seizures, developmental delay, and intellectual disability with no diagnosis, and the diagnosis was established in the second child. Prenatal diagnosis in two subsequent pregnancies revealed one affected fetus that was terminated. The maternal aunt had 11 miscarriages and 2 infantile deaths (male).
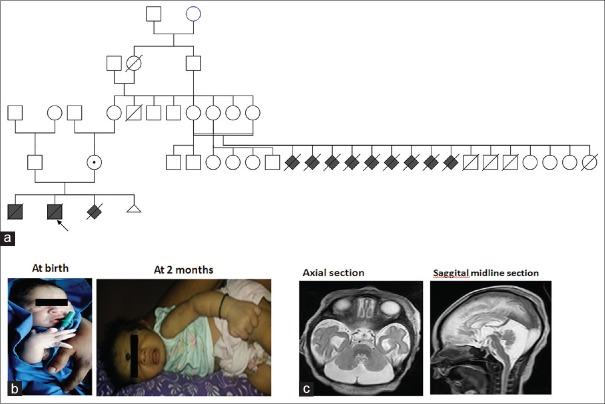
The proband, second in birth order, full term, was born to nonconsanguineous parents by Cesarean section due to failure in the progress of labor. Birth weight was 3.1 kg and length was 50 cm. Neonatal hypotonia, hyperkinesia, and postnatal microcephaly with occipito frontal circumference at −2 SD at 2 months of age was observed. Facial features at birth were normal, and at 2 months, mild coarse facial features were observed [Figure 1]. Seizures were present since birth and electroencephalogram was abnormal. The metabolic screening was normal. At 2 months, the child was not fixing the eye and not following the light. Brain magnetic resonance imaging showed axial (T2WI image) cerebellar hypoplasia with inferior vermis hypoplasia and prominent temporal horn with pontine atrophy [Figure 1c]. Sagittal T2WI midline section showed significant thinning of the brainstem with cerebellar hypoplasia with inferior vermis hypoplasia and prominent temporal horn with pontine atrophy [Figure 1c]. Exome sequencing was carried out. The child expired with recurrent seizures at the age of 2 months.
Figure 1.
Pedigree and phenotypic presentation of the proband. (a) Pedigree analysis of the proband. The pedigree depicting early infantile deaths and first trimester abortions in the family. (b) Phenotypic presentation of the proband. At birth: the child appeared normal and at the age of 2 months: child had hypotonia, hyperkinesia, and microcephaly. (c) Brain MRI of the proband. Axial and sagittal sections revealed cerebellar hypoplasia with inferior vermis hypoplasia and prominent temporal horn with pontine atrophy
The CASK variant detected in the proband is novel and is not present in both the 1,000 genomes and ExAC databases and is predicted to be probably damaging by PolyPhen-2 and damaging by SIFT and Mutation Taster. The region is conserved across species. The mutation was further confirmed by Sanger sequencing.
The mutation occurred in the Guanylate Kinase (GK)-like domain of CASK. Although the GK-domain of CASK lacks kinase activity, it plays a pivotal role in mediating specific protein–protein interactions. This domain was reported to interact with the C2B domain of rabphilin3a, a presynaptic protein involved in synaptic vesicular exocytosis.[3] Further, it also interacts with Krüppel-like zinc finger protein B-cell lymphoma/COUP-TF-interacting protein 1 (Bcl11A), thus playing a vital role in axonogenesis.[4] Loss of GK-domain of CASK might affect axonogenesis, thus resulting in defects in brain anatomy.
The MICPCH is postulated to be a genetically heterogeneous condition with loss-of-function mutations in CASK contributing to majority of cases with more severe phenotypes, whereas non-CASK mutations exhibiting milder microcephaly.[5] Recent studies revealed that the phenotypic spectrum of CASK mutations is expanding beyond the typical and classical categorization proposed by Moog et al. and documented the association of CASK mutation with autism spectrum disorders[6] and developmental disorder.[7]
To conclude, we report the first case of an Indian infant with a novel mutation in the GK-domain of CASK with severe pontocerebellar hypoplasia and seizures.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank the family of the proband in providing consent and co-operating for this study.
REFERENCES
- 1.Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008;40:1065–7. doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- 2.Moog U, Bierhals T, Brand K, Bautsch J, Biskup S, Brune T, et al. Phenotypic and molecular insights into CASK-related disorders in males. Orphanet J Rare Dis. 2015;10:44. doi: 10.1186/s13023-015-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Luan Z, Liu A, Hu G. The scaffolding protein CASK mediates the interaction between rabphilin3a and beta-neurexins. FEBS Lett. 2001;497:99–102. doi: 10.1016/s0014-5793(01)02450-4. [DOI] [PubMed] [Google Scholar]
- 4.Kuo TY, Hong CJ, Chien HL, Hsueh YP. X-linked mental retardation gene CASK interacts with Bcl11A/CTIP1 and regulates axon branching and outgrowth. J Neurosci Res. 2010;88:2364–73. doi: 10.1002/jnr.22407. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi S, Uehara DT, Tanimoto K, Mizuno S, Chinen Y, Fukumura S, et al. Comprehensive investigation of CASK mutations and other genetic etiologies in 41 patients with intellectual disability and microcephaly with pontine and cerebellar hypoplasia (MICPCH) PLoS One. 2017;12:e0181791. doi: 10.1371/journal.pone.0181791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–8. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]