Abstract
Background:
Parkinson's disease (PD) patients are at a higher risk of malnutrition with the overall prevalence estimated to be 3%–60%, but there are limited data in India regarding nutritional assessment of PD.
Aim:
This study aims to assess nutritional status of PD patients and correlate the disease factors and gastrointestinal tract (GIT) symptoms with nutritional status.
Materials and Methods:
The PD cohort was assessed for demographic factors, nutritional assessment was done by Mini-Nutritional Assessment (MNA) Scale, and GI symptoms were assessed by validated scales. Age- and gender-matched cohort controls were randomly selected to correlate the GIT symptoms influencing nutritional status. The study population was divided into two groups according to the MNA score; Group I malnourished/at risk of malnutrition (score <23.5) or Group II normal nutrition (>23.5). The two subgroups were then compared.
Results:
We assessed 75 patients of PD and 35 age- and gender-matched controls. According to anthropometric criteria, 23% of the PD population was underweight, and according to biochemical assessment, 17.3% had hypoalbuminemia along with anemia. According to MNA scale, 12% were malnourished and 45.3% were at risk of malnutrition. Hence, a total of 57.3% patients in Group I (with abnormal nutrition) as compared to 14% of the controls were at risk of malnutrition while none was found to be malnourished. In our study, GIT symptoms, such as sialorrhea and dysphagia was reported by 29.3% each and constipation by 41.3% patients. While comparing GI symptoms within the two MNA groups, there was statistically significant relationship of all GI manifestations, sialorrhea (P = 0.041), dysphagia (P = 0.00081), and constipation (P = 0.0042) with malnutrition. There was no statistical significant difference between groups for age (P = 0.54), gender (P = 0.903), and duration of disease (P = 0.743).
Conclusions:
The data suggest that about 45% of PD patients are at risk of malnourishment. MNA Score is a validated nutritional assessment tool and anthropometric or biochemical measures alone cannot identify all the malnourished population. PD patients at risk of malnutrition or malnourished do have symptoms of dysphagia, sialorrhea, and constipation as compared to PD patients with normal nutrition.
Keywords: Gastrointestinal symptoms, malnutrition, Mini-Nutritional Assessment score, Parkinson's disease
INTRODUCTION
Parkinson's disease (PD) is the second most common neurodegenerative disease that usually develops late in life affecting 1% of the population over 65 worldwide, with an incidence of 14–100 cases/100,000 a year.[1,2] The motor symptoms progressively lead to severe disability and together with some nonmotor complications can contribute substantially to the changes in the nutritional status during the course of the disease.[3,4,5]
Nutritional status is an important contributor to a good quality of life and the general state of everyday life in PD patients.[6] There is a growing body of evidence suggesting about 3%–60% of PD patients are reported to be at risk of malnutrition. It has been noted that many symptoms of PD as well as treatment side effects have nutritional implications.[7] The possible factors responsible for the loss of weight in these patients could be low dietary intake due to dysphagia and/or anorexia, lowering of absorption caused by slow gastric emptying, the increase of energy consumption due to high muscular activity, especially tremors, and cognitive impairment.[8] Moreover, as the disease advances patients typically become totally dependent on caretakers who can further lead to progression of nutrition-related problems like malnutrition.
Malnutrition in PD patients adds to the morbidity by prolonging the risk of hospitalization and recovery from illness, increasing risk of osteoporosis and likelihood of falls, decline in the quality of life, and thus, overall poor outcomes. Hence, regular assessment of nutritional status plays a major role in the prevention of malnutrition and its complications in PD patients. There is number of nutrition-related aspects associated with PD, but there is a paucity of quality evidence on the true extent of malnutrition prevalent in these patients, especially from developing countries. The objective of this work was to assess the nutritional status of PD patients in a developing country like India and correlate the disease factors and gastrointestinal tract (GIT) symptoms.
MATERIALS AND METHODS
A prospective observational study was conducted, including all consecutive adults between 30 and 80 years with idiopathic Parkinson's who attended the Outpatient Department of Neurology of our tertiary care teaching hospital in Northern India, between July 1, 2016 and June 30, 2017. PD was diagnosed by the United Kingdom Parkinson's Disease Society Brain Bank Criteria.[9] Neurological disease severity was assessed using the Hoehn and Yahr (H and Y) staging.[10] The H and Y classifications were split into two groups (mild/moderate PD H and Y: 0–2.5 and severe PD H and Y: >3). Patients with diabetes, renal disorders, congestive heart failure, secondary PD, protein–energy malnutrition, and malignancy were excluded from the study. Age- and gender-matched control population without any disease influencing the nutritional status was also enrolled. The controls were preferably the members from the patient's family so as to have uniformity in socioeconomic status, culture, and eating patterns.
Each patient was assessed through a clinical history, where data were obtained regarding demographic information, disease symptoms, drug intake/treatment history (daily medication dosage was expressed as levodopa equivalent dose [LED]), and data on GI symptoms.
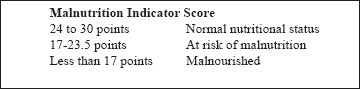
Nutritional status assessment was done using (1) anthropometrics – (a) weight (to the nearest 0.1 kg using calibrated weighing scale), (b) height (to the nearest cm using common measuring scale), (c) body mass index (BMI) – weight (kg)/height2 (cm) where BMI of <18.5 kg/m2 was considered as undernutrition, 18.5–22.9 as normal, 23–−24.9 as overweight, 25–29.9 as preobese, and >30 as obese as per the WHO classification for Asian population,[11] (d) mid-upper arm circumference – measured in cm at the mid-point between the olecranon and acromion process of the right arm using standard measuring tape, (e) Calf circumference – measured in cm at the point of maximum thickness at mid-calf using standard measuring tape; (2) biochemical parameters – venous blood samples were drawn for the determination of hemoglobin, total serum proteins, and serum albumin. The threshold values considered indicative of impairment in nutritional status were hemoglobin <11 g/dl, total serum proteins <6 g/dl, and albumin <3.5 g/dl.[12] Patients fulfilling all three criteria were considered malnourished; (3) Mini-Nutritional Assessment (MNA) Scale – a well-validated scale that was used as an additional nutritional assessment tool.[13] MNA scale has two parts, Part 1 – the screening tool and Part 2 – total MNA for nutritional assessment. The total score of 30 from 18 questions within four assessment categories (anthropometric, global, dietary, and subjective) was done to classify respondents into one of three categories: Normal nutritional status (>24), at risk of undernutrition (17–23.5), and malnourished (<17) [Appendix 1].[13] We divided the study population into two groups, that is Group I with abnormal nutritional status (those at risk of malnutrition as well as malnourished) whose MNA score was <23.5 and Group II with normal nutritional status whose MNA score >24 [Figure 1].
Figure 1.
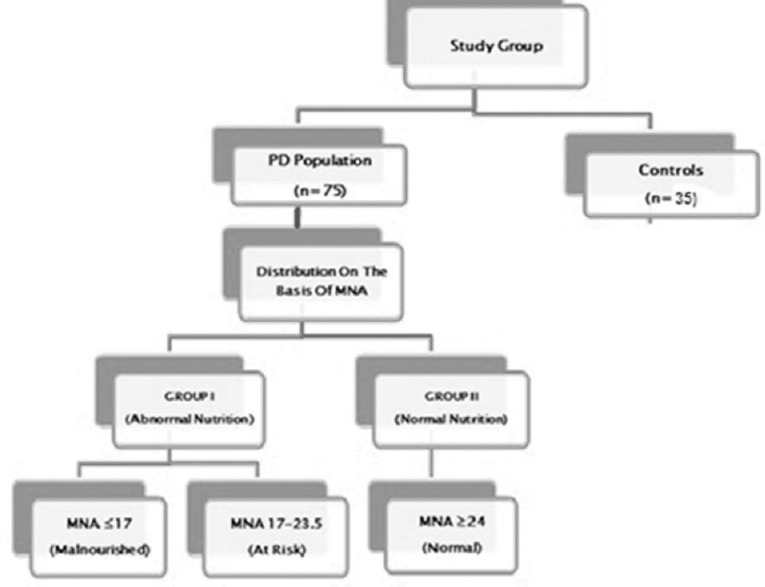
Distribution of the studied population
GI symptoms of nutritional interest studied were dysphagia, sialorrhea, and chronic constipation. Dysphagia was assessed by Swallowing Disturbance Questionnaire [Appendix 2][14] which includes 14 items, each with a score ranging from 0 to 3, except one question with a score ranging from 0.5 to 2.5. The total score varies between 0.5 and 44·5. A score 0.5 means that the patients experience some degree of difficulty in swallowing and a score >2 was arbitrarily considered as threatening. A cutoff score >11 was considered for the diagnosis of dysphagia needing treatment. Sialorrhea was assessed by Sialorrhea Clinical Scale for PD [Appendix 3].[15] The scale consists of seven questions, each with a score ranging from 0 to 3, resulting in a total score of 0–21. A cutoff score of >11 was suggested for the diagnosis of sialorrhea needing treatment; however, any score higher than 0 meant that the patient had sialorrhea. Chronic constipation was assessed by ROME III Criteria [Appendix 4].[16] Patients were diagnosed with functional constipation if they scored >2 points answering this questionnaire.
Continuous data were expressed as mean ± standard deviation. Categorical variables were expressed as percentage. Comparison between the control and cases were made using paired t-test and Mann–Whitney U-test for nonparametric data. Categorical variables were compared by Chi-square test. Groups were compared by Students t-test and value of P < 0.05 was considered as statistically significant. Analyses were performed using a spreadsheet program (Excel 2010, Microsoft and a statistical software package).
RESULTS
Seventy-five PD patients were studied along with 35 controls. Forty PD patients were male (53.3%) and 35 (46.6%) were female, while in the control group, there were 18 male (51.5%) and 17 female (48.5%). The mean age of the study group and control group was 63 + 10.5 years and 62 + 9.46 years, respectively. According to the severity of the disease, 41.3% had mild-to-moderate disease (H and Y stage: 0–2.5) and remaining 58.6% had advanced disease (H and Y > 3). Table 1 shows the demographic and clinical characteristics of the studied subjects and controls.
Table 1.
The demographic and clinical characteristics of the studied subjects and controls
| Factors | PD group (n=75) | Control group (n=35) |
|---|---|---|
| Age (years) | 63+10.5 | 62+9.46 |
| Gender (male) | 40 (53.3%) | 18 (51.4%) |
| Anthropometric Criteria | ||
| Normal (BMI 25-29.9) | 38 (50.6%) | 15 (43.3%) |
| Malnourished (underweight) | 17 (22.6%) | 2 (5.7%) |
| Pre-obese/Obese | 20 (26.6%) | 18 (51%) |
| Biochemical Criteria | ||
| Hb (<11 gm/dl) + Total proteins (<6 g/dl) + Albumin (<3.5g/dl) | 13 (17.3%) | 3 (8.5%) |
| Mini Nutritional Assessment | ||
| Normal | 32 (42.6%) | 30 (85.7%) |
| At risk malnourishment/Malnourished | 43 (57.3%) | 5 (14.2%) |
| GIT symptoms | ||
| Constipation | 31 (41.3%) | 1 (2.8%) |
| Dysphagia | 22 (29.3%) | 1 (2.8%) |
| Sialorrhea | 22 (29.3%) | 0 |
PD=Parkinson’s disease, BMI=Body mass index, HB=Hemoglobin, GIT=Gastrointestinal tract
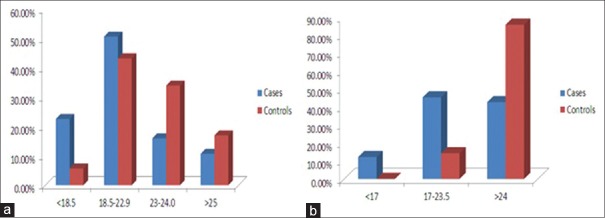
According to anthropometric criteria, 23% of the PD population was underweight, and 26.6% were preobese/obese according to the WHO parameters for Asian countries. The remaining 50% were normal as compared to 43% of the controls that were normal [Figure 2a]. The anthropometric criteria detected 5.7% (2 patients) malnourishment in the control group.
Figure 2.
Comparison of cases and controls according to different nutritional assessment tools (a) on the basis of anthropometric criteria (b) on the basis of Mini-Nutritional Assessment Scale
According to biochemical assessment, 17.3% (13 patients) of the PD patients had hypoalbuminemia along with anemia as compared to 8.5% (3 patients) in the control group.
According to MNA scale for nutritional assessment, among the 75 PD patients, 9 (12%) were malnourished and 34 (45.3%) were at risk of malnutrition. Hence, a total of 43 (57.3%) patients in Group I (with abnormal nutrition) as compared to 5 patients (14%) of the control population were at risk of malnutrition while none of them was found to be malnourished [Figure 2b]. Table 2 shows the demographic profile, disease severity, and characteristics and GI symptoms among Group I and Group II. The mean age of Group I was 64 + 9.5 years and Group II was 62 + 10.5 years. Twenty-seven patients were male (62.7%) and 16 (37.2%) were female, while in Group II, there were 20 male (62.5%) and 12 female (37.5%). The disease duration was comparable in both the groups. Twenty-five percent of patients in Group I had mild-to-moderate disease (H and Y stage 0-2.5) as compared to 56.5% patients in Group II. On the other hand, 32 patients (74.4%) in Group I had advanced disease (H and Y >3) as compared to 34.3% patients in Group II. This difference was statistically significant. The LED of Group I was 585.91 while that of Group II was 512.21. The number of falls as reported by the UPDRS score >3 was much higher in Group I (27.9%) as compared to Group II (4.65%) and this difference was statistically significant. In our study, there was no correlation of nutritional status according to MNA with respect to age (P = 0.826), gender (P = 0.903), duration of disease (P = 0.8312), and LED (P = 0.43).
Table 2.
Comparison of demographic factors, disease severity and characteristics and gastrointestinal symptoms among Group I (Mini Nutritional Assessment <23.5) and Group II (Mini Nutritional Assessment >24)
| PD group | Group I (43) | Group II (32) | P |
|---|---|---|---|
| Age (years) | 64+9.5 | 62+10.5 | 0.54 |
| Gender (male) | 27 (62.7%) | 20 (62.5%) | 0.903 |
| Duration of disease (years) | 5.19+3.5 | 5.03+3.7 | 0.743 |
| H&Y<3 | 11 (25.5%) | 18 (56.5%) | 0.009 |
| H&Y>3 | 32 (74.4%) | 11 (34.3%) | |
| LED | 585.91 | 512.21 | 0.431 |
| Falls (> 2 III UPDRS-13) | 12 | 2 | 0.0374 |
| Constipation | 31 (72.1%) | 9 (28.1%) | 0.0004 |
| Dysphasia | 22 (51.2%) | 4 (11.7%) | 0.0008 |
| Silarrhoea | 22 (51.2%) | 9 (28.1%) | 0.041 |
PD=Parkinson’s disease, LED=Levodopa equivalent dose, UPDRS=Unified Parkinson’s Disease Rating Scale
We also compared the nutritional status with regard to the presence of nonmotor GI symptoms. Among the PD patients, the symptoms of constipation, dysphagia, and sialorrhea were present in 31 patients (41.3%), 22 patients (29.3%), and 22 patients (29.3%), respectively, as compared to 1 person (2.8%) in the control group who had constipation and dysphagia each and none had sialorrhea. On comparison of the gastrointestinal symptoms within the two MNA groups in the PD population, constipation (P = 0.0042), dysphagia (P = 0.00081), and sialorrhea (P = 0.041) were significantly associated with Group I (at risk of malnourishment and malnourished patients) as compared to those with normal nutritional status (MNA >24), as shown in Table 2.
DISCUSSION
India is a developing country with high prevalence of malnutrition. Patients with Parkinson's disease are at more risk of malnutrition, but there is a scarcity of data from India regarding nutritional assessment of PD patients. Malnutrition results in poor health outcomes and it remains underrecognized and underdiagnosed in the community. Hence, it was worthwhile to find the trends of malnutrition in a cohort of PD patients versus the normal community-dwelling population. To the best of our knowledge, this is the first study from India on the nutritional problems prevalent in PD patients.
Different studies from Western literature have reported the prevalence of malnutrition in patients with PD ranging from 3% to 60%.[17,18] This wide variation could be attributed to the differences in methodology, criteria of classification of weight loss and malnutrition, or characteristics of the patient studied. We used three assessment tools for the diagnosis of malnutrition-anthropometric criteria, biochemical assessment, and MNA score. Our results show that 23% of the PD population was identified as underweight according to anthropometric criteria, 17.3% were malnourished as per biochemical assessment, and 57.3% had abnormal nutritional status as per the MNA score [Figure 3].
Figure 3.
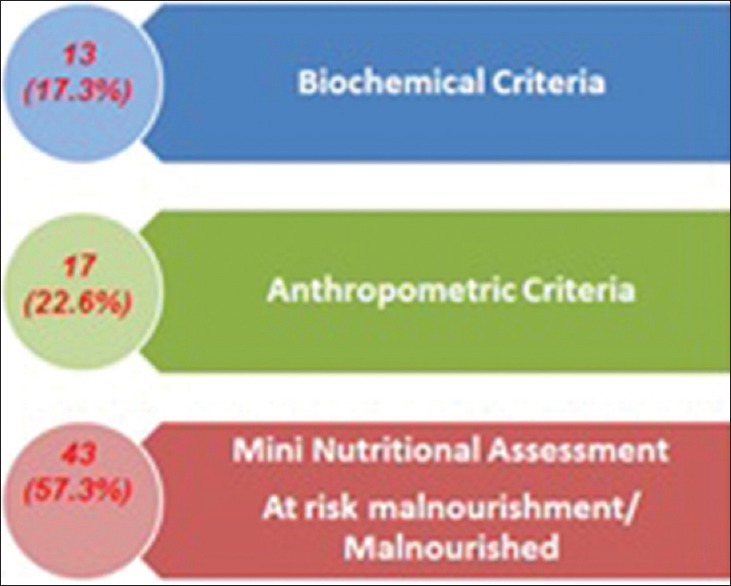
Comparision of prevalence of malnutrition using different assessment methods
Using anthropometric criteria as an indicator of nutritional risk in general population is historically accepted, but its use in older population, the cutoff value is debatable.[19] The risk of mortality decreases with increasing BMI in older age, and hence, using the same cutoff to diagnose malnutrition may lead to inaccurate results. Furthermore, it has been recently proposed that due to ethnic differences Asians have higher weight-related disease risks at lower BMIs; hence separate classifications have been proposed. Second, clinically significant weight loss may sometimes not result in change in BMI classification, decreasing its sensitivity as a tool for detecting malnutrition. Patients at risk of malnourishment or malnourished may be classified as overweight or obese according to BMI. Barichella et al. reported 0% as underweight, 40.8% as overweight, and 25% as obese according to BMI classification, whereas MNA score resulted in detection of 22% patients at risk of malnutrition in the same group of PD patients. Third, changes in BMI can be masked by decrease in height that occurs in PD patients as a result of osteoporosis which is quiet prevalent in this group. Finally, measurement of height for calculation of BMI is also sometimes difficult in the PD patients who have poor balance, rigidity, and are confined to wheelchair or are bedridden. Considering all these reasons, depending on anthropometric criteria alone as a tool for diagnosing malnutrition may result in fewer people being detected as malnourished.
MNA Score is a validated nutritional assessment tool that has high sensitivity and specificity for predicting nutritional status as compared to a single tool.[20] It is a wholistic tool that incorporates anthropometry, weight loss, dietary intake, and physical signs of malnutrition. The clinical guidelines for nutritional support in adults published by the National Institute for Health and Clinical Excellence recommend the use of variety of parameters when accessing malnutrition because anyone is not sufficiently sensitive.[21] Although these tools have been validated in other populations, they have not been specifically validated for use in PD patients. A systemic review published recently reports that only 2 out of the 11 reviews used MNA and 20% and 34% of the participants were at risk of malnutrition (MNA score >17), while only 2% and 0% were malnourished (MNA score <17).[22]
Hence, our results are coherent with other studies that suggest that anthropometric or biochemical measures alone cannot identify all the malnourished population, but a multifactorial approach to the diagnosis including recent weight loss, dietary intake, nutrition impact symptoms along with assessment of muscle, and fat status is a valid approach for the true detection of malnourishment. MNA as a nutritional assessment tool is applicable to any age as it includes weight changes, physical examination, and presence of symptoms likely to influence intake in people with PD. Fereshtehnejad et al. used MNA scale and reported that more than a quarter of PD population was at risk of malnutrition, necessitating more attention toward nutritional assessment in PD.[23] In our results also, 57.3% had abnormal nutritional status where 12% were malnourished and 45.3% were at risk of malnutrition as per MNA score whereas incidence of abnormal nutrition was only 14% in controls.
The combination of number of factors may lead to poor intake in PD patients and hence are at greater nutritional risk. There was statistically significant relation of GI symptoms such as constipation, dysphagia, and sialorrhea with malnutrition in PD patients in our study. Park et al. have shown that GI symptoms such as constipation, dysphagia, sialorrhea, and weight loss were significantly more frequent in more severe PD patients.[24] While there is limited research on the predictors of malnutrition, Wang et al. have reported that constipation and depression are significant predictors of malnutrition.[25]
GI disturbances are common in PD and virtually affect all levels of the GIT. GI symptoms in PD can be the result of both motor and nonmotor impairment. Contrary to the belief, GI disturbances are seen early in the course of the disease and constipation is one of the earliest manifestations that can precede the motor symptoms in PD patients by years.[26] Dysphagia or difficulty in swallowing food or liquid can make PD population prone to increased risk of aspiration. GI symptoms such as sialorrhea are not only incapacitating but also distressing for the patient. Hence, clinicians should pay due attention to these GI symptoms during evaluation of PD patients. These should be timely identified and promptly treated so as to reduce the risk of associated complications like malnutrition and hence increased morbidity.
Our study results need to be interpreted with caution as they are the results of a single center with small study group. Second, majority of patients in our study population fell within H and Y stage >3, patients with severe disability could not attend the OPD due to disability, so our study population may inadequately reflect the effects of severe motor disability on GI symptoms. Third, due to the geographical location of our center, most of our patients belonged to urban areas. People in rural areas may have limited access to medical facilities. Finally, most of our assessment was questionnaire based, therefore, the study has a limitation of recall bias.
CONCLUSIONS
We may say that PD is an independent risk factor for malnutrition as incidence of abnormal nutrition was significantly higher in cases than control population. MNA has higher sensitivity and specificity for predicting nutritional status in PD. Appropriate identification and intervention for malnutrition may prevent/delay the associated poor outcomes. Hence, it is important to screen for malnutrition at the time of PD diagnosis and on follow-up.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY DATA
APPENDIX1. MINI NUTRITIONAL ASSESSMENT SCALE
Screening
-
A. Has food intake declined over the past three months due to loss of appetite, digestive problems, chewing or swallowing difficulties?
0 = Severe decrease in food intake
1 = Moderate decrease in food intake
2 = No decrease in food intake 膕膕膕膕 □
-
B. Weight loss during the last 3 months?
0 = Weight loss greater than 3 kg (6.6 pounds)
1 = Does not know
2 = Weight loss between 1 and 3 kg (2.2 and 6.6 pounds)
3 = No weight loss 膕膕膕膕 □
-
C. Mobility?
0 = Bed or chair bound
1 = Able to get out of bed/chair, but does not go out
2 = Goes out 膕膕膕膕 □
-
D. Has the patient suffered psychological stress or acute disease in the past three months?
0 = Yes
1 = No 膕膕膕膕 □
-
E. Neuropsychological problems?
0 = Severe dementia or depression
1 = Mild dementia
2 = No psychological problems 膕膕膕膕 □
-
F. Body mass index (BMI)? (weight in kg / height in m2)
0 = BMI less than 19
1 = BMI 19 to less than 21
2 = BMI 21 to less than 23
3 = BMI 23 or greater 膕膕膕膕 □
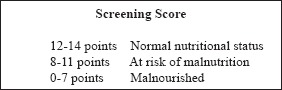
The screening section is now complete. Add the numbers to obtain the Screening Score (max. 14 points)
Screening Score 膕膕膕膕 □ □
Assessment
-
G. Lives independently (not in hospital or nursing home)
1=yes
0=no 膕膕膕膕 □
-
H. Takes more than 3 prescription drugs per day?
0=yes
1=no 膕膕膕膕 □
-
I. Pressure sores or skin ulcers?
0=yes
1=no 膕膕膕膕 □
-
J. How many full meals does the patient eat daily?
0=1 meal
1= 2 meals
2=3 meals 膕膕膕膕 □
-
K. Selected consumption markers for protein intake
•At least 1 serving of dairy products (milk, cheese, yoghurt) per day □ yes □ no
•Two or more servings of legumes or eggs per week □ yes □ no
•Meat fish or poultry everyday □ yes □ no
0.0 = if 0 or 1 yes
0.5 = if 2 yes
1.0 = if 3 yes 膕膕膕膕 □ □
-
L. Consumes 2 or more servings of fruits or vegetables per day?
0 = no
1 = yes 膕膕膕膕 □
-
M. How much fluid (water, juice, coffee, tea, milk, etc) is consumed per day?
0.0= less than 3 cups
0.5 = 3-5cups
1.0= more than 5 cups 膕膕膕膕 □ □
-
N. Mode of feeding
0=unable to eat without assistance
1= self fed with some difficulty
2= self fed without any problem 膕膕膕膕 □
-
O. Self view of nutritional status
0= views self as being malnourished
1= is uncertain of nutritional status
2= views self as having no nutritional problem 膕膕膕膕 □
-
P. In comparison with other people of the same age, how does the patient consider his/her own health status?
0.0= not as good
0.5 = does not know
1.0= as good
2= better 膕膕膕膕 □□
-
Q. Mid arm circumference (MAC) in cm
0.0= MAC less than 21
0.5 = MAC 21-22
1.0= MAC more than 22 □□
Assessment Score (max. 16 point) 膕膕膕膕 □□□
Total Assessment (max. 30 point) 膕膕膕膕 □□□
APPENDIX2. STANFORD HOSPITAL AND CLINICS SWALLOWING DISTURBANCE QUESTIONNAIRE
| 1. | Do you experience difficulty chewing solid food like apple, cookie, or cracker? | 0 | 1 | 2 | 3 |
| 2. | Are there any food residues in your mouth, cheeks, under your tongue, or stuck to the roof of your mouth after swallowing? | 0 | 1 | 2 | 3 |
| 3. | Does food come out of your nose or mouth when you eat or drink? | 0 | 1 | 2 | 3 |
| 4. | Does chewed up food dribble from your mouth? | 0 | 1 | 2 | 3 |
| 5. | Do you feel you have too much saliva in your mouth (do you drool or have difficulty in swallowing your saliva)? | 0 | 1 | 2 | 3 |
| 6. | Do you swallow chewed up food several times before it goes down your throat? | 0 | 1 | 2 | 3 |
| 7. | Do you experience difficulty in swallowing solid food (do apples or crackers get stuck in your throat)? | 0 | 1 | 2 | 3 |
| 8. | Do you experience difficulty in swallowing pureed food? | 0 | 1 | 2 | 3 |
| 9. | While eating, do you feel as if a lump of food is stuck up in your throat? | 0 | 1 | 2 | 3 |
| 10. | Do you cough while swallowing liquids? | 0 | 1 | 2 | 3 |
| 11. | Do you cough while swallowing solid food? | 0 | 1 | 2 | 3 |
| 12. | Immediately after eating or drinking, do you experience a change in your voice, such as hoarseness or wetness? | 0 | 1 | 2 | 3 |
| 13. | Other than during meals, do you experience coughing or difficulty in breathing as a result of saliva entering your windpipe? | 0 | 1 | 2 | 3 |
| 14. | Do you experience difficulty in breathing during meals? | 0 | 1 | 2 | 3 |
| 15 | Have you suffered from respiratory infection (such as pneumonia, bronchitis) in the past years? (circle one) | Yes | No | ||
APPENDIX3. SIALORRHEA CLINICAL SCALE FOR PD (SCS-PD)
-
A. During the day, when do you feel there is more saliva in your mouth?
0 = Never.
1 = At meal times.
2 = Throughout the day, not related to meals.
3 = All the time, even when I am asleep.
-
B. When you are asleep, how much saliva is there in your mouth?
0 = I don’t notice an increase in saliva.
1 = I notice increased amounts of saliva in my mouth, but my pillow doesn’t get wet.
2 = My pillow gets wet.
3 = My pillow and other bedclothes get wet.
-
C. While you are awake,
0 = I don’t drool.
1 = Saliva wets my lips.
2 = Saliva accumulates on my lips, but I don’t drool.
3 = I drool.
-
D. Does accumulation of saliva in your mouth impair your speech?
0 = No.
1 = I must swallow frequently to avoid difficulties.
2 = I have trouble speaking.
3 = I can’t speak at all.
-
E. Does accumulation of saliva in your mouth impair your eating ability?
0 = No.
1 = I must swallow frequently to avoid difficulties.
2 = I have trouble eating.
3 = I can’t eat at all.
-
F. How many times do you drool during the daytime?
0 = Never.
1 = Not more than 3 times.
2 = Often. I have to carry a handkerchief with me all the time.
3 = Permanently.
-
G. When you go out or on social occasions, does saliva accumulation bother you?
0 = No.
1 = I notice an accumulation, but it does not bother me.
2 = I realize other people notice it, but I can control the situation (for example, with a handkerchief).
3 = I have stopped attending social meetings.
APPENDIX 4.ROME III DIAGNOSTIC CRITERIA FOR FUNCTIONAL CONSTIPATION
Symptom onset more than 6 months prior to the diagnosis, with the following criteria fulfilled for the past 3 months:
-
1. Two or more of the following criteria must be met:
b. Less than three bowel movements per week
c. Manual maneuvers necessary to facilitate defecation more than 25% of the time.
d. Hard or lumpy stools more than 25% of the time
e. Sensation of incomplete evacuation more than 25% of the time
f. Sensation of anorectal obstruction more than 25%of the time
g. Straining with defecation more than 25% of the time
2. Loose stools rarely present without the use of laxatives
3. Insufficient criteria met to establish a diagnosis of irritable bowel syndrome
REFERENCES
- 1.Ułamek-Kozioł M, Bogucka-Kocka A, Kocki J, Pluta R. Good and bad sides of diet in Parkinson's disease. Nutrition. 2013;29:474–5. doi: 10.1016/j.nut.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Gaba A. Recent studies on nutrition and Parkinson's disease prevention: A systematic review. Open J Prev Med. 2015;5:197–205. [Google Scholar]
- 3.Quinn N. Parkinsonism – Recognition and differential diagnosis. BMJ. 1995;310:447–52. doi: 10.1136/bmj.310.6977.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ, et al. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333–41. doi: 10.1212/01.wnl.0000266593.50534.e8. [DOI] [PubMed] [Google Scholar]
- 5.Lorefät B, Ganowiak W, Wissing U, Granérus AK, Unosson M. Food habits and intake of nutrients in elderly patients with Parkinson's disease. Gerontology. 2006;52:160–8. doi: 10.1159/000091825. [DOI] [PubMed] [Google Scholar]
- 6.Neumann SA, Miller MD, Daniels L, Crotty M. Nutritional status and clinical outcomes of older patients in rehabilitation. J Hum Nutr Diet. 2005;18:129–36. doi: 10.1111/j.1365-277X.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Pålhagen S, Lorefält B, Carlsson M, Ganowiak W, Toss G, Unosson M, et al. Does L-dopa treatment contribute to reduction in body weight in elderly patients with Parkinson's disease? Acta Neurol Scand. 2005;111:12–20. doi: 10.1111/j.1600-0404.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer RF, Quigley EM. Gastrointestinal mobility problems in patients with Parkinsoson's disease-epidemiology, pathophysiology and guidelines for management. Cent Nerv Syst Drugs. 1999;11:435–48. [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov Disord. 2004;19:1020–8. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 11.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 12.Barichella M, Villa MC, Massarotto A, Cordara SE, Marczewska A, Vairo A, et al. Mini nutritional assessment in patients with Parkinson's disease: Correlation between worsening of the malnutrition and increasing number of disease-years. Nutr Neurosci. 2008;11:128–34. doi: 10.1179/147683008X301441. [DOI] [PubMed] [Google Scholar]
- 13.Guigoz Y. The Mini-Nutritional Assessment (MNA® ) Review of the Literature - What does it tell us? J Nutr Health Aging. 2006;10:466–487. [PubMed] [Google Scholar]
- 14.Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson's disease. Mov Disord. 2007;22:1917–21. doi: 10.1002/mds.21625. [DOI] [PubMed] [Google Scholar]
- 15.Evatt ML, Chaudhuri KR, Chou KL, Cubo E, Hinson V, Kompoliti K, et al. Dysautonomia rating scales in Parkinson's disease: Sialorrhea, dysphagia, and constipation – Critique and recommendations by movement disorders task force on rating scales for Parkinson's disease. Mov Disord. 2009;24:635–46. doi: 10.1002/mds.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barichella M, Cereda E, Cassani E, Frazzitta G, Pezzoli G. A focus on Rome III criteria for the assessment of constipation in Parkinson's disease. Mov Disord. 2017;32:630. doi: 10.1002/mds.26974. [DOI] [PubMed] [Google Scholar]
- 17.Jaafar AF, Gray WK, Porter B, Turnbull EJ, Walker RW. A cross sectional study of the nutritional status of community-dwelling people with idiopathic Parkinson's disease. BMC Neurol. 2010;10:124. doi: 10.1186/1471-2377-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Malnutrition in a sample of community-dwelling people with Parkinson's disease. PLoS One. 2013;8:e53290. doi: 10.1371/journal.pone.0053290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Obesity: Prevention and Managing the Global Epidemic. Geneva: World Health Organization; 2000. [Google Scholar]
- 20.Watterson C, Fraser A, Banks M, Isenring E, Miller M, Silvester C, et al. Evidence based practice guidelines for the nutritional management of malnutrition in adult patients across the continuum of care. Nutr Diet. 2009;66:1–34. [Google Scholar]
- 21.National Centre for Classification in Health. International Statistical Classification of Diseases and Related Health Problems. 10th Revision, Australian Modification (ICD-10-AM) Sydney: National Centre for Classification in Health; 2008. p. 26. [Google Scholar]
- 22.Sheard JM, Ash S, Silburn PA, Kerr GK. Prevalence of malnutrition in Parkinson's disease: A systematic review. Nutr Rev. 2011;69:520–32. doi: 10.1111/j.1753-4887.2011.00413.x. [DOI] [PubMed] [Google Scholar]
- 23.Fereshtehnejad SM, Ghazi L, Sadeghi M, Khaefpanah D, Shahidi GA, Delbari A, et al. Prevalence of malnutrition in patients with Parkinson's disease: A comparative study with healthy controls using mini nutritional assessment (MNA) questionnaire. J Parkinsons Dis. 2014;4:473–81. doi: 10.3233/JPD-130323. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Lee JY, Shin CM, Kim JM, Kim TJ, Kim JW. Characterization of gastrointestinal disorders in patients with Parkinsonian syndromes. Parkinsonism Relat Disord. 2015;21:455–60. doi: 10.1016/j.parkreldis.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Wan Y, Cheng Q, Xiao Q, Wang Y, Zhang J, et al. Malnutrition and associated factors in Chinese patients with Parkinson's disease: Results from a pilot investigation. Parkinsonism Relat Disord. 2010;16:119–23. doi: 10.1016/j.parkreldis.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Jost WH. Gastrointestinal dysfunction in Parkinson's disease. J Neurol Sci. 2010;289:69–73. doi: 10.1016/j.jns.2009.08.020. [DOI] [PubMed] [Google Scholar]