Abstract
Background
Patients with advanced non-small cell lung cancer (NSCLC) treated with cisplatin, also termed cis-diamminedichloroplatinum (CDDP) or diamminedichloroplatinum (DDP), may develop chemoresistance. This study aimed to investigate the role of long non-coding RNA (lncRNA) X-inactive specific transcript (XIST) and multidrug resistance-1 (MDR1) in tumor tissue samples and the chemoresistant human NSCLC cell lines, H460/DDP and A549/DDP, and in a murine A549/DDP tumor xenograft.
Material/Methods
Tissue samples were from patients with NSCLC who responded cisplatin (DDP-sensitive) (n=24), patients with NSCLC unresponsive to cisplatin (DDP-resistant) (n=30), and normal lung tissue (n=25). In H460/DDP and A549/DDP cells, expression of XIST, microRNA (miR)-144-3p, MDR1, and multidrug resistance-associated protein 1 (MRP1) were detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR) and Western blot. The MTT assay measured cell survival and proliferation, a transwell assay evaluated cell migration, and flow cytometry measured apoptosis. Luciferase reporter, RNA immunoprecipitation (RIP), and RNA pull-down assays examined the relationship between XIST and miR-144-3p. Tumor xenografts from A549/DDP cells were studied in BALB/c nude mice.
Results
In tissue from patients with DDP-resistant NSCLC and the mouse A549/DDP tumor xenograft, lncRNA-XIST expression was upregulated and miR-144-3p expression was inhibited. In A549/DDP and H460/DDP cells, down-regulation of lncRNA-XIST and upregulation of miR-144-3p reduced cell survival, proliferation, migration, induced apoptosis and suppressed MDR1 and MRP1 expression.
Conclusions
Upregulation of lncRNA-XIST was associated with cisplatin resistance in NSCLC by downregulating miRNA-144-3p in H460/DDP and A549/DDP cells, a murine A549/DDP tumor xenograft, and human tumor tissues from patients with cisplatin-resistant NSCLC.
MeSH Keywords: Antineoplastic Agents; Carcinoma, Non-Small-Cell Lung; Cisplatin; MicroRNAs
Background
Worldwide, lung cancer is the leading cause of cancer-related death. Non-small-cell lung cancer (NSCLC) accounts for about 85% of all cases of lung cancer. Chemotherapy is an important treatment option for advanced NSCLC, and usually includes cisplatin, also termed cis-diamminedichloroplatinum (CDDP) or diamminedichloroplatinum (DDP) [1,2]. However, chemoresistance may be an obstacle for chemotherapy for NSCLC. Therefore, further studies are required to determine the mechanisms of chemoresistance in NSCLC so that treatment options can be improved and effective treatments selected for individual patients with NSCLC.
Long noncoding RNAs (lncRNAs) are a class of non-coding RNAs with transcripts of more than 200 nucleotides. Recently, several lncRNAs have been shown to act as important regulatory molecules that participate in cellular processes and drug-resistance in several types of cancers, including hepatocellular carcinoma, NSCLC, breast cancer, gastric cancer and colorectal cancer [3–5]. Han et al. reported that in colorectal cancer, the differential expression of lncRNA contributed to cell proliferation and chemoresistance [5]. Upregulation of lncRNA-H19 has been shown to promote resistance to paclitaxel treatment in estrogen receptor (ER)-positive breast cancer [6]. Also, the upregulation of lncRNA-SNGHG14 has been shown to increase chemoresistance to trastuzumab in breast cancer [4]. These findings indicate that lncRNAs are associated with chemoresistance in multiple types of cancer.
The findings from a recent study showed that lncRNA X-inactive specific transcript (XIST) was upregulated and modulated chemoresistance by interacting with miR-29c via transcription factor specificity protein 1 (SP1) and DNA repair protein O6-methylguanine-DNA methyltransferase in glioma cells [7]. Also, lncRNA-XIST has been shown to be upregulated in NSCLC tumor tissues and significantly increased in cisplatin-resistant A549 lung adenocarcinoma cells, or A549/DDP cells. However, knockdown of XIST restored the chemosensitivity of A549/DDP cells to cisplatin, which was reversed by the microRNA-17 (miR-17) inhibitor and overexpression of ATG7, indicating that XIST was involved in chemoresistance in NSCLC [8], However, the regulation mechanisms of XIST in tumorigenesis in NSCLC and chemotherapy resistance remain poorly understood.
The lncRNAs act as competing endogenous RNAs (ceRNAs) to regulate tumor chemoresistance by sponging miRNAs. Recent studies have shown that miRNAs play important roles in cell proliferation, apoptosis, and chemoresistance in several types of cancer. For example, miR-19 and miR-34a, are involved in the regulation of chemoresistance in breast cancer [9,10]. Also, miR-193a-3p expression increases chemoresistance to multiple agents in bladder cancer [11]. Pang et al. reported that overexpression of miR-146a enhances cisplatin sensitivity in NSCLC [12]. Also, miR-144-3p has been reported to serve as a miRNA tumor suppressor involved in the development of cancer [13,14]. Down-regulation of miR-144-3p has been reported in NSCLC cells [15]. However, the role of miR-144-3p in NSCLC has not been fully investigated and the roles for lncRNA-XIST and miR-144-3p in the development of chemosensitivity or chemoresistance in NSCLC has not been determined.
Therefore, this study aimed to investigate the role of lncRNA-XIST expression and multidrug resistance-1 (MDR1) in the chemoresistant human lung carcinoma cell lines, H460/DDP and A549/DDP, in a murine A549/DDP tumor xenograft, and in tissue samples from patients with surgically resected NSCLC with and without resistance to cisplatin.
Material and Methods
Ethics approval and patient consent
This study was approved by the Ethics Committee of the First Peoples’ Hospital of Lanzhou City. Informed consent was obtained from all patients and their guardians. The animal experiments were approved by the Animal Care and Ethics Committee of the First Peoples’ Hospital of Lanzhou City.
Patient tissue samples
Tissue samples were obtained from patients with non-small cell lung cancer (NSCLC) who responded cisplatin (DDP-sensitive) (n=24), patients with NSCLC who were unresponsive to cisplatin (DDP-resistant) (n=30), and normal lung tissue from healthy individuals (n=25). All study participants had undergone surgery at the First Peoples’ Hospital of Lanzhou City Hospital. No patients underwent radiotherapy or immunotherapy before surgery. All tissue samples were stored at −80°C.
Cell culture and transient transfection
Cisplatin-resistant human NSCLC cell lines, H460/DDP and A549/DDP, were obtained from RiboBio Co. (Guangzhou, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA), penicillin and and streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C with 5% CO2. Culture medium was changed every 2 days.
The negative control (NC) mimic, microRNA (miR)-144-3p mimic, NC inhibitor and miR-144-3p inhibitor were purchased from GenePharma Co., Ltd. (Shanghai, China). Short-hairpin (sh)-NC, sh-XIST, pc-NC and pc-XIST were constructed by GenePharma Co., Ltd. (Shanghai, China). All vectors and oligonucleotides were transfected into H460/DDP and A549/DDP cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tumor tissues, tumor-adjacent tissues or cells individually using TRIzol (Invitrogen, Carlsbad, CA, USA) and RNA concentration was detected by NanoDropND-1000 spectrophotometer. For XIST, M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) was used to reverse-transcribe to cDNAs. For miRNA, the TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) was used to reverse transcribe into cDNAs. XIST and miR-144-3p expression were determined using the SYBR® Green assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. GAPDH and U6 small nuclear RNA (snRNA) were used as reference gene for XIST and miR-144-3p. Fluorescence was detected using the iQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The relative expression of XIST and miR-144-3p was calculated by the 2−ΔΔCt method. The following primer sequences were used:
U6, forward: 5′-CTCGCTTCGGCAGCACA-3′;
U6, reverse: 5′-AACGCTTCACGAATTTGCGT-3′;
miR-144-3p, forward: 5′-TACTGCATCAGGAACTGACTGGA-3′;
miR-144-3p, reverse: 5′-GTGCAGG GTCCGAGGT-3′;
XIST, forward: 5′-ACGCTGCATGTGTCCTTAG-3′;
XIST, reverse: 5′-GAGCCTCTTATAAGCTGTTTG-3′;
GAPDH, forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′;
GAPDH, reverse: 5′-GGCTGTTGTCATACT TCTCATGG-3′.
Western blot
Tissues and cells were washed with Tris-buffered saline (TBS). RIPA buffer was added with constant agitation for 30 min, then centrifuged for 15 min at 4°C at 12,000 rpm and the supernatant was transferred to new tubes for subsequent experiments. The protein concentrations were measured using Nanodrop2000 (Thermo Fisher Scientific, San Jose, CA, USA). Proteins (20 mg) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blocking with 5% dried skimmed milk powder in TBS, the membranes were incubated with specific primary antibodies against MDR1, (1: 2000 dilution) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and GAPDH (1: 2000 dilution) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 4°C overnight. The membrane was washed five times for 25 min with TBS and Tween-20 (TBST), followed by incubation with horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (1: 2000 dilution) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The protein signals were detected using Pierce™ ECL Western blot substrate (Thermo Fisher Scientific, San Jose, CA, USA).
The MTT assay
Cell survival and proliferation were measured using an MTT assay kit (Sigma-Aldrich, St. Louis MO, USA) according to the manufacturer’s protocol. Cells (2×103) were seeded into 96-well plates (Corning Costar, Corning, NY, USA). Then 30 μl of serum-free media with MTT solution was added to each well and incubated for 4 h at 37°C. After discarding the media, 150 μl of MTT solvent (4 mM HCl, 0.1% NP40 in isopropanol) was added into each well and then incubated for 3 h at 37°C. Then the absorbance (A) value of each well at an optical density (OD) of 450 nm was measured using a microplate reader (MG LabTech, Durham, NC, USA).
Luciferase reporter assay
The binding sites of XIST and miR-144-3p were predicted using miRcode (http://mircode.org/index.php). The fragments of wild-type XIST (XIST WT) or mutated miR-144-3p binding sites (XIST MUT) were amplified and cloned into a site downstream of pGL3-Control Vector (Promega, Madison, WI, USA). All vectors were verified via DNA sequencing and were co-transfected with miR-144-3p mimics into H460/DDP and A549/DDP cells using Lipofectamine 2000. After transfection 48h, luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA), according to the manufacturer’s instructions.
RNA immunoprecipitation (RIP) and RNA pulldown assays
RNA immunoprecipitation (RIP) and RNA pulldown assays were conducted in H460/DDP and A549/DDP cells. RIP assays were determined using the EZ-Magna RIP kit (Merck Millipore, Burlington, MA, USA) following the manufacturer’s instruction. Anti-AGO2 (Abcam, Cambridge, MA, USA) and anti-IgG (Abcam, Cambridge, MA, USA) antibodies were used. For RNA pulldown assays, biotinylated XIST was incubated with BGC-823 cell protein extracts and RT-qPCR was used to evaluate the level of miR-144-3p.
Transwell assays
Transwell assays were performed to evaluate cell invasion and migration. Cells transfected with above plasmids or oligonucleotides were adjusted to 1×105 cells/mL and 150 μL of cell suspension was added into the transwell chamber (Merck Millipore, Burlington, MA, USA) with a porous membrane pre-coated with or without migration Matrigel solution (Sigma-Aldrich, St. Louis MO, USA). Complete medium (500 mL) was added to the lower chamber. Cells were incubation for 48 h. Cells that had traversed the lower compartment were fixed with methanol, stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis MO, USA) and counted by light microscopy.
Cell apoptosis
Cell apoptosis was determined using the Annexin V-FITC apoptosis detection kit (Beyotime, Shanghai, China). Cells were cultured in DMEM with 10% FBS for 48 h. The cells (1×106) were collected and washed with phosphate-buffered saline (PBS). After re-suspending in Annexin V-fluorescein isothiocyanate (FITC) binding solution, cells were stained with Annexin V-FITC and propidium iodide. Finally, cell apoptosis was detected using flow cytometry (BD Biosciences, San Jose, CA, USA).
In vivo tumor xenograft
The animal experiments were approved by animal care and Ethics Committee of the First People’s Hospital of Lanzhou City. A549 cell stably transfected with sh-NC and sh-XITS. Moreover, sh-NC or sh-XITS was stably co-transfected with NC inhibitor or miR-144-3p inhibitor into A549 cell. Then, 3.0×106 cells were suspended in 100 μl of PBS and injected subcutaneously into the right side of the posterior flank of female BALB/c nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd. China) at 4–5 weeks of age. Tumor volume was detected every 3 days for 15 days. After 15 days, mice were euthanized for further studies and the tumors were weighed.
Statistical analysis
Analysis of the statistical significance between the two groups was performed using Student’s t-test, and the relationship between XIST and miR-144-3p was determined by Pearson’s correlation analysis. All data were presented as the mean ± standard deviation (SD) and were analyzed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). P<0.05 was considered to be statistically significant.
Results
The expression of X-inactive specific transcript (XIST) was upregulated in tumor tissue from patients with chemoresistant non-small cell lung cancer (NSCLC)
Tumor tissues from 24 patients with NSCLC that were DDP-sensitive, from 30 patients with NSCLC that were DDP-resistant, and 25 normal lung tissue controls were studied. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) showed that XIST expression was upregulated in tissue from NSCLC tumors that were DDP-resistant compared with DDP-sensitive NSCLC tumor tissues, suggesting that XIST was associated with NSCLC chemoresistance (Figure 1).
Figure 1.
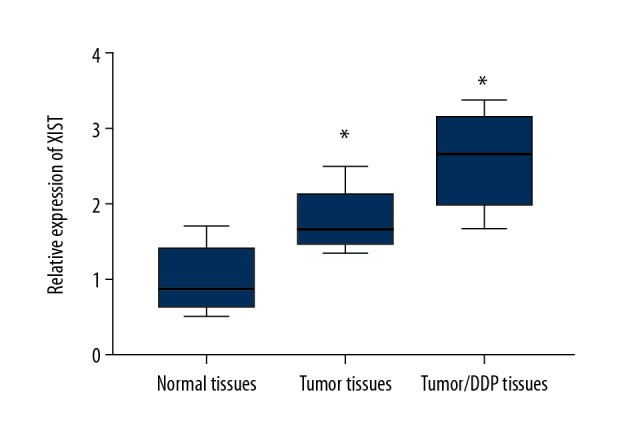
The expression of XIST was upregulated in non-small cell lung cancer (NSCLC) tissues. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed to detect the expression of XIST in normal tissues, tumor tissue, and in NSCLC tumor tissues from DDP-sensitive and DDP-resistant patients. * p<0.05.
Knockdown of XIST enhanced DDP sensitivity in H460/DDP and A549/DDP cells
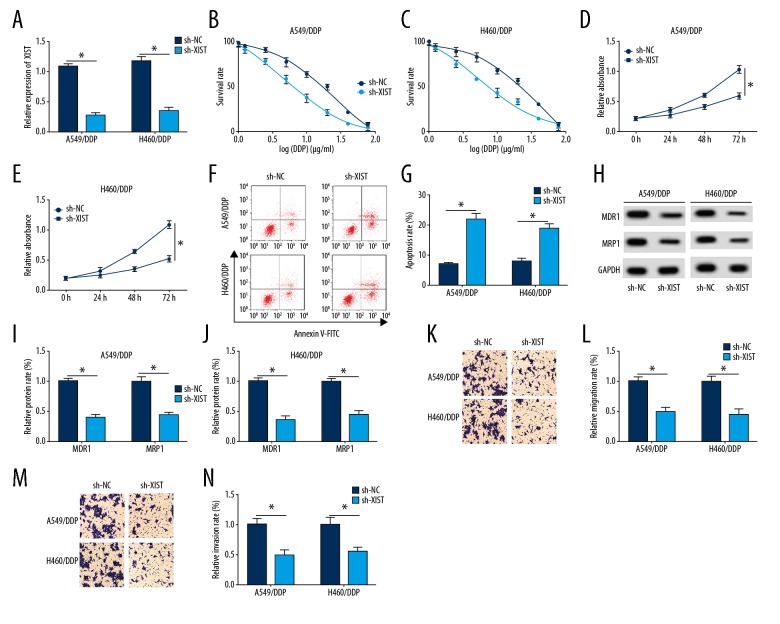
To further explore the role of XIST, sh-NC and sh-XIST were transfected into H460/DDP and A549/DDP cells. As shown in Figure 2A, XIST expression was significantly decreased in the sh-XIST group compared with the sh-NC group. An increased concentration of DPP significantly reduced the cell survival rate of the sh-XIST group compared with the sh-NC group (Figure 2B, 2C). Cell proliferation was significantly inhibited in the sh-XIST group compared with the sh-NC group (Figure 2D, 2E). Knockdown of XIST significantly increased cell apoptosis (Figure 2F).
Figure 2.
Knockdown of XIST enhanced chemosensitivity to DDP in H460/DDP and A549/DDP cells. (A) The expression of XIST in short-hairpin negative control (sh-NC) and sh-XIST groups in H460/DDP and A549/DDP cells was detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR). (B–E) The survival rate and proliferation were determined in H460/DDP and A549/DDP cells using MTT assay. (F, G) Cell apoptosis was examined in H460/DDP and A549/DDP cells using flow cytometry. (H–J) The protein levels of MDR1 and MRP1 in A549/DDP and H460/DDP cells were measured by Western blot. (K–N) Cells migration and invasion abilities were elevated in cells transfected with sh-NC and sh-XIST. * p<0.05.
MDR1 and MRP1 are common chemoresistance genes. Western blot showed that down-regulated XIST in A549/DDP cells significantly inhibited the protein levels of MDR1 and MRP1 (Figure 2G–2I). Also, downregulation of XIST inhibited cell migration of DPP-treated cells. These findings indicated that knockdown of XIST enhanced chemosensitivity in NSCLC cells.
XIST interacted with miR-144-3p
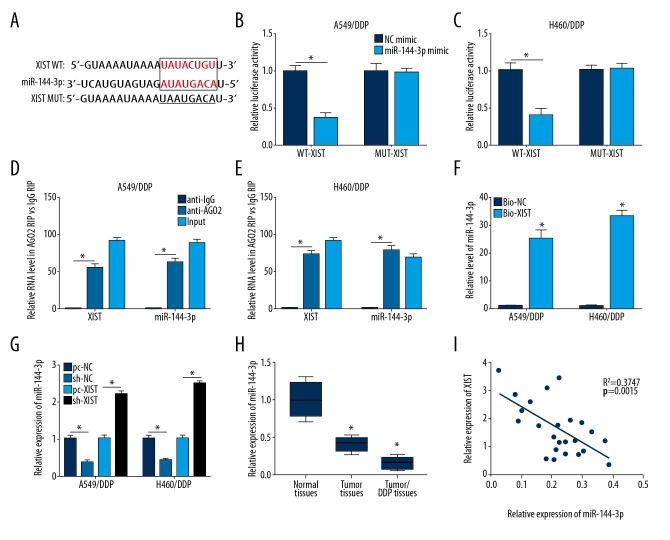
Bioinformatics analysis predicted miR-144-3p as a target of XIST (Figure 3A). The luciferase reporter assay was performed to verify whether XIST could bind to miR-144-3p. As shown in Figure 3B and 3C, XIST wild-type (WT) or XIST mutation (MUT) were co-transfected with miR-144-3p mimic, respectively. The results showed that the miR-144-3p mimic significantly reduced luciferase activity by binding to XIST WT compared with XIST MUT.
Figure 3.
XIST interacted with miR-144-3p. (A) The binding sites between XIST and miR-144-3p were predicted using the miRCode database. (B, C) The luciferase reporter assay was used to detect luciferase activity of H460/DDP cells and A549/DDP cells co-transfected with XIST wild-type (WT) or XIST mutated (MUT) and miR-143-3p mimic or negative control (NC) mimic. (D, E) The expressions of miR-143-3p and XIST were detected in A549/DDP and H460/DDP cells after Ago2 or IgG RIP assay. (F) RNA pull-down experiment was performed to determine the relationship between XIST and miR-143-3p. (G) The expression of miR-144-3p in A549/DDP and H460/DDP cells with different treatment was detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR). (H) The expression of miR-144-3p was performed using RT-qPCR in normal tissues, tumor tissue, and tumor/DDP tissues. (I) The relationship between XIST and miR-144-3p were determined in tumor tissue by Pearson’s correlation analysis. * p<0.05.
The RNA immunoprecipitation (RIP) assay was performed using an antibody against AGO2 or IgG. As shown in Figure 3D and 3E, miR-144-3p was enriched in cells transfected with the XIST overexpression plasmid in the anti-AGO2 group and was compared with that in the anti-IgG group. The same phenomenon of XIST overexpression was found in the miR-144-3p group. Also, a significant increase in miR-144-3p was found in the RNA pull-down experiment (Figure 3F). The results of RT-qPCR showed that the expression of miR-144-3p was down-regulated in A549/DDP cells transfected with pc-XIST, whereas miR-144 expression was upregulated in A549/DDP cells transfected with sh-XIST (Figure 3G). Also, miR-144-3p expression was down-regulated in tumor tissues and tumor/DDP tissues compared with normal tissues (Figure 3H) and Pearson’s correlation analysis showed that XIST expression was significantly negatively correlated with the expression of miR-144-3p (Figure 3I). These findings demonstrated that miR-144-3p was a target of XIST and interacted with XIST in A549/DDP and H460/DDP cells.
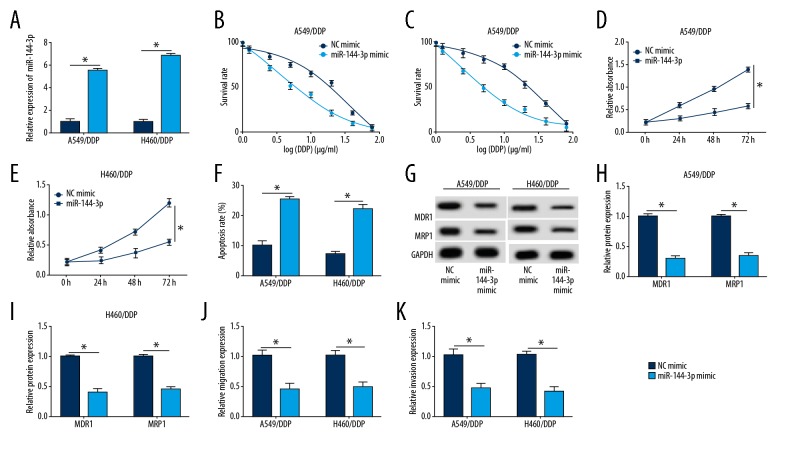
Overexpression of miR-144-3p increased sensitivity to DDP in H460/DDP and A549/DDP cells
To further investigate the regulatory effect of miR-144-3p on the sensitivity to DDP in H460/DDP and A549/DDP cells, they were transfected with NC mimic or miR-144-3p mimic. The expression of miR-144-3p was induced by miR-144-3p mimic transfection (Figure 4A), and the effects of miR-144-3p overexpression were similar to the effects of XIST knockdown on cell function. Upregulation of miR-144-3p reduced the cell survival rate and cell proliferation compared with the NC mimic group (Figure 4B–4E). Cell apoptosis was also inhibited in the group that overexpressed miR-144-3p (Figure 4F). The protein levels of MDR1 and MRP1 were reduced in the miR-144-3p mimic group (Figure 4G–4I). Also, miR-144-3p overexpression reduced cell migration and invasion in DPP-treated cells (Figure 4J, 4K). These findings showed that increased expression of miR-144-3p could increase the chemosensitivity of NSCLC cells.
Figure 4.
Overexpression of miR-144-3p increased chemosensitivity to DDP in H460/DDP and A549/DDP cells. (A) miR-144-3p mimic or NC mimic were transfected into H460/DDP and A549/DDP cells. The quantitative reverse transcription polymerase chain reaction (RT-qPCR) assay was performed to measure the level of miR-144-3p. (B–E) The survival rate and proliferation were elevated in H460/DDP and A549/DDP cells using the MTT assay. (F) Cell apoptosis was examined in H460/DDP and A549/DDP cells using flow cytometry. (G–I) The protein levels of MDR1 and MRP1 in A549/DDP and H460/DDP cells were measured by Western blot. (J, K) Cell migration and invasion abilities were increased in cells transfected with miR-144-3p mimic or negative control (NC) mimic. * p<0.05.
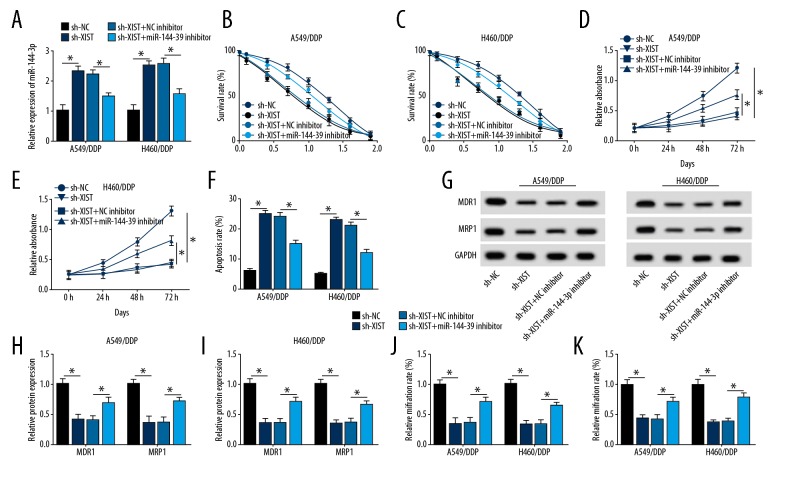
miR-144-3p inhibition reduced sensitivity to DDP induced by XIST knockdown
The negative control (NC) inhibitor or miR-144-3p inhibitor was transfected with sh-XIST or sh-NC into the A549/DDP and H460/DDP cells, which showed that miR-144-3p expression was significantly increased in the sh-XIST group compared with the sh-NC group. However, miR-144-3p expression was significantly decreased in the miR-144-3p inhibitor and the sh- XIST group compared with the sh- XIST and the NC inhibitor group (Figure 5A). The findings of the MTT assay showed that with the increase of DPP concentration, the down-regulation of miR-144-3p significantly reduced sh-XIST inhibition of survival (Figure 5B, 5C). Downregulation of miR-144-3p reduced the effect of low XIST expression on cell proliferation in A549/DDP and H460/DDP cells (Figure 5D, 5E).
Figure 5.
Decreased miR-144-3p reduced chemosensitivity to DDP induced by knockdown of XIST. (A) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed to measure the level of miR-144-3p in A549/DDP cells or H460/DDP cells transfected with short-hairpin-negative control (sh-NC), sh-XIST, sh-XIST and NC inhibitor, and miR-144-3p inhibitor and sh-XIST. (B–E) The cell survival rate (B, C) and cell proliferation rate (D, E) were measured by the MTT assay in A549/DDP cells or H460/DDP cells. (F) Cell apoptosis was examined in the H460/DDP and A549/DDP cells using flow cytometry. (G–I) The protein levels of MDR1 and MRP1 in A549/DDP and H460/DDP cells was measured by Western blot. (J, K) Cell migration and invasion abilities were increased as shown by the transwell assay. *p <0.05.
Flow cytometry showed that down-regulation of XIST significantly increased cell apoptosis in A549/DDP and H460/DDP cells. However, cell apoptosis was reduced by the down-regulation of miR-144-3p expression (Figure 5F). Western blot showed that down-regulation of XIST significantly inhibited the expression of MDR1 and MRP1, while the decreased level of miR-144-3p reversed the inhibitory effect of low XIST expression on MDR1 and MRP1 expression in A549/DDP and H460/DDP cells (Figure 5G–5I). Cell migration and invasion reduced following down-regulation of XIST expression, and this effect was reversed by transfection with miR-144-3p inhibitor (Figure 5J, 5K). Therefore, knockdown of XIST inhibited cell survival, proliferation, migration, and invasion, induced cell apoptosis, and suppressed the expression of MDR1 and MRP1. However, miR-144-3p inhibitor transfection restored the effects of reduced XIST expression on A549/DDP and H460/DDP cells. These results indicate that XIST regulated cell progression in H460/DDP and A549/DDP cells by sponging miR-144-3p, and that XIST may affect sensitivity to DDP in NSCLC cells by inhibiting miR-144-3p.
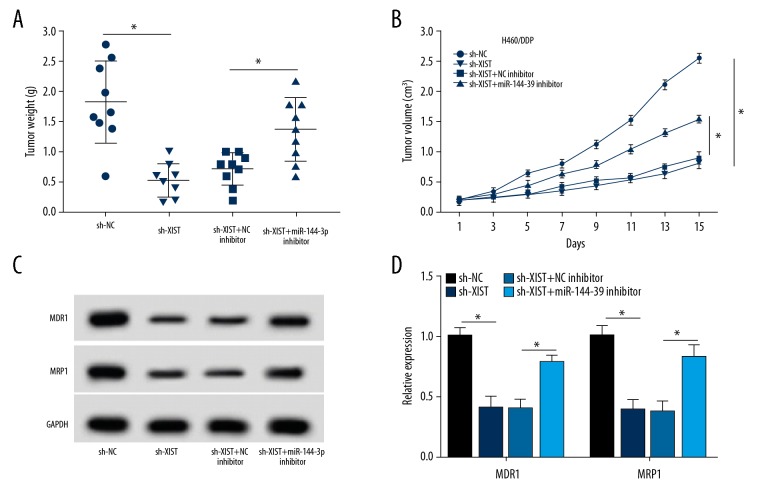
Effects of the XIST/miR-144-3p axis on tumor growth in A549/DDP cells
To determine the biological function of the XIST/miR-144-3p axis in vivo, A549/DDP cells were stably transfected with sh-NC, sh-XIST, sh-XIST and NC inhibitor, and sh-XIST and miR-144-3p inhibitor and were subcutaneously injected into BALB/c nude mice. Tumor volume was measured every three days until 15 days after treatment. As shown in Figure 6A, 6B, tumor volume and weight in the sh-XIST group was lower than that of the sh-NC group, and were increased in the sh-XIST and the miR-144-3p inhibitor group compared with the sh-XIST and NC inhibitor group. Consistent with the in vitro findings, sh-XIST transfection significantly inhibited the expression of MDR1and MRP1, while down-regulation of miR-144-3p expression rescued the effect of XIST knockdown on the expression of MDR1and MRP1 in vivo (Figure 6C, 6D). Therefore, the XIST/miR-144-3p axis affected tumor growth and regulated the expression of MDR1and MRP1 in vivo. The results demonstrated that XIST/miR-144-3p axis regulated tumor growth in NSCLC with DDP treatment.
Figure 6.
The effects of XIST/miR-144-3p axis on tumor growth in A549/DDP cells. (A, B) Tumor weight (A) and volume (B) were calculated in mice in the short-hairpin-negative control (sh-NC), sh-XIST, sh-XIST and NC inhibitor, or sh-XIST and miR-144-3p inhibitor groups. (C, D) The protein levels of MDR1 and MRP1 were measured using Western blot for mice in the sh-NC, sh-XIST, sh-XIST and NC inhibitor, or sh-XIST and miR-144-3p inhibitor groups. * p<0.05.
Discussion
Cisplatin, also known as cis-diamminedichloroplatinum (CDDP) or diamminedichloroplatinum (DDP), is a chemotherapy agent used in the treatment of human malignancy, including advanced non-small cell lung cancer. However, the underlying mechanisms of chemoresistance to cisplatin, or DDP, in NSCLC remains unclear. Therefore, an increased understanding of the mechanism of chemoresistance in NSCLC might improve the selection and efficacy of chemotherapy for patients with advanced NSCLC.
The findings from recently published studies have shown that the expression of long non-coding RNA (lncRNA) X-inactive specific transcript (XIST) is upregulated in NSCLC cells in vitro and in human tumor tissues and that lncRNA-XIST may be involved in the progression of NSCLC and chemoresistance. Knockdown of lncRNA-XIST has been shown to inhibit cell proliferation and TGF-β1-induced apoptosis and enhanced chemosensitivity in NSCLC [8,16,17]. In the present study, the chemoresistant human NSCLC cell lines, H460/DDP and A549/DDP, were transfected with short-hairpin (sh)-XIST to investigate the role of XIST in chemoresistance in NSCLC cells. The findings showed that down-regulation of XIST reduced cell survival of A549/DDP and H460/DDP cells. Knockdown of XIST resulted in suppression of cell proliferation and migration, induced cell apoptosis, and reduced the expression of multidrug resistance-1 (MDR1) and multidrug resistance-associated protein 1 (MRP1) expression in A549/DDP and H460/DDP cells. These findings indicated that knockdown of XIST increased chemosensitivity in A549/DDP and H460/DDP cells.
The interaction between long noncoding RNA (lncRNA) and microRNA (miRNA) affects cell progression, tumor growth, and drug resistance in human cancer, including NSCLC [10,18,19]. Jiang et al. showed that lncRNA-XIST inhibited cell viability and invasion by regulating miT-137/PXN in NSCLC [20]. Li et al. reported that lncRNA-XIST promoted TGF-β1-induced epithelial-mesenchymal transition (EMT) by regulating the miR-367/141-ZEB axis [21]. Also, XIST has previously been shown to exhibits oncogenic properties by targeting miR-449a [22]. However, whether XIST is implicated in chemoresistance in NSCLC by sponging miR-144-3p has been not previously investigated.
In NSCLC, miRNAs also play an important role in tumor cell proliferation and progression, inflammation, and drug resistance [23–25]. For example, miR-34a has been shown to be down-regulated in NSCLC tissues and to inhibit tumor growth [26]. Also, miR-144-3p has been previously shown to be expressed at low level in NSCLC tissues, but the function and regulatory mechanism involved in the effects of miR-144-3p were not investigated [15,18]. In the present study, the results of the luciferase reporter, RNA immunoprecipitation (RIP), and RNA pulldown assays demonstrated that miR-144-3p interacted with XIST. In this study, A549/DDP and H460/DDP cells were transfected with a miR-144-3p mimic to address the effect of miR-144-3p on chemosensitivity in NSCLC. The results showed that upregulation of miR-144-3p decreased cell survival rate, inhibited cell progression, induced apoptosis, and reduced MDR1 and MRP1 expression in A549/DDP and H460/DDP cells. Therefore, upregulation of miR-144-3p increased the chemosensitivity of A549/DDP and H460/DDP cells, suggesting that miR-144-3 was involved in chemoresistance in NSCLC.
However, in the present study, down-regulation of miR-144-3p reduced the effects of inhibition of XIST on A549/DDP and H460/DDP cells. Therefore, XIST regulated chemosensitivity of NSCLC cells by sponging miR-144-3p in vitro. Also, the findings of this study also showed that the XIST/miR-144-3p axis affected tumor growth and that the XIST/miR-144-3p axis regulated chemosensitivity of NSCLC in vivo. These results showed the potential mechanisms for the effects of XIST on chemosensitivity in NSCLC. However, this study only explored the roles of miR-144-3p and XIST in cisplatin chemoresistance in NSCLC, and further future studies are needed to investigate the signaling pathways involved.
Conclusions
The findings of this study showed that long noncoding RNA (lncRNA)-XIST was associated with cisplatin resistance in non-small cell lung cancer (NSCLC) by downregulating miRNA-144-3p in H460/DDP and A549/DDP cells, a murine A549/DDP tumor xenograft, and human tumor tissues. Knockdown of XIST reduced cell proliferation, induced apoptosis, and inhibited the expression of drug-resistance genes by sponging miR-144-3p. A novel axis of XIST/miR-144-3p was shown to be required for the development of chemosensitivity to DDP in NSCLC. These preliminary findings require validation with further prospective clinical studies in patients treated with cisplatin for NSCLC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2017;37(1):630–39. doi: 10.1080/14737140.2018.1409624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388:1012–24. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 3.Xiao JLY, Jin F, et al. LncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell Physiol Biochem. 2017;43(5):1926–38. doi: 10.1159/000484116. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Wang W, Chen R, et al. Exosome-mediated transfer of lncRNA-SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int J Oncol. 2018;53(3):1013–26. doi: 10.3892/ijo.2018.4467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Han P, Li J, Zhang B, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol Cancer. 2017;16:9. doi: 10.1186/s12943-017-0583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si X, Zang R, Zhang E, et al. LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget. 2016;7:81452–62. doi: 10.18632/oncotarget.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du P, Zhao H, Peng R, et al. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci Rep. 2017;37 doi: 10.1042/BSR20170696. BSR20170696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun W, Zu Y, Fu X, et al. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol Rep. 2017;38:3347–54. doi: 10.3892/or.2017.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Z, Li Y, Huang K, et al. Regulation of miR-19 to breast cancer chemoresistance through targeting PTEN. Pharm Res. 2011;28:3091–100. doi: 10.1007/s11095-011-0570-y. [DOI] [PubMed] [Google Scholar]
- 10.Park EY, Chang E, Lee EJ, et al. Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance. Cancer Res. 2014;74:7573–82. doi: 10.1158/0008-5472.CAN-14-1140. [DOI] [PubMed] [Google Scholar]
- 11.Lv L, Li Y, Deng H, et al. MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. Cancer Lett. 2015;357:105–13. doi: 10.1016/j.canlet.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Pang L, Lu J, Huang J, et al. Upregulation of miR-146a increases cisplatin sensitivity of the non-small cell lung cancer A549 cell line by targeting JNK-2. Oncol Lett. 2017;14:7745–52. doi: 10.3892/ol.2017.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Su C, Chen Y, et al. MiR-144-3p promotes the tumor growth and metastasis of papillary thyroid carcinoma by targeting paired box gene 8. Cancer Cell Int. 2018;18:54. doi: 10.1186/s12935-018-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Li X, Zhang D, et al. IL-1β-mediated up-regulation of WT1D via miR-144-3p and their synergistic effect with NF-κB/COX-2/HIF-1α pathway on cell proliferation in LUAD. Cell Physiol Biochem. 2018;48:2493–502. doi: 10.1159/000492687. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Ma Y, Jiang Z, et al. Lico a causes ER stress and apoptosis via up-regulating miR-144-3p in human lung cancer cell line H292. Front Pharmacol. 2018;9:837. doi: 10.3389/fphar.2018.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You B, Fu Y, Li X, et al. Long non-coding RNA Xist promotes progression of non-small-cell lung cancer (NSCLC) by modulating miR-103a and MAP3K3 pathway. Int J Clin Exp Pathol. 2017;10:1348–55. [Google Scholar]
- 17.Wang X, Zhang G, Cheng Z, et al. Knockdown of LncRNA-XIST suppresses proliferation and TGF-β1-induced EMT in NSCLC through the Notch-1 pathway by regulation of miR-137. Genet Test Mol Biomarkers. 2018;22:333–42. doi: 10.1089/gtmb.2018.0026. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Shi H, Gao M, et al. Long non-coding RNA CASC2 improved acute lung injury by regulating miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell Biosci. 2018;8:15. doi: 10.1186/s13578-018-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J, Lv Y, Jin F, et al. LncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell Physiol Biochem. 2017;43:1926–38. doi: 10.1159/000484116. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Zhang H, Hu X, et al. Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Int J Biol Macromol. 2018;111:623–31. doi: 10.1016/j.ijbiomac.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Wan L, Liu Z, et al. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018;418:185–95. doi: 10.1016/j.canlet.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YL, Li XB, Hou YX, et al. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. Acta Pharmacol Sin. 2017;38:443. doi: 10.1038/aps.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei L, Huang Y, Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep. 2013;30:2897–902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Lin Z, Kai F, et al. miR-27b targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol Cell Biochem. 2014;390:85–91. doi: 10.1007/s11010-013-1959-1. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Zhang B, Sun X, et al. MiR-204 inhibits human NSCLC metastasis through suppression of NUAK1. Br J Cancer. 2014;111:2316–27. doi: 10.1038/bjc.2014.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Liu C, Liu X, et al. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9:e90022. doi: 10.1371/journal.pone.0090022. [DOI] [PMC free article] [PubMed] [Google Scholar]