Abstract
Background.
The authors’ aim in this systematic review was to evaluate the validity of using preoperative serum C-terminal cross-linking telopeptide (CTX) levels as a predictive factor of increased risk of developing medication-related osteonecrosis of the jaw (MRONJ) in patients receiving bisphosphonate (BP) therapy who underwent invasive dental procedures.
Types of Studies Reviewed.
The authors searched PubMed, MEDLINE, and Web of Science and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. The authors conducted a meta-analysis on the risk ratio. The authors used the methodological index for nonrandomized studies and Quality Appraisal of Reliability Studies checklist to assess quality.
Results.
The authors included 18 clinical trials involving 2,301 patients. Most patients received alendronate or risedronate for an average of 62.14 months. The average serum CTX level in patients who received BP before surgery was 198.25 picograms per milliliter. Meta-analysis results showed that the cutoff in CTX level (150 pg/mL) was not predictive of MRONJ risk. The sensitivity of CTX values lower than 150 pg/mL was 34.26%, and the specificity was 77.08%.
Conclusions and Practical Implications.
The use of CTX levels to diagnose MRONJ risk after dental procedures in patients receiving BP is not justified. The cutoff of 150 pg/mL in serum CTX levels is not predictive of MRONJ. Further studies are needed to develop other reliable biomarkers.
Keywords: Bisphosphonate-associated osteonecrosis, clinical protocols, alveolar bone, bone
To our knowledge, the first report of bisphosphonate (BP)-related osteonecrosis of the jaw (BRONJ) was published in 2003.1 In 2009, the American Association of Oral and Maxillofacial Surgeons (AAOMS) defined BRONJ as exposed or necrotic bone in the maxilla or mandible that has persisted for more than 8 weeks in a patient with a history of BP use who has no history of radiotherapy in the head and neck region.2,3 In a 2014 position article, AAOMS reported that clinicians should consider BRONJ in patients receiving BP therapy who seek care for exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for more than 8 weeks (stage 1) or any nonspecific clinical or radiologic findings of osteonecrosis of the jaw, even without visibly exposed bone (stage 0).2,4 Reports of these severe adverse effects, as well as atypical femoral fractures, have led to a substantial decrease in the prescription rates of BPs, in what has been termed a “crisis in osteoporosis treatment.”5 AAOMS recommended changing the nomenclature from BRONJ to medication-related osteonecrosis of the jaw (MRONJ) to accommodate the substantial increase in osteonecrosis of the jaw with other antiresorptive and antiangiogenic therapies.2
The prevalence of MRONJ ranges from 0.7% to 12% in patients receiving intravenous (IV) BP6 to 0.01% to 0.1% in patients receiving oral BPs.6–9 Risk factors for developing MRONJ can be drug related, local, systemic, or genetic.3 Study investigators listed the most significant risk factors as tooth extraction,10 preexisting dental or periodontal disease,11 cumulative doses of IV BPs,12–14 mandibular location,3 smoking,15 and concomitant steroid use.3,10–17
Osteonecrosis and related infections significantly decrease patients’ quality of life, with no definitive strategy for prevention or management.18 Clinicians usually recommend that patients considered at risk should be dentally fit before starting any BP therapy.19,20 In addition, clinicians should examine patients with dentures frequently for any oral mucosa trauma.21,22 Although there is no established consensus, some investigators recommended that treatment should be discontinued after dental extraction or other oral surgeries until the extraction site fully heals.20,22
Investigators have used tests of the serum levels of C-terminal cross-linking telopeptide (CTX) reliably as a biomarker for bone turnover.23 During bone resorption, type I collagen is degraded, releasing CTX, so when bone turnover is suppressed by a BP, then the CTX level is expected to decrease. However, there is wide individual variation in the effect of the same dose of BP on CTX levels.24 Marx and colleagues25 reported that the fasting serum level of CTX was a useful tool for risk assessment and treatment planning. However, their study was based on a small sample size of 30 patients receiving only oral BPs. They reported that fasting CTX levels higher than 150 picograms per milliliter were associated with minimal risk, 100 through 150 pg/mL with moderate risk, and 100 pg/mL with high risk of developing osteonecrosis after dentoalveolar surgery. Consequently, they recommended that clinicians should not perform surgeries in patients with CTX levels lower than this cutoff and that they should suspend BP therapy for several months until a CTX level of 150 pg/mL or higher is achieved.25
Investigators have proposed measuring the CTX level as a possible predictive and prognostic test for monitoring the BP inhibition of bone turnover. Because investigators suggested the inhibition of such bone turnover as the main cause for MRONJ, they have used low levels of CTX as an indication of high susceptibility to MRONJ. However, investigators have reached no consensus about the extent of its actual accuracy and reliability. Our aim in this article was systematically to review the existing literature and analyze the quality of evidence for and against the use of CTX levels (lower than versus higher than 150 pg/mL) as a predictive biomarker for MRONJ risk.
METHODS
In this systematic review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.26 This review also fulfilled the quality checklist as reported in the Cochrane Handbook for Systematic Reviews of Interventions.27
Population, intervention, comparison, outcome criteria
Population: patients receiving BPs who were scheduled for oral or dental surgeries;
Intervention: CTX level measurement before the oral surgical procedures;
Comparison: patients who did not receive BP treatment;
- Outcomes:
- primary outcome: risk of developing MRONJ after dentoalveolar surgery in patients with preoperative CTX levels lower than 150 pg/mL;
- secondary outcomes: difference in CTX levels between patients who developed MRONJ and those who did not and calculation of the sensitivity and specificity of the CTX level cutoff as a predictive tool for MRONJ development in patients receiving oral BPs versus that in patients receiving IV BPs.
Inclusion criteria
We considered for inclusion all prospective and retrospective observational cohort studies (case-control, cohort, cross-sectional) published in the English language in which the authors investigated the usefulness of serum CTX levels as a predictive test for developing MRONJ. Exclusion criteria included language other than English, article not being available, or data or study being duplicate. We excluded some articles by reviewing the inclusion criteria in the title or abstract. All other studies required full-text review to determine relevance.
Exclusion criteria
We excluded nonhuman studies, narrative reviews, and predictive interventions not including measuring CTX levels. Study inclusion required the reviewers (M.E.A., C.S., J.J.) to agree. The 3 reviewers resolved disagreements by means of direct communication among themselves.
Search methodology
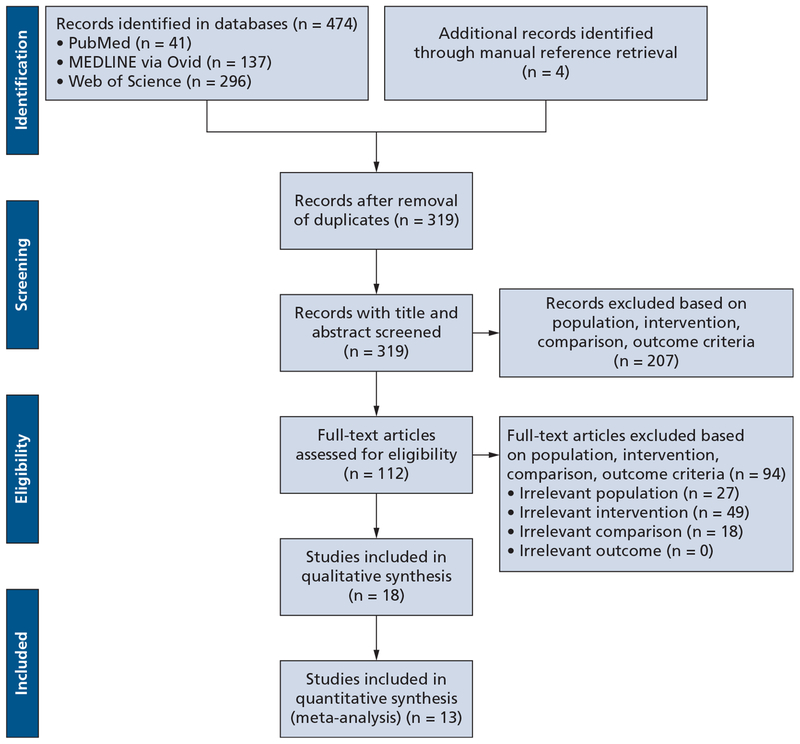
On August 25 and 26, 2018, we performed a detailed comprehensive literature search for all relevant articles in 3 online databases: PubMed, MEDLINE via Ovid, and Web of Science. The Boolean operations and key words we used for the search were “Bisphosphonate-Associated Osteonecrosis of the Jaw”[Medical Subject Headings] AND “Marker”[All Fields] AND “Bisphosphonate-Associated Osteonecrosis of the Jaw”[Medical Subject Headings] AND “collagen type I trimeric cross-linked peptide”[Supplementary Concept]. To ensure inclusion of all available relevant evidence, we also searched the references of available studies for any studies that met our inclusion criteria. Figure 1 is a flowchart showing study identification, inclusion, and exclusion and studies requiring manual retrieval.
Figure 1.
Flowchart showing study identification, inclusion, and exclusion.26
Data extraction
Two of us (M.E.A., J.J.) extracted information from all eligible publications independently. We established a data collection sheet to sort quantitative and qualitative information for our analysis. We extracted the data from the clinical trials by using the following variables: demographic characteristics (author; study design; year of publication; patient number, age, and sex), BP therapy (type, route, duration), surgical procedure, and CTX level characteristics.
Quality assessment of included studies
We used the methodological index for nonrandomized studies (MINORS)28 to assess the quality of the included studies. We scored 12 items as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). Two reviewers (M.E.A., C.S.) independently assessed the quality of the included studies.
Assessment based on Quality Appraisal of Reliability Studies checklist
We used the Quality Appraisal of Reliability Studies (QAREL) checklist29 on included studies to identify their reliability according to 11 items. The QAREL checklist provides a quality assessment for the spectrum of participants and examiners, examiner blinding, order effects of examination, suitability of the time interval among repeated measurements, appropriate test application and interpretation, and appropriate statistical analysis. The checklist also can help assess the variability in performance and reporting of physical examination procedures. We classified the quality of the included studies on the basis of QAREL scores: high quality for a score of 67% or more, moderate quality for a score of 50% through 66%, and low quality for a score of less than 50%.
Synthesis of results
We used standard meta-analytic methods to combine the results of all studies that provided sufficient data to obtain overall effect size estimates and the corresponding forest plots. In the presence of homogeneity (I2 < 50%), we used a fixed-effects model to estimate the overall effects. If there was significant heterogeneity among included studies, we used a random-effects model. We performed the meta-analysis by using software (Review Manager, Version 5.3, Cochrane Community).
Statistical analysis
We also used software (Prism, Version 5.0.0, graphpad) to analyze the data. We reported descriptive statistics such as the mean, standard deviation, range, median, and interquartile range. We used a Pearson correlation coefficient for normally distributed data. We generated mean differences with their corresponding 95% confidence intervals (CIs) for continuous outcome data and calculated I2 values to estimate heterogeneity among the included studies. We analyzed sensitivity and specificity by using an online tool (MedCalc Diagnostics, MedCalc Software).29
RESULTS
Search results and study selection
Using the previously mentioned key words, we obtained 474 relevant citations from the 3 online databases (PubMed, MEDLINE via Ovid, and Web of Science). The selection process yielded 18 clinical trials25,30–46 after removal of duplicates and further screening. These 18 clinical trials25,30–46 involved patients who had a history receiving BP therapy and who were scheduled for oral or dental surgery. These 18 studies included 6 prospective observational cohort studies, 3 retrospective observational cohort studies, 6 case-control studies, and 3 case series (eTable 1).25,30–46
Included studies
The search involved data from 2,301 patients enrolled in the 18 clinical trials.25,30–46 The mean age across the 18 trials25,30–46 was 67.6 years. Most of the recruited patients were receiving BPs for the treatment of osteoporosis or to reduce skeletal-related events, such as pathologic fractures, metastasis, or hypercalcemia, associated with malignancy (eTable 1).25,30–46
Prospective Cohort Studies
Six studies31–36 included 1,338 patients with a mean age of 66.8 years. Ninety-six percent of these patients received oral BPs for an average therapy duration of 45.6 months before study enrollment. The underlying conditions for BP indication were osteoporosis, bone metastasis, and multiple myeloma, in 97%, 3%, and 1%, respectively. Sixty percent and 38% of patients received alendronate and risedronate, respectively. Zoledronate, ibandronate, and pamidronate were administered in 2% of patients. Most of the included patients were scheduled for tooth extraction, and the others were scheduled for other minor surgeries (for example, implant placement or grafts).
Retrospective Cohort Studies
Three studies37–39 included 186 patients with a mean age of 75.6 years. All patients received oral BPs for an average therapy duration of 47.7 months. The underlying conditions for BP indication were osteoporosis, bone metastasis, and multiple myeloma, in 97%, 3%, and 1% of patients, respectively. All patients received alendronate and risedronate. Most of the included patients were scheduled for tooth extraction.
Case-Control Studies
Six studies30,40–44 included 677 patients with a mean age of 68 years. Eighty percent of these patients received oral BPs for an average therapy duration of 55.7 months. The underlying conditions for BP indication were osteoporosis, bone metastasis, and multiple myeloma in 77%, 15%, and 6% of patients, respectively. Forty percent and 33% of patients received alendronate and risedronate, respectively. Zoledronate and pamidronate were administered in 17% and 10% of patients, respectively.
Case Series
Three studies25,45,46 included 100 patients with a mean age of 63 years. Eighty percent received oral BPs for an average therapy duration of 47.6 months, and 20% received IV BP. The underlying conditions for BP indication were osteoporosis, bone metastasis, and multiple myeloma in 60%, 24%, and 14% of patients, respectively.
Investigators measured serum CTX levels in the included patients who were scheduled for oral or dental surgeries. The average of serum CTX level of patients receiving BPs across the included studies before surgery was 198.25 pg/mL (range, 87.3–316.51 pg/mL).
Most patients had long history of BP therapy and had undergone an oral or dental procedure. Approximately 90% of procedures were tooth extractions. Approximately 2% of patients underwent implant placement, and 7% underwent other procedures, such as bone graft or mucogingival surgery.
Levels of evidence
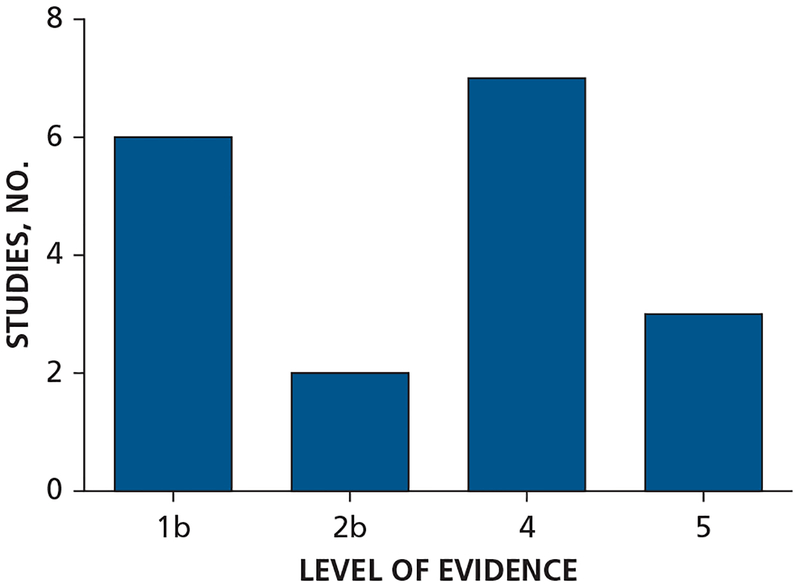
According to the Oxford Centre for Evidence-Based Medicine’s levels of evidence scheme,47 most studies were of level 4 evidence (7 studies). The rest of the studies were of level 1b evidence (6 studies), level 2b evidence (2 studies), and level 5 evidence (3 studies) (Figure 2).47
Figure 2.
Level of evidence of the included studies according to the Oxford Centre for Evidence-Based Medicine’s levels of evidence scheme.47
MINORS
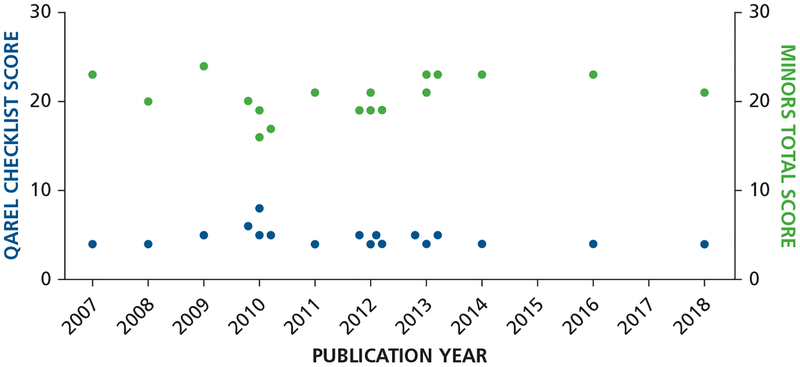
Although nonrandomized trials are lower in the hierarchy of evidence than are randomized controlled trials (RCTs), the quality of most of the included non-RCTs was satisfactory on the basis of the MINORS scale, with a mean (standard deviation) score of 20.38 (2.29) (total, n = 24) and a range of 16 through 24 (mean difference, 0.97; 95% CI, 19.4 to 21.36). eTable 228 shows the average MINORS methodology score for each item and the total MINORS methodology score. The following parameters were the most likely to receive a lower score:
prospective collection of data: the investigators had not collected most of the data according to an established protocol before the beginning of the study;
unbiased assessment of the study end point: the investigators did not use blinding in the evaluations and did not state clearly the rationale for not doing so;
prospective calculation of the study size: the investigators did not perform a power analysis before starting the study;
contemporary groups: there were some historical comparisons (that is, control and study groups were not treated at the same time).
Assessment based on QAREL checklist
Although the included studies showed a high average score on the MINORS scale, we appraised the methodological quality and reliability of the included studies by using the QAREL checklist (Figure 3).28,29 The total ratings of the methodological quality of reliability studies ranged from 4 through 8, of a total score of 11. Among the 18 studies included, 1 study was of high quality, 1 study was of moderate quality, and 16 studies were of low quality (eTable 3).29
Figure 3.
Total methodological index for nonrandomized studies (MINORS) score28 and Quality Appraisal of Reliability Studies (QAREL) checklist score29 for the included studies plotted against the publication year.
Results of meta-analysis
Nonstratified Analysis
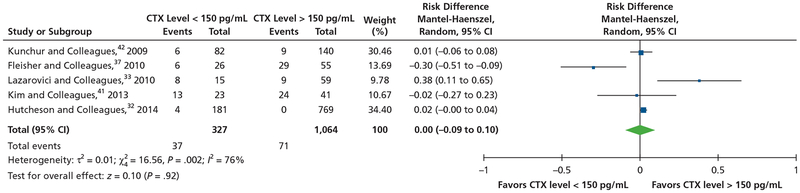
For the meta-analysis of a serum CTX level of 150 pg/mL as a risk factor for the development of MRONJ, investigators in 5 studies32,33,37,41,42 reported data on the number of patients who developed MRONJ in both CTX levels lower than 150 pg/mL group and CTX levels higher than 150 pg/mL. There was significant statistical heterogeneity among the 5 studies (I2 76%; P = .002). When we used the random-effects model, the pooled results of the 5 studies showed no significant difference in the prevalence of MRONJ between patients with CTX levels lower than 150 pg/mL and patients with CTX levels higher than 150 pg/mL (risk ratio, 0.00; 95% CI, 0.09 to 0.10; z = 0.1; P = .92) (Figure 4).32,33,37,41,42
Figure 4.
Meta-analysis of serum C-terminal cross-linking telopeptide (CTX) level of 150 picograms per milliliter as a risk factor for development of medication-related osteonecrosis of the jaw. CI: Confidence interval.
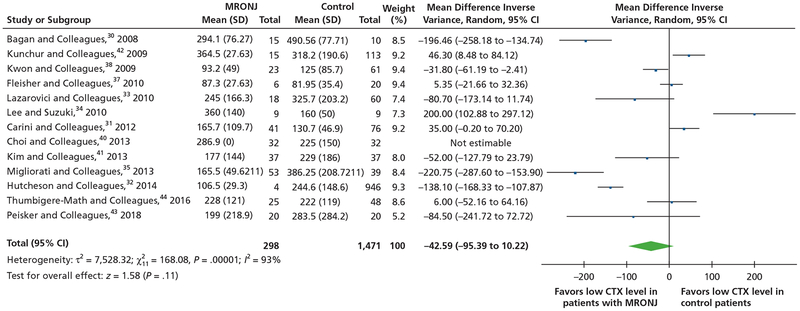
For the meta-analysis of the mean difference of serum CTX levels between patients who developed MRONJ and patients who did not, investigators in 13 studies,30–33,35,37–44 which included 2,015 patients, reported the data of mean serum CTX levels between patients with MRONJ and control participants. There was significant statistical heterogeneity among the 13 studies (I2 = 93%; P < .00001). When we used the random-effects model, the pooled results did not show a significant difference in mean CTX levels between the 2 groups (mean difference, −42.59; 95% CI, −95.39 to 10.22; z = 1.58; P = .11) (Figure 5).30–35,37,38,40–44
Figure 5.
Meta-analysis of mean difference of serum C-terminal cross-linking telopeptide (CTX) levels between patients who developed medication-related osteonecrosis of the jaw (MRONJ) and patients who did not. CI: Confidence interval.
Stratified Analysis
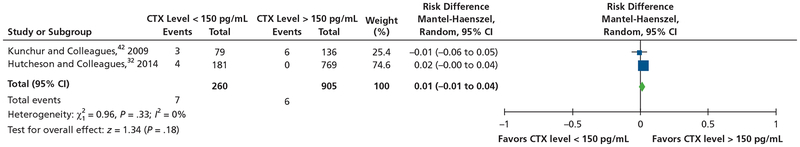
For the meta-analysis of a serum CTX level of 150 pg/mL as a risk factor for the development of MRONJ in the population receiving oral BPs, investigators in only 2 studies32,42 reported data on the number of patients receiving oral BPs who developed MRONJ in both CTX levels lower than 150 pg/mL and CTX levels higher than 150 pg/mL. There was no significant heterogeneity across both studies (I2 = 0%; P = .33). When we used the fixed-effects model, the pooled results of the 2 studies showed no significant difference in the prevalence of MRONJ between patients with CTX levels lower than 150 pg/mL and that in patients with CTX levels higher than 150 pg/mL (risk ratio, 0.01; 95% CI, −0.01 to 0.04; z = 1.34; P = .18) (Figure 6).32,42
Figure 6.
Meta-analysis of serum C-terminal cross-linking telopeptide (CTX) level of 150 picograms per milliliter as a risk factor for development of medication-related osteonecrosis of the jaw in the population receiving oral bisphosphonate. CI: Confidence interval.
Sensitivity of CTX level lower than 150 pg/mL as a predicting tool for MRONJ
Nonstratified Analysis
Investigators in 5 studies32,33,37,41,42 (2 prospective, 2 case-control, and 1 retrospective) reported the prevalence of MRONJ development in patients with CTX levels lower and higher than 150 pg/mL (eTable 4).32–34,36,37,41,42 The sensitivity of a CTX level lower than 150 pg/mL as a predicting tool for MRONJ across the studies was 34.26%, and the specificity was 77.08%. The average positive and negative likelihood ratios were 1.49 and 0.85, respectively (eTable 4).32–34,36,37,41,42 eTable 525,30–46 presents the correlation of CTX levels lower than 150 pg/mL with MRONJ according the included studies.
Stratified Analysis
eTable 632,33,35,36,42,44 presents the subgroup stratified analysis and correlation between serum CTX levels and the prevalence of MRONJ in patients receiving oral BPs versus that in patients receiving IV BP. Investigators in 3 studies34,36,42 reported the prevalence of MRONJ in patients receiving oral BPs for an average therapy duration of 41.3 months for those with CTX levels lower than and those with CTX levels higher than 150 pg/mL. The sensitivity of CTX levels lower than 150 pg/mL as a predicting tool for MRONJ across the studies was 33.33%, and the specificity was 63.33%. The average positive and negative likelihood ratios were 0.91 and 1.05, respectively (eTable 4).32–34,36,37,41,42
Investigators in 2 studies36,42 reported the prevalence of MRONJ in patients receiving IV BPs for an average therapy duration of 17.8 months for those with CTX levels lower and those with CTX levels higher than 150 pg/mL. The sensitivity of a CTX level lower than 150 pg/mL as a predicting tool for MRONJ across the studies was 50%, and the specificity was 66.67%. The average positive and negative likelihood ratios were 1.50 and 0.75, respectively (eTable 4).32,34,36,37,41,42
DISCUSSION
Our primary aim in this systematic review and meta-analysis was to test the reliability of preoperative CTX levels lower than 150 pg/mL as a risk indicator for development of MRONJ after an invasive dental procedure in patients receiving BP treatment. The secondary outcomes were to test the difference in CTX levels between patients who developed MRONJ and those who did not and to extract from eligible studies the sensitivity and specificity of the CTX level cutoff in predicting subsequent MRONJ.
CTX is a by-product of the breakdown of type I collagen; therefore, investigators have used its serum levels as a biomarker of the rate of bone remodeling in clinical and experimental settings.48 The use of CTX levels to indicate MRONJ risk in patients undergoing antiresorptive treatment was based on the premise that the primary pathogenic mechanism of this adverse effect was drug-related impairment in bone remodeling, which causes an impaired repair mechanism after dental procedures, eventually leading to bone necrosis. Marx and colleagues25 suggested that blood levels of CTX progressively decreased with antiresorptive treatment and increased after the treatment stopped. Investigators proposed a serum level lower than 100 pg/mL as an indication of high risk and a serum level higher than 150 pg/mL as an indication of low risk.25,49,50 Many clinicians all over the world continue to measure CTX serum levels routinely before dental procedures in patients receiving antiresorptive treatment. However, there has been wide variability in reference values, which makes the CTX test difficult to apply across different ages and sexes. There is no reference range for women older than 50 years or for any patient older than 68 years.51–53 In addition, the variability in baseline CTX levels and the dose patterns of antiresorptive agents across studies makes it difficult to compound data from different studies.52
In our study, we found no statistically significant difference in preoperative CTX levels between patients who developed MRONJ and patients who did not. The quality of the evidence was acceptable, despite the low number of prospective studies with matched control participants. Investigators in most of the studies did not match the participants for age, sex, and other confounding variables, a common limitation in clinical studies. Heterogeneity between the studies was also significant, signifying that the experimental conditions were variable between studies. Therefore, we used a random-effects model for meta-analysis.
In a 2016 systematic review, Enciso and colleagues54 reached the same conclusion; they provided an update, stratified the BP populations according to route of administration, and reported the sensitivity and specificity calculations in the stratified groups. Furthermore, they provided quality analysis of the literature. Patients who developed MRONJ tended to have lower preoperative CTX levels than did patients who did not, although the difference was not statistically different. The clinical relevance of this finding, however, is worth noting, because such perceived differences seem to have influenced the clinical decision to continue using CTX testing, especially given the lack of a reliable alternative biomarker. A close look at the distribution of CTX levels in the included studies reveals that MRONJ developed in many patients with CTX levels remarkably higher than 150 pg/mL. Across the included trials, the average serum CTX level in patients receiving BP before surgery was 198.25 pg/mL, suggesting that a cutoff value of 150 pg/mL may be too low. Furthermore, many patients with low CTX levels did not develop any complications. Together, these findings bring into question the basic assumption that an impairment in systemic bone remodeling primarily causes MRONJ. Investigators in many studies have suggested that localized and patient-specific factors may play an important role in the pathogenesis.55,56
Our study also involved the calculation of sensitivity, specificity, and positive and negative likelihood ratios of the CTX level cutoff of 150 pg/mL. The sensitivity of the test was low, but the specificity was more promising. However, the positive and negative likelihood ratios were also low, and their ranges involved the ratio of 1. The mean CTX level was lower in the population receiving oral BPs than in the population receiving IV BP. The CTX test appeared to have higher sensitivity in the population receiving IV BP, likely because of the small number of patients in this group. Taken together, the overall value of this cutoff in predicting MRONJ becomes unjustified. Some investigators questioned the validity of the test itself, regardless of the cutoff value. Lee and Suzuki34 reported that 21 patients had preoperative CTX levels lower than 100 pg/mL and elected to proceed with surgery without a drug holiday. Postoperatively, none of these patients developed MRONJ. There are no RCTs in the literature addressing this question. Although the quality of the non-randomized studies was acceptable on the MINORS scale, the QAREL checklist showed that most of these studies lack adequate reliability.
In patients with low CTX levels, the investigators typically initiated a drug holiday to bring the levels up to the “safe” range. However, multiple study investigators questioned the validity of this strategy.2,57 In 2017, the American Academy of Oral Medicine stated that there was not enough evidence to justify the use of CTX levels in predicting the risk of developing MRONJ after a dental procedure.58
The timing of CTX level measurement was variable in the available studies. Investigators in a few studies did not specify the exact timing for CTX level measurement at all; none of which were included in the primary outcome analysis. Investigators in most of the included studies mentioned that the CTX test was performed the morning before the surgery. Investigators in 2 studies measured CTX levels as part of a regular osteoporotic examination or at the first visit, not immediately before surgery.25,38
In 2007, Marx and colleagues25 reported that the fasting serum level of CTX was a useful tool for risk assessment and treatment planning in 30 patients receiving oral BPs. However, this small sample size could not represent the overall population, and their conclusion might have been biased. The preliminary results Bagan and colleagues30 presented showed a decreased CTX serum level in patients with MRONJ, but they recommended further studies to confirm the cutoff value proposed by Marx and colleagues.25 Kwon and colleagues38 found a significant correlation between the severity pf MRONJ and the risk assessment using serum CTX levels. However, Kunchur and colleagues42 found that CTX test results were not predictive of MRONJ development in individual patients, although the test may be useful for identifying those at risk and assisting in the development of a treatment plan that proceeds accordingly.
In 2010, Lazarovici and colleagues33 found that a CTX level lower than 150 pg/mL was associated significantly with MRONJ development. However, the authors cautiously concluded that the CTX level was not a definitive predictor of the development of MRONJ, although it may be an adjuvant marker in risk assessment. Furthermore, Lee and Suzuki34 argued that the original study by Marx and colleagues25 was underpowered and that the results showed that there was no precise and direct correlation between the presurgical values of the morning fasting serum level of CTX and the risk of a patient developing MRONJ after the procedure. Fleisher and colleagues37 suggested that radiographic periodontal ligament widening may be a better indicator of the risk of developing MRONJ after surgery than is CTX testing in a patient receiving BP treatment.
Atalay and colleagues45 used the CTX level cutoff to categorize patients receiving BP treatment before dental procedures and found no significant correlation between serum CTX levels and healing. Similarly, investigators in other studies found no correlation between CTX levels and osteonecrosis.35,36,39,46
Conversely, Carini and colleagues31 maintained that the CTX level is still a relevant indicator for the risk of developing osteonecrosis and should be used as a consideration in treatment planning. In a prospective study, Hutcheson and colleagues32 found that a CTX level lower than 150 pg/mL could be associated with a 3-fold greater risk of developing MRONJ.
Thumbigere-Math and colleagues44 found no difference in serum markers in patients with an MRONJ diagnosis who had discontinued IV BP therapy (drug holiday) versus those in a control group of patients with cancer without MRONJ. However, when they accounted for age and total number of BP infusions, the levels of inflammation and angiogenesis markers appeared to be different.
There are some limitations in our meta-analysis because of detectable biases in the included studies. Language bias may be 1 of these limitations in this review because we included only studies published in the English language. However, there is no evidence of a systematic bias as a result of the language restrictions in medical meta-analyses.59,60 Most included studies lacked correlations among the dosage, route, and duration of treatment and MRONJ incidence. Reporting outcome measures without stratification according to these important risk factors could be misleading. Further studies are needed to address this knowledge gap. The lack of information about the surgical procedures in the included studies is 1 of the limitations of this review. The individual risk ratio for developing MRONJ remains undetermined for each invasive procedure, such as multiple surgical extractions of affected teeth, implant placement, bone grafting, and mucogingival surgery. However, dentoalveolar surgeries are convenient to reduce bone manipulation to a minimum and are associated with good healing despite being invasive dental procedures.61 Also, as outlined earlier, heterogeneity may reflect an effect of confounding variables such as the dose and length of administration of BPs, surgical approaches, and the timing of CTX level measurement. Finally, we included only 6 prospective studies, which limited the sample size of patients.
CONCLUSIONS
Although investigators have used CTX level measurements widely for identifying patients at risk of developing MRONJ, the strategy cannot be justified by evidence. Specifically, the proposed CTX level cutoff of 150 pg/mL is not associated with an increase in the prevalence of osteonecrosis. There is a dire need for developing other more reliable biomarkers and preventive strategies in these patients, ideally without sacrificing the therapeutic benefits of antiresorptive medications. Further studies, including well-designed RCTs with sufficiently large sample sizes, are needed.
Supplementary Material
Acknowledgments
Dr. Elsalanty received program project grant 1R15DE025134-01 from the National Institute of Dental and Craniofacial Research, National Institutes of Health.
ABBREVIATION KEY
- AAOMS
American Association of Oral and Maxillofacial Surgeons
- BP
Bisphosphonate
- BRONJ
Bisphosphonate-related osteonecrosis of the jaw
- CTX
C-terminal cross-linking telopeptide
- IV
Intravenous
- MINORS
Methodological index for nonrandomized studies
- MRONJ
Medication-related osteonecrosis of the jaw
- QAREL
Quality Appraisal of ReliabilityStudies
- RCT
Randomized controlled trial
Footnotes
Disclosure. None of the authors reported any disclosures.
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Supplemental material is available online.
SUPPLEMENTAL DATA
Supplemental data related to this article can be found at: https://doi.org/10.1016/j.adaj.2019.03.006.
Contributor Information
Mohamed E. Awad, Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta University, Augusta, GA..
Christina Sun, Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta University, Augusta, GA..
Joshua Jernigan, Dental College of Georgia, Augusta University, Augusta, GA..
Mohammed Elsalanty, Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta University, 1120 15th St., Room CB 2404E, Augusta, GA 30904,.
References
- 1.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–1117. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw: 2014 update [published corrections appear in J Oral Maxillofac Surg. 2015;73(7):1440 and 2015;73(9):1879]. J Oral Maxillofac Surg. 2014;72(10):1938–1956. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw: 2009 update. Aust Endod J. 2009;35(3):119–130. [DOI] [PubMed] [Google Scholar]
- 4.Mawardi H, Treister N, Richardson P, et al. Sinus tracts: an early sign of bisphosphonate-associated osteo-necrosis of the jaws? J Oral Maxillofac Surg. 2009;67(3): 593–601. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res. 2016;31(8):1485–1487. [DOI] [PubMed] [Google Scholar]
- 6.Bedogni A, Bettini G, Totola A, Saia G, Nocini PF. Oral bisphosphonate-associated osteonecrosis of the jaw after implant surgery: a case report and literature review. J Oral Maxillofac Surg. 2010;68(7): 1662–1666. [DOI] [PubMed] [Google Scholar]
- 7.Ikebe T. Pathophysiology of BRONJ: drug-related osteoclastic disease of the jaw. Oral Sci Int. 2013; 10(1):1–8. [Google Scholar]
- 8.Lo JC, O’Ryan FS, Gordon NP, et al. ; for the Predicting Risk of Osteonecrosis of the Jaw with Oral Bisphosphonate Exposure (PROBE) Investigators. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010; 68(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65(3): 415–423. [DOI] [PubMed] [Google Scholar]
- 10.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;24(6):945–952. [DOI] [PubMed] [Google Scholar]
- 11.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research [editorial]. J Bone Miner Res. 2007;22(10):1479–1491. [DOI] [PubMed] [Google Scholar]
- 12.Bilezikian JP. Osteonecrosis of the jaw: do bisphosphonates pose a risk? N Engl J Med. 2006;355(22):2278–2281. [DOI] [PubMed] [Google Scholar]
- 13.Hoff AO, Toth BB, Altundag K, et al. Osteonecrosis of the jaw in patients receiving intravenous bisphospho-nate therapy [abstract 8528]. J Clin Oncol. 2006;24(18 suppl). [Google Scholar]
- 14.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23(6):826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66(4):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Goodger NM, Pogrel MA. Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg. 2003;61(9):1104–1107. [DOI] [PubMed] [Google Scholar]
- 17.Villa A, Castiglioni S, Peretti A, Omodei M, Ferrieri GB, Abati S. Osteoporosis and bisphosphonate-related osteonecrosis of the jaw bone. ISRN Rheumatol. 2011;2011:654027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miksad RA, Lai KC, Dodson TB, et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist. 2011;16(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer JS, Beck N, Kiefer J, Stockmann P, Wichmann M, Eitner S. Awareness and education of patients receiving bisphosphonates. J Craniomaxillofac Surg. 2012;40(3):277–282. [DOI] [PubMed] [Google Scholar]
- 20.Patel V, McLeod NM, Rogers SN, Brennan PA. Bisphosphonate osteonecrosis of the jaw: a literature review of UK policies versus international policies on bisphosphonates, risk factors and prevention. Br J Oral Maxillofac Surg. 2011;49(4):251–257. [DOI] [PubMed] [Google Scholar]
- 21.Reich W, Bilkenroth U, Schubert J, Wickenhauser C, Eckert AW. Surgical treatment of bisphosphonate-associated osteonecrosis: prognostic score and long-term results. J Craniomaxillofac Surg. 2015;43(9):1809–1822. [DOI] [PubMed] [Google Scholar]
- 22.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369–376. [DOI] [PubMed] [Google Scholar]
- 23.Rosen HN, Moses AC, Garber J, et al. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000;66(2):100–103. [DOI] [PubMed] [Google Scholar]
- 24.Khosla S. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2008;66(6):1320–1321. [DOI] [PubMed] [Google Scholar]
- 25.Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65(12):2397–2410. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. Available at: http://handbook-5-1.cochrane.org/. Accessed March 31, 2019. [Google Scholar]
- 28.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9): 712–716. [DOI] [PubMed] [Google Scholar]
- 29.Lucas NP, Macaskill P, Irwig L, Bogduk N. The development of a quality appraisal tool for studies of diagnostic reliability (QAREL). J Clin Epidemiol. 2010; 63(8):854–861. [DOI] [PubMed] [Google Scholar]
- 30.Bagan JV, Jimenez Y, Gomez D, Sirera R, Poveda R, Scully C. Collagen telopeptide (serum CTX) and its relationship with the size and number of lesions in osteonecrosis of the jaws in cancer patients on intravenous bisphosphonates. Oral Oncol. 2008;44(11):1088–1089. [DOI] [PubMed] [Google Scholar]
- 31.Carini F, Saggese V, Porcaro G, Barbano L, Baldoni M. Surgical protocol in patients at risk for bisphosphonate osteonecrosis of the jaws: clinical use of serum telopetide CTX in preventive monitoring of surgical risk. Ann Stomatol (Roma). 2012; 3(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 32.Hutcheson A, Cheng A, Kunchar R, Stein B, Sambrook P, Goss A. A C-terminal crosslinking telo-peptide test-based protocol for patients on oral bisphosphonates requiring extraction: a prospective single-center controlled study. J Oral Maxillofac Surg. 2014; 72(8):1456–1462. [DOI] [PubMed] [Google Scholar]
- 33.Lazarovici TS, Mesilaty-Gross S, Vered I, et al. Serologic bone markers for predicting development of osteonecrosis of the jaw in patients receiving bisphosphonates. J Oral Maxillofac Surg. 2010;68(9):2241–2247. [DOI] [PubMed] [Google Scholar]
- 34.Lee CY, Suzuki JB. CTX biochemical marker of bone metabolism: is it a reliable predictor of bisphosphonate-associated osteonecrosis of the jaws after surgery? part II: a prospective clinical study. Implant Dent. 2010;19(1): 29–38. [DOI] [PubMed] [Google Scholar]
- 35.Migliorati CA, Saunders D, Conlon MS, et al. Assessing the association between bisphosphonate exposure and delayed mucosal healing after tooth extraction. JADA. 2013;144(4):406–414. [DOI] [PubMed] [Google Scholar]
- 36.O’Connell JE, Ikeagwani O, Kearns GJ. A role for C-terminal cross-linking telopeptide (CTX) level to predict the development of bisphosphonate-related osteonecrosis of the jaws (BRONJ) following oral surgery? Ir J Med Sci. 2012;181(2):237–242. [DOI] [PubMed] [Google Scholar]
- 37.Fleisher KE, Welch G, Kottal S, Craig RG, Saxena D, Glickman RS. Predicting risk for bisphosphonate-related osteonecrosis of the jaws: CTX versus radiographic markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):509–516. [DOI] [PubMed] [Google Scholar]
- 38.Kwon YD, Kim DY, Ohe JY, Yoo JY, Walter C. Correlation between serum C-terminal cross-linking telopeptide of type I collagen and staging of oral bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(12):2644–2648. [DOI] [PubMed] [Google Scholar]
- 39.Lee JJ, Cheng SJ, Wang JJ, et al. Factors predicting the prognosis of oral alendronate-related osteonecrosis of the jaws: a 4-year cohort study. Head Neck. 2013;35(12): 1787–1795. [DOI] [PubMed] [Google Scholar]
- 40.Choi SY, An CH, Kim SY, Kwon TG. Bone turnover and inflammatory markers of bisphosphonate-related osteonecrosis of the jaw in female osteoporosis patients. J Oral Maxillofac Surg Med Pathol. 2013;25(2): 123–128. [Google Scholar]
- 41.Kim JW, Kong KA, Kim SJ, Choi SK, Cha IH, Kim MR. Prospective biomarker evaluation in patients with osteonecrosis of the jaw who received bisphosphonates. Bone. 2013;57(1):201–205. [DOI] [PubMed] [Google Scholar]
- 42.Kunchur R, Need A, Hughes T, Goss A. Clinical investigation of C-terminal cross-linking telopeptide test in prevention and management of bisphosphonate-associated osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(6):1167–1173. [DOI] [PubMed] [Google Scholar]
- 43.Peisker A, Raschke GF, Fahmy MD, et al. Cross-sectional study of four serological bone turnover markers for the risk assessment of medication-related osteonecrosis of the jaw. J Craniofac Surg. 2018;29(2): e137–e140. [DOI] [PubMed] [Google Scholar]
- 44.Thumbigere-Math V, Michalowicz BS, Hughes PJ, et al. Serum markers of bone turnover and angiogenesis in patients with bisphosphonate-related osteonecrosis of the jaw after discontinuation of long-term intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2016; 74(4):738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atalay B, Yalcin S, Emes Y, et al. Bisphosphonate-related osteonecrosis: laser-assisted surgical treatment or conventional surgery? Lasers Med Sci. 2011;26(6):815–823. [DOI] [PubMed] [Google Scholar]
- 46.Flichy-Fernández AJ, Alegre-Domingo T, González-Lemonnier S, et al. Study of serum CTX in 50 oral surgical patients. Med Oral Patol Oral Cir Bucal. 2012;17(3):e367–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine: levels of evidence (March 2009). Available at: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed October 21, 2018.
- 48.Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63(2):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedlander AH, Hazboun RC. Bisphosphonate therapy: C-terminal telopeptide testing facilitates devising more accurate consent for extraction. J Oral Maxillofac Surg. 2015;73(3):377–378. [DOI] [PubMed] [Google Scholar]
- 50.Friedlander AH, Chang TI, Hazboun RC, Garrett NR. High C-terminal cross-linking telopeptide levels are associated with a minimal risk of osteonecrosis of the jaws in patients taking oral bisphosphonates and having exodontia. J Oral Maxillofac Surg. 2015;73(9):1735–1740. [DOI] [PubMed] [Google Scholar]
- 51.Hannon RA, Eastell R. Bone markers and current laboratory assays. Cancer Treat Rev. 2006;32(suppl 1): 7–14. [DOI] [PubMed] [Google Scholar]
- 52.Hellstein JW, Adler RA, Edwards B, et al. ; for the American Dental Association Council on Scientific Affairs Expert Panel on Antiresorptive Agents. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. JADA. 2011; 142(11):1243–1251. [DOI] [PubMed] [Google Scholar]
- 53.Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62(10):781–792. [DOI] [PubMed] [Google Scholar]
- 54.Enciso R, Keaton J, Saleh N, Ahmadieh A, Clark GT, Sedghizadeh PP. Assessing the utility of serum C-telopeptide cross-link of type 1 collagen as a predictor of bisphosphonate-related osteonecrosis of the jaw: a systematic review and meta-analysis. JADA. 2016;147(7): 551.e11–560.e11. [DOI] [PubMed] [Google Scholar]
- 55.Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2011;8(2):90–96. [DOI] [PubMed] [Google Scholar]
- 56.Elsayed R, Abraham P, Awad ME, et al. Removal of matrix-bound zoledronate prevents post-extraction osteonecrosis of the jaw by rescuing osteoclast function. Bone. 2018;110: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baim S, Miller PD. Assessing the clinical utility of serum CTX in postmenopausal osteoporosis and its use in predicting risk of osteonecrosis of the jaw. J Bone Miner Res. 2009;24(4):561–574. [DOI] [PubMed] [Google Scholar]
- 58.AAOM clinical practice statement: subject—the use of serum C-terminal telopeptide cross-link of type 1 collagen (CTX) testing in predicting risk of osteonecrosis of the jaw (ONJ). Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(4):367–368. [DOI] [PubMed] [Google Scholar]
- 59.Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. [DOI] [PubMed] [Google Scholar]
- 60.Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol. 2005;58(8):769–776. [DOI] [PubMed] [Google Scholar]
- 61.Di Fede O, Panzarella V, Mauceri R, et al. The dental management of patients at risk of medication-related osteonecrosis of the jaw: new paradigm of primary prevention. Biomed Res Int. 2018;2018:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.