Abstract
Purpose of this review:
The goal of the review is to provide an updated understanding of the pathophysiology of glucocorticoid induced osteoporosis, and treatment recommendations.
Recent findings:
Glucocorticoids reduce osteoblast and osteocyte lifespan and activity and reduce the vascularity of bone that together may explain the greater reductions in bone strength than those of bone mass. Treatments with parathyroid hormone fragments appear to reverse glucocorticoid induced bone loss and fracture risk partially through maintaining bone vascularity and bone strength.
Summary:
This review identifies how glucocorticoids anti-osteogenic and vascular effects together may reduce bone strength. It also provides guidance to clinicians on rationale treatment for glucocorticoid induced osteoporosis.
Keywords: Glucocorticoids, bone cells, bone vascularity, osteonecrosis, parathyroid hormone
Introduction
Glucocorticoids are anti-inflammatory medications that are often used for the treatment of inflammatory non-infectious diseases including rheumatoid arthritis, systemic lupus erythematous, organ transplantation, asthma, and malignancies. Despite their anti-inflammatory efficacy and rapid onset of action, GC treatment is associated with osteoporosis and fractures and is the most common cause of medication induced osteoporosis, with over 30% of glucocorticoid treated subjects experiencing an osteoporotic fracture, and over 10 percent develop osteonecrosis (1). Interestingly, the bone loss from glucocorticoids is rapid from sites rich in trabecular bone, slow and continual from cortical bone sites which over time results in an increased fracture risk. Subjects (ages 18 to 64 years) treated with prednisone at 10mg/day for more than 90 days had a more than 15-fold risk of vertebral fractures and 7-fold risk of hip fractures than individuals not treated with glucocorticoids [2].
A unique observation in glucocorticoid treated subjects is that they often fracture with higher bone density that osteoporotic subjects. Van Stat and colleagues studied the incident fracture risk of the lumbar spine and hip in post-menopausal women and women treated with glucocorticoids (3). At the end of the one-year study, compared to the postmenopausal women, glucocorticoid treated subjects had a significantly higher fracture incidence, and the fractures were at higher BMD values. This observation suggested that glucocorticoids may alter bone strength in addition to bone mass. This bone strength to bone mass discrepancy became a topic of investigation for our research group.
Pathophysiology
The pathophysiology of glucocorticoid induced osteoporosis is complex due to their effects on both hematopoietic and mesenchymal derived bone cells. Glucocorticoids reduce osteogenesis from mesenchymal stem cells (MSCs) and direct their differentiation to adipocytes. In addition, glucocorticoids reduce the maturation, lifespan, and function of osteoblasts which eventually can lead to bone loss (1). To improve our understanding of the GC effect on bone cells, Yao et al. exposed mice to GC excess through slow release methylprednisolone pellets and evaluated for gene expression and microarchitecture changes at days 0,7,28 and 56. (4). GC excess resulted in an early up-regulation of genes involved with osteoclast activation, osteoclast function, and adipogenesis up to day seven while genes associated with osteoclast cytoskeleton reorganization and bone matrix degradation remained high until day 28. Interestingly from day 28 to 56, genes associated with osteoblast maturation and activation significantly decreased from the baseline values, and Wnt/Beta Catenin antagonists including Dickkopf-1 and sclerostin were increased. Also, osteocyte specific genes associated with bone mineralization including dentin matrix protein-1 (DMP-1), Phosphate regulating gene with homologues to endopeptidases on the X chromosome (PHEX-1) were higher at day 28 and 56. The microarray results with reverse transcription polymerase chain reaction (4). These changes in expression paralleled changes in the biochemical markers of bone turnover and bone mass with early increases in the bone resorption marker, CTX-1 and a reduction in trabecular bone volume of the lumbar spine. Also, reduction in bone formation was not present until day 28 with a reduction in serum osteocalcin that was sustained through day 56.
Despite the documented changes of early elevations of osteoclast activity followed by delayed and continued reduction in osteoblast activity and bone mass, these observations did not explain the reduction in bone strength observed in glucocorticoid treated subjects. Yao et al. focused their attention to the osteocyte, and they found osteocytic gene expression with glucocorticoid use. Osteocytes are terminally differentiated osteoblasts that lie within the bone matrix and connect with other osteocytes, blood vessels, and the bone surface. They are responsible for orchestrating the transmission of the mechanical forces experienced by the skeleton into skeletal bone remodeling. Yao and others had identified Wnt inhibitor genes expressed by osteocytes with GC exposure suggesting that these medications were influencing osteocyte function (5,6). Additional evaluation of the GC treated and control trabecular bone matrix by scanning probe microscopy revealed the osteocyte lacunae were enlarged after glucocorticoid exposure; a term referred to “osteocytic osteolysis” (7). While the exact cause of the expansion of the osteocyte lacunae has not been fully determined, immunohistochemical evaluation determined that osteocytes, and to a much less extent osteoblasts, undergo autophagy in the presence of glucocorticoids. In addition, a number of the osteocytes and osteoblasts undergo apoptosis. Autophagy is a process in which the cell can survive stress by breaking down internal proteins and expelling the waste through the cell membrane, or “autophagic flux” (5,6). The osteocytes of the mice treated with glucocorticoids undergo autophagy, and cultured autophagic osteocytes release cathepsin K into the culture media in vitro (8). Cathepsin K is an enzyme that can remove collagen I from the bone matrix, and it is also an enzyme within the lysosomes. When the lysosomes fuse with the autophagosome, cell proteins are broken down through the autophagic process. Release of the autophagasome contents from the osteocyte through exocytosis maybe able to breakdown the bone matrix adjacent to the osteocyte, and this could partially explain the observed increased size of the osteocyte lacunae size with exposure to glucocorticoids. Osteocytes and their surrounded perilacunar matrix appear to sense the mechanical loading of bone, deflect and absorb bone forces, and activate remodeling (9). It is possible that the large osteocyte lacunae observed in mice treated with glucocorticoids may be less efficient in absorbing the bone strains as larger osteocyte lacunae reduce the load bearing bone surface leading to increased bone fragility. However, this hypothesis has yet to be tested.
Other investigators have reported, that glucocorticoids induce apoptosis in osteoblasts and osteocytes (10) in both the cortical and trabecular sites; however, not all studies find apoptosis. The differences in mouse strains, duration, dose, and glucocorticoid used may influence these results.
Bone weight is composed of nearly 20–25% water, and bound bone water is reduced with age, and a reduction of about 9% in bone water is associated with a reduction in bone strength (11). Weinstein and colleagues performed a study in glucocorticoid treated mice and reported a reduction in bone hydration, and this was accompanied by a reduction in bone vascularity, bone blood flow, and bone strength compared to control mice (12,13). We analysed bone vascular density in mice treated for 28 days with methylprednisolone pellets (2.8mg/kg/day) by intravenous administration of Microfil prior to sacrifice. The bones are then decalcified and then scanned by MicroCT as vascular density (vessel volume/total volume) is calculated (14). Interesting, glucocorticoid treatment reduced vascular density by approximately 50% compared to control mice, and this was associated with a reduction in serum VEGF levels and a reduction in bone strength (14). Therefore, our results support those of Weinstein in that glucocorticoids reduce bone vascularity, and this may be a mechanism whereby glucocorticoids reduce bone strength.
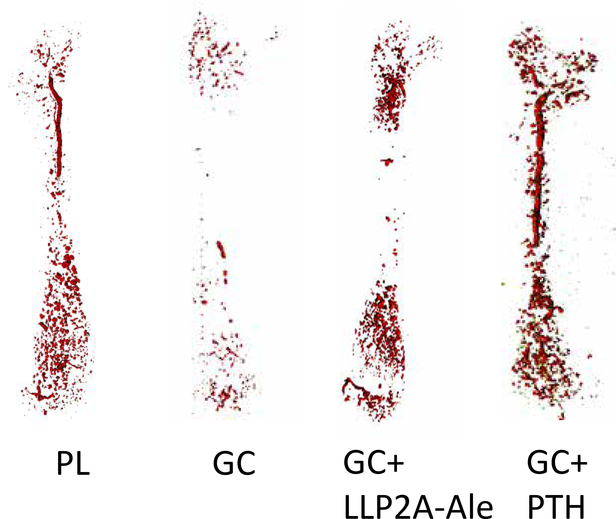
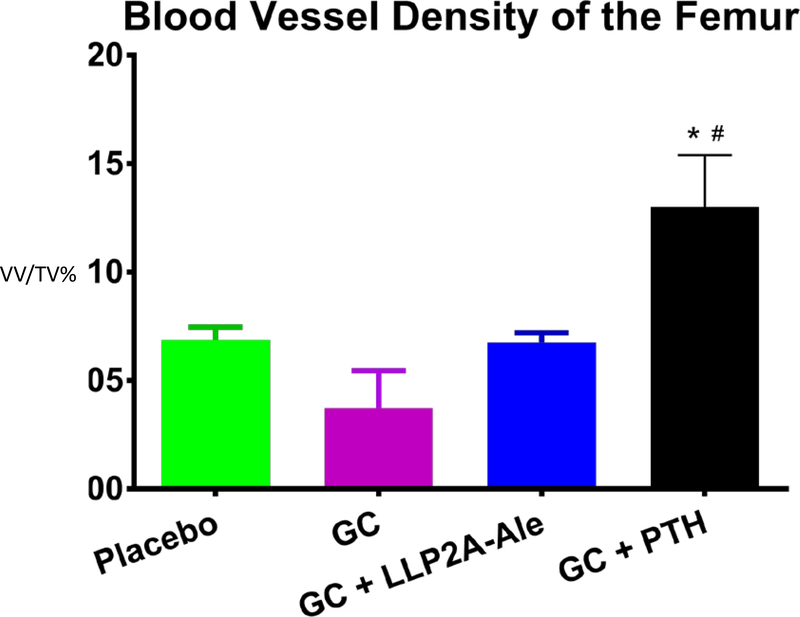
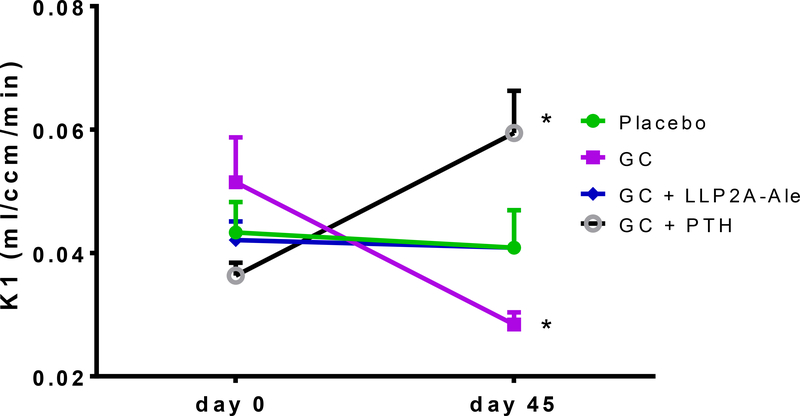
In addition to the changes in bone strength mediated by reduced bone blood flow and hydration, reduced bone vascularity is also observed with glucocorticoid exposure; this presents in clinical medicine as osteonecrosis or avascular necrosis. We recently studied a mouse model of glucocorticoid induced osteonecrosis in our laboratory to study the effect of glucocorticoids on bone vascularity, the incidence of osteonecrosis, and the response of osteonecrosis to treatments known to increase bone mass in the presence of glucocorticoids. We utilized the protocol by Relling et al (15) in which 6–8 week old male mice were randomized into three treatment groups and a control group. The three treatment groups were 1)oral dexamethasone 4mg/kg per day, 2) oral dexamethasone plus subcutaneous PTH (1–34) 40ug/kg 5 times a week 3)oral dexamethasone plus a hybrid compound LLP2A-Ale, administered intravenously at 250ug/kg and 500ug/kg on Day 1, 14 and 28, that directs MSCs to the bone surface for angiogenesis and bone formation (16). After 45 days, we determined in vivo changes in blood flow in the mouse tibias with 18NaF PET/CT and bone vascular density in mouse femurs with Microfil at sacrifice. In addition, the prevalence of glucocorticoid induced osteonecrosis was determined in the distal femurs by histology, bone microarchitecture by microCT and bone strength of the lumbar vertebrae with compression testing. At 45 days, glucocorticoid treatment resulted in a nearly 50% reduction in both the femoral and tibial blood flow assessed by Microfil or 18 NaF PET/CT (Figure 1a and 1b). Interestingly, concurrent treatment with either hPTH (1–34) or LLP2A-Ale prevented the glucocorticoid induced reduction in bone blood flow (Figure 1). We also evaluated the distal femoral epiphyses for evidence of osteonecrosis, trabecular bone volume, and adipocyte volume. The oral dexamethasone treatment model produced osteonecrosis in nearly 40% of glucocorticoid treated mice. Glucocorticoid treatment was associated with a significant reduction in epiphyseal and distal femur trabecular bone volume and a dramatic increase in adipocyte volume in those same regions. Surprisingly, neither human parathyroid hormone 1–34 (hPTH 1–34) or LLP2A-Ale prevented glucocorticoid induced osteonecrosis. While neither treatment significantly reduced the prevalence of osteonecrosis, both agents maintained the bone vascularity and maintained epiphyseal and trabecular bone mass of the distal femur in the presence of glucocorticoids (Figure 1c). Additional studies are needed in other animal models of glucocorticoid induced osteonecrosis to determine if there are angiogenic agents that will maintain bone vascularity in the presence of glucocorticoids and prevent osteonecrosis.
Figure 1.
Mice were treated with oral dexamethasone alone or with LLP2A-Ale (500ug/kg at day 0, 15, and 30) or PTH (1–34) (40ug/kg) 5 days/week for 45 days.
Figure 1a. Images of blood vessels within the mouse femur
At day 45, the mice had an intravenous infusion of Microfil (MV-120, Flow Tech, Inc., Carver, Massachusetts), and after perfusion, the animals were stored at 4°C overnight to allow polymerization. The following day, the femurs were dissected, fixed in 10% formalin, decalcified thoroughly and scanned using a micro-CT (VivaCT 40; Scanco Medical, Bassersdorf, Switzerland), with an isotropic resolution of 10.5 μm. The entire femur was used in the analysis for blood vessel density (VV)(14). Treatment with glucocorticoids appeared to reduce the bone vascularity while treatment with either LLP2A-Ale (500ug/kg at day 1, 14,28) or PTH (1–34) 40ug/kg 5x/wk maintained bone vascularity.
Figure 1b.
Blood vessel density of mouse femurs was reduced with glucocorticoid treatment and was maintained with either hPTH (1–34) or with a hybrid compound LLP2A-Ale An algorithm of thresholding 150–200, sigma 0.8 and support 1 were used for the quantification of the vascularization. Vascular density was presented as vessel volume per tissue volume (VV/TV) Treatment with glucocorticoid alone significantly reduced vascular density in the femur and treatment with either LLP2A-Ale (500ug/kg at day 1, 14,28 days) or PTH(1–34) (40ug/kg/5x wk) preserved the vascular density.
Figure 1c.
Bone Blood flow in the Proximal Tibia measured by 18F-fluoride by PET/CT. Mice were treated with oral dexamethasone alone or with LLP2A-Ale (500ug/kg at day1,14,28) or PTH (1–34) 5 days/week for 45 days. At day −1, and day 45 mice from each treatment group (n=6) had PET/CT scanning with the radiotracer 18F-fluoride to measure blood flow in vivo. Dynamic PET data were analyzed via a two-compartment, four-parameter model, and values of the rate constant K1 were compared between groups (Mean ± SD). At baseline, there were no differences in the K1 between the treatment groups. At day 45, compared to the placebo group, the GC group had a significant reduction in blood flow, and GC + PTH group had a significant increase compared to the placebo group.
Parathyroid hormone fragments are available to treat patients with osteoporosis and are known to increase bone formation, trabecular thickness, and bone strength both in estrogen deficiency and in glucocorticoid-induced osteoporosis. The observation that glucocorticoids reduce vascular density in mice and treatment with hPTH (1–34) maintained the vascular density in the femur, suggests a novel observation mechanism by which PTH fragments maintain and improve bone strength in the presence of glucocorticoids.
Recently, PTH (1–34) was reported to promote angiogenesis directly via endothelial cell migration and blood vessel formation in vitro. In addition, in a rat model of implant osteointegration, PTH (1–34)increased osteoclast participation in bone remodeling by secreting angiogenic and osteogenic growth factors to induce early vascularization (17). In a PTH knockout (PTHKO) mouse, the rate of fracture healing was delayed compared to the control animals, and the expression levels of protein kinase A (PKA), phosphorylated-serine/threonine protein kinase (pAKT), hypoxia-inducible factor-1α (HIF1α) and VEGF were significantly decreased in bone marrow mesenchymal stem cell derived osteoblasts from PTHKO mice (18). The expression levels of HIF1α, VEGF, runt-related transcription factor 2, osteocalcin and alkaline phosphatase were also decreased in PTHKO mice, and fracture healing was delayed. In conclusion, lack of endogenous PTH may reduce VEGF expression in BMSC-derived osteoblasts by downregulating the activity of the PKA/pAKT/HIF1α/VEGF pathway, thus affecting endochondral bone formation through a reduction in angiogenesis and osteogenesis, and ultimately leading to delayed fracture healing (18). Since glucocorticoids are known to reduce the differentiation of BMSC- derived osteoblasts and angiogenesis, treatment with PTH may maintain bone vascularity by allowing for the production of VEGF and HIF1α. This, in turn, may restore the supply of osteoblasts and nutrients to the bone surface allowing for bone formation in occur in the presence of glucocorticoids.
Update on Fracture Risk Assessment in Glucocorticoid Treated Patients
Fracture Risk assessment in patients treated with glucocorticoids can be somewhat challenging as the risk factors for fractures are multiple. The Fracture Risk Assessment (FRAX) has been used for over a decade to calculate hip fracture and major osteoporotic fracture risk (hip, proximal humerus, wrist, and clinical vertebral fracture) in subjects with low bone mass that are not osteoporotic by T score (DXA). The risk factors for the assessment include age, weight, BMD, family history, and other clinical risk factors such as rheumtoid arthitis, secondary osteoporosis and glucocorticoid exposure. However, the FRAX instrument question about GC use is answered as either yes or no, without regard to GC doses, GC duration or other risk factors that are strong predictors of fractures in glucocorticoid treated patients such as falls, vertebral deformities or underlying inflammatory diseases (19). A modification of the FRAX tool has been developed for use in glucocorticoid treated subjects. According to the new modified FRAX calculations, patients treated with low dose prednisolone (< 2.5mg per day) should have the fracture probability decreased by about 20% and for those treated with high dose prednisolone (> 7.5mg per day) the fracture risk should be increased by about 15%. At this time, there is no modification for fracture risk for individuals treated with prednisolone 2.5 to 7.5mg per day (19). This modification is useful when health care providers are trying to estimate 10-year fracture risk in glucocorticoid treated subjects. However, this new glucocorticoid dose adjustment does not incorporate fall risk or consider the severity of the underlying inflammatory disease. That said, this adjustment can help both clinicians and patients when deciding on whether pharmacologic therapy to prevent or treat bone loss in the setting of glucocorticoid treatments appropriate(19)
Guidelines and Recommendations for the prevention and treatment of glucocorticoid induced osteoporosis
Since the approval of alendronate for the prevention and treatment of glucocorticoid induced osteoporosis, there have been guidelines published for the management of glucocorticoid induced osteoporosis. In 2017, the American College of Rheumatology revised their guidelines for the prevention and treatment of glucocorticoid induced osteoporosis. This guideline provides guidance for initial assessment, continuing reassessment and medication choice in patients initiating GC therapy and those chronically treated (>= 3 months) with glucocorticoids. (21) The group utilized the grading of recommendations assessment, development and evaluation (GRADE) methodology that required a thorough literature review, followed by an expert panel of rheumatologists and internists who reviewed “straw patients” and voted on treatment recommendations. The majority of the recommendations the committee approved were “conditional” due to concerns regarding the benefits and harms of the currently approved treatments. The new guideline divides subjects into two groups by age (adults < 40 years and > 40 years of age), GC status (initiating or continuing glucocorticoids) and fracture risk (low, medium, high). The guideline identified adults > 40 years that had a prevalent or an osteoporotic T score ( <−2.5 at the hip or spine) or a FRAX with a risk of greater than 20% over 10 years for a major osteoporotic fracture ( hip, humerus, clinical fracture, ankle X) or 3% for a hip fracture as high risk. The guideline included children and adults < 40 years together. High risk of fracture in this younger age group was defined as a history of a prior fracture. Moderate fracture risk was designated if subjects were expected to be treated with glucocorticoids >= 7.5mg/day for > = 6 months and have a BMD Z score at the hip or lumbar spine of < −3 or have rapid decline in BMD of > 10% over 12 months while treated with glucocorticoids (20,21).
Treatments to prevent glucocorticoid induced osteoporosis are both nonpharmacologic and pharmacologic.
Non-pharmacologic treatments include adequate intake of calcium and vitamin D, and exercise. Glucocorticoids create a negative calcium balance with reduced gastrointestinal absorption and elevated urinary excretion. Also, glucocorticoids weaken muscles, and vitamin D, in addition to augmenting calcium absorption from the gastrointestinal tract, may also strengthen muscles and improve balance. The recommendations for daily doses of calcium and vitamin D supplementation vary and range from calcium 1000 to 1500mg a day and at least 400 to 800 IU per day with either vitamin D2 or vitamin D3 to maintain vitamin D after normal levels are achieved ( >=30ng/ml). Regular weight bearing exercise with an emphasis on strength training may prevent loss of muscle mass and strength, as well as potentially prevent falls in glucocorticoids treated subjects. However, studies to confirm the utility of these recommendations in this patient group are lacking (20,21)
Pharmacologic Prevention of Fractures during glucocorticoid treatment.
Initial randomized, controlled, clinical trials that evaluated bisphosphonates for the prevention and treatment of osteoporosis were able to compare the active medication to placebo treated subjects (22,23)). All of the bisphosphonates evaluated significantly increased bone mineral density of the lumbar spine and hip compared to placebo treatment in both prevention (22,23) and treatment studies (22,23) and reduced incident vertebral fractures in the treatment group. More recently, Reid et al compared zoledronic acid ( 5mg intravenous) to risedronate and reported greater gains in BMD in the spine and hip with zoledronic acid compared to risedronate, with no differences in incident fractures (24). The mechanism of action for this improved bone density might seem counterintuitive as bisphosphonates reduce osteoclast lifespan and activity which then reduces bone turnover. In vivo studies of mice treated with glucocorticoids alone or glucocorticoids with the bisphosphonate risedronate, demonstrated that glucocorticoids alone reduced the osteoblast and osteocytic mineralization gene expression (DMP1, Phex and Spp1 ), while concurrent treatment with risedronate reversed the inhibition of mineralization gene expression, suggesting that restoring the mineralization process in new bone was associated with increased bone mineral density and improved bone strength (25).
Since, one of the major factors related to glucocorticoid induced bone loss is a reduction in osteoblast maturation, lifespan and activity, studies with hPTH (1–34) in glucocorticoid treated subjects were performed to determine if this bone anabolic agent could override the suppressive effects of glucocorticoids on bone formation and restore bone mass. Studies initially by Lane et el (26) in postmenopausal women concurrently treated with glucocorticoids and estrogen replacement were randomized to treatment with either hPTH (1–34) 40ug per day or placebo for one year. Treatment with hPTH (1–34) resulted in an increase in trabecular bone mineral density measured by quantitative computed tomography (QCT )of about 35% and DXA of the lumbar spine of about 10% compared to the placebo treated subjects (25). This study was followed by a larger study in glucocorticoid treated subjects that were randomized to either oral alendronate (10mg per day) or teriparatide (rhPTH 1–34)(20ug per day) and endpoints were evaluated at 18 and 36 months. There were significantly greater increases in the bone mineral density of the lumbar spine in the teriparatide treated group compared to the alendronate group, and although incident fractures were recorded as adverse events, there were significantly less fractures in the teriparatide treated group. (27).
Recently, denosumab, an antibody to RANKL that inhibits osteoclast maturation, lifespan and activity, has been studied and approved for the prevention and treatment of glucocorticoid induced bone loss. Saag et al studied subjects either initiating glucocorticoid treatment (less than 3 months of treatment) or continuing chronic glucocorticoid treatment ( > 3 months) and randomized subjects to either risedronate (5mg/day) and subcutaneous placebo every 6 months or denosumab (60mg every 6 months by subcutaneous injection) and daily oral placebo tablets for 12 months (28). Subjects treated with denosumab, had significantly increased bone mineral density at both the lumbar spine and hip compared to risedronate and most adverse events were mild to moderate in severity. Denosumab has not been approved the Food and Drug Administration (FDA) for the treatment of glucocorticoid-induced osteoporosis. While denosumab is effective in preventing bone loss and improving bone mass in glucocorticoid treated subjects, when denosumab is discontinued there can be a rapid loss of bone mass and potentially increased risk of fractures (27). Therefore, it is critical that patients who discontinue denosumab be educated about this issue and that appropriate use of an anti-resorptive medication such as a bisphosphonate be prescribed in the short term to prevent this complication.
Glucocorticoids treatment has a number of effects on bone. Glucocortiocids can increase bone resorption reduce osteoblast maturation and over a short period of time these changes in bone activity results in bone loss, reduction in bone strength and an increase in bone fractures. In addition, glucocorticoids reduce bone blood flow which may be one of the factors associated with osteonecrosis. Since glucocorticoid induced bone fragility can develop within a few months of initiating glucocorticoid therapy, it is important to that clinicians initiate therapy to either prevent or reduce the loss of bone density. Since glucocorticoids major effect is to reduce bone formation, teriparatide is the rationale intervention is a subject with low bone mass or a fracture while on glucocorticoids. Treatment with anti-resorptive agents in glucocorticoid treated subjects will prevent additional bone loss and improve bone strength, however they will not reverse the changes in the bone structure induced by glucocorticoid treatment. Therefore, glucocorticoid treated subjects with low bone mass or fractures may best be served by initiating treatment with hPTH (1–34) type of medications that can restore trabecular bone mass and structure, then to maintain this new bone mass and structure with the use of an anti-resorptive agent.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Nancy Lane reports having a patent (LLP2A-ale) issued.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinology and metabolism clinics of North America. 2012;41:595–611. 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15:323–328. 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003. November;48(11):3224–9. 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 4.Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58:1674–1686. 10.1002/art.23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao W, Dai W, Jiang JX, Lane NE. Glucocorticoids and osteocyte autophagy. Bone. 2013. June;54(2):279–84. 10.1016/j.bone.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia X, Kar R, Gluhak-Heinrich J, Yao W, Lane NE, Bonewald LF, Biswas SK, Lo WK, Jiang JX. Glucocorticoid-induced autophagy in osteocytes. Bone Miner Res. 2010. November;25(11):247–988. 10.1002/jbmr.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006. March;21(3):466–76. 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R, Bonewald L, Jiang JX, Lane NE. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011. October;25(10):3366–76. 10.1096/fj.11-182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uda Y, Azab E, Sun N, Shi C, Pajevic PD. Osteocyte Mechanobiology. Curr Osteoporos Rep. 2017. August;15(4):318–325. 10.1007/s11914-017-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998. July 15;102(2):274–82. 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyman JS, Roy A, Shen X, Acuna RL, Tyler JH, Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39(5):931–8. 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010. April;9(2):147–61. 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinstein RS. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone. 2010. March;46(3):564–70. 10.1016/j.bone.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan G, Lay EY, Berka H, Ringwood L, Kot A, Chen H, Yao W, Lane NE. A Novel Hybrid Compound LLP2A-Ale Both Prevented and Rescued the Osteoporotic Phenotype in a Mouse Model of Glucocorticoid-Induced Osteoporosis. Calcif Tissue Int. 2017. January;100(1):67–79. 10.1007/s00223-016-0195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Boyd K, Kaste SC, Kamdem L, Rahija RJ, Relling MV. A Mouse Model for Glucocorticoid-Induced Osteonecrosis: Effect of a Steroid Holiday. J Orthop Res. 2009. February; 27(2): 169–175. 10.1002/jor.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane NE, Mohan G, Yao W, Shidara K, Lay YE, Junjing J, Dubrovsky A, Kimmel DB. Prevalence of glucocorticoid induced osteonecrosis in the mouse is not affected by treatments that maintain bone vascularity. Bone Rep. 2018. 9:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang L, Zhang W, Wei L, Zhou Q, Yang G, Qian N, Tang Y, Gao Y, Jiang X. Early effects of parathyroid hormone on vascularized bone regeneration and implant osseointegration in aged rats. Biomaterials. 2018. June 26;179:15–28. 10.1016/j.biomaterials.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Ding Q, Sun P, Zhou H, Wan B, Yin J, Huang Y, Li Q, Yin G, Fan J. Lack of endogenous parathyroid hormone delays fracture healing by inhibiting vascular endothelial growth factor-mediated angiogenesis. Int J Mol Med. 2018. July;42(1):171–181. 10.3892/ijmm.2018.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Güler-Yüksel M, Hoes JN, Bultink IEM, Lems WF. Glucocorticoids, Inflammation and Bone. Calcif Tissue Int. 2018. May;102(5):592–606. 10.1007/s00223-017-0335-7. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011. March;22(3):809–16. 10.1007/s00198-010-1524-7. [DOI] [PubMed] [Google Scholar]
- 21.Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Byun Robinson A, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017. American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Care Res (Hoboken). 2017 August;69(8):1095–1110. 10.1002/acr.23279.* Guidelines for the treatment of glucocorticoid induced osteoporosis were revised. The methodology utilized was case based and recommendations are made for young, middle age, and older men and women.
- 22.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998. July 30;339(5):292–9. 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 23.Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, Doherty SM, Maricic M, Rosen C, Brown J, Barton I, Chines AA. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000. October;67(4):277–85. 10.1007/s002230001146. [DOI] [PubMed] [Google Scholar]
- 24.Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola T, Mesenbrink P, Sambrook PN; HORIZON investigators. Zoledronic acid and risedronate in the prevention and treatment of GIOP (HORIZON): a multicenter, double-blind, double-dummy, randomized controlled trial. Lancet. 2009. April 11;373(9671):1253–63. 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- 25.Yao W, Cheng Z, Pham A, Busse C, Zimmermann EA, Ritchie RO, Lane NE. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 2008. November;58(11):3485–97. 10.1002/art.23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998. October 15;102(8):1627–33. 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007. November 15;357(20):2028–39. 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 28.Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, Chapurlat R, Wang A, Pannacciulli N, Lems WF. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018. June;6(6):445–454. 10.1016/S2213-8587(18)30075-5.* This randomized, double blind, active control study demonstrated that in glucocorticoid treated subjects, denosumab increased bone mass at the lumbar spine after 12 months significantly more than subjects treated with residronate. This result was observed in both subjects initiating glucocorticoid treatment or chronic glucocorticoid therapy.
- 29.Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res. 2018. February;33(2):190–198. 10.1002/jbmr.3337.This post-hoc analysis of the extension of the phase 3 FREEDOM trial, determined that subjects that discontinued densoumab had an increase risk of vertebral fractures that was similar to the untreated subjects. Also many of the subjects that had an incident vertebral fracture after discontinuation of denosumab had multiple vertebral fractures, and subjects with a prior fracture had a greater risk. These results led to the recommendation that subjects discontinuing denosumab should transition to another anti-resorptive agent for a period of time.