Abstract
Next-generation forms of HIV pre-exposure prophylaxis (PrEP) currently in development, including long-acting injectables (LAIs), rectal microbicides (RMs), antibody infusions (AIs), and subdermal implants (SIs), may address barriers to daily oral PrEP uptake and adherence. The purpose of this study was to evaluate barriers to oral PrEP, preferences for next-generation PrEP modalities, sociodemographic characteristics and sexual behaviors associated with preferences, and reasons for wanting or not wanting each formulation among a sample of men who have sex with men (MSM). We administered a cross-sectional survey to a diverse sample of MSM currently taking oral PrEP (n = 108) at two sexually transmitted disease clinics. We used logistic multivariate analyses to explore preferences, relative to oral PrEP, for each formulation across sociodemographic and sexual behaviors. The most commonly endorsed barriers were finding a PrEP provider and making appointments to get PrEP. Participants were most likely to prefer the SI (45%), followed by the LAI (31%), pill (21%), RM (1%), and AI (1%). Black/African American and Hispanic/Latino MSM were more likely to prefer the LAI over daily oral PrEP (odds ratio: 2.45, 95% confidence interval: 0.86–6.89), and sexual behaviors were most commonly associated with preference for the SI. Top reasons for wanting or not wanting each formulation were most commonly related to perceived ease of use. These findings demonstrate variations in preferences for next-generation PrEP modalities, highlighting a need to ensure comprehensive access to all formulations once they become available.
Keywords: pre-exposure prophylaxis, men who have sex with men, HIV prevention, next-generation biomedical interventions
Introduction
While new HIV diagnoses in the United States have declined in recent years, disparities persist. In 2017, gay, bisexual, and other men who have sex with men (MSM) accounted for 66% of all new HIV diagnoses.1 Further subgroup disparities exist among MSM, with black/African American and Hispanic/Latino MSM (hereinafter referred to as black and Hispanic MSM) disproportionately affected among people living with HIV. Specifically, white MSM have a 1 in 11 lifetime risk of HIV, which increases to 1 in 5 for Hispanic MSM and 1 in 2 among black MSM.2 Pre-exposure prophylaxis (PrEP) is highly effective at preventing HIV among MSM and has the potential to mitigate disparities. However, uptake remains suboptimal with common barriers, including lack of access to PrEP care, low HIV risk perception, cost, stigma, and concerns about side effects.3–7 Those who do initiate PrEP face additional barriers to adherence and retention in care, both of which are necessary to ensure efficacy.8
Importantly, emerging racial and ethnic disparities in PrEP care may perpetuate existing inequalities.9 Black and Hispanic MSM are dramatically underrepresented at every level of the PrEP care continuum, including uptake, retention in care, and adherence.10–13 Prior research shows that these disparities persist even after structural barriers are addressed.14 Focused research and public health interventions informed by structural barriers, including racism and the historical abuses of communities of color by medical institutions, are urgently needed to promote PrEP use and reduce HIV transmission among MSM of color.13,15,16
Ongoing development of the next generation of PrEP formulations has the potential to reduce barriers to uptake, adherence, and retention in care. Nonoral forms of PrEP currently in development include long-acting injectables (LAIs), rectal microbicides (RMs), antibody infusions (AIs), and subdermal implants (SIs).17 These modalities may appeal to populations that disproportionately experience barriers to daily oral PrEP. For example, formulations that do not require a daily pill may reduce PrEP stigmas associated with being HIV positive, gay, or “sexually irresponsible.”18 Long-acting formulations may also make it easier for people with more life chaos who experience difficulty taking a daily pill, picking up prescriptions, or getting to appointments.19 Further, certain characteristics of alternative modalities may appeal to others who have not yet initiated PrEP.20 Next-generation products are in development and anticipated to become approved in the next few years. Once these formulations become available, implementation success will depend on their acceptability among populations of focus and particularly MSM of color.
Research on next-generation PrEP modality acceptability among MSM thus far has primarily focused on LAIs.21–23 Some studies have also included RMs and SIs.24–26 Findings from this research suggest that MSM are interested in and willing to use next-generation PrEP formulations and that LAIs are preferred over daily oral PrEP. Few of these studies have compared preferences across modalities, and none have compared all modalities currently in development to daily oral PrEP. In addition, black and Hispanic MSM are generally underrepresented in next-generation acceptability research even though these populations are at high risk for HIV infection and most likely to benefit from PrEP.
This research builds upon prior work to evaluate next-generation PrEP preferences across modalities among MSM currently taking oral PrEP. The objectives of this study were to evaluate barriers to oral PrEP use and adherence, preferences for various next-generation PrEP modalities, sociodemographic characteristics and sexual behaviors associated with preferences, and reasons for wanting or not wanting each formulation among a racially and ethnically diverse sample of MSM.
Methods
Study participants and procedures
We administered a cross-sectional survey to a sample of MSM (n = 114) at two safety-net sexually transmitted disease (STD) clinics in Providence, Rhode Island and Boston, Massachusetts in 2017. Eligibility criteria included the following: (1) being 18 years of age or older, (2) identifying as male, (3) reporting sex with another man in the previous 12 months, (4) receiving care at one of the participating STD clinics, (5) being able to provide written informed consent, and (6) speaking English or Spanish. Trained research assistants conducted informed consent, answered any questions or concerns, administered the surveys, and provided compensation of $20 to all participants. The Miriam Hospital and the Boston University Medical Campus Institutional Review Boards reviewed and approved this research.
Study instrument
We collected information on sociodemographic characteristics, sexual behaviors, barriers to oral PrEP, and preferences for each next-generation modality. Sexual and risk behavior questions were adapted from prior research and included number of sex partners, STD diagnoses, receptive sex, condomless sex, and sex with an HIV-positive partner at any point in the past 6 months.27
Participants were asked whether they experienced any of 14 established barriers to obtaining daily oral PrEP with the following question: “Parts of the process of getting and taking PrEP may be easy or difficult for different people. How difficult is each of the following for you?” Barriers are based on prior research and covered topics, including finding information, finding and talking to a PrEP provider, attending appointments, taking PrEP every day, coping with side effects, and paying for PrEP care.28–30 We assessed barriers using a 5-point scale (1 = “Very Easy” to 5 = “Very Difficult”). Likert responses were dichotomized for ease of descriptive presentation, and participants who identified a barrier as “medium,” “difficult,” or “very difficult” were classified as endorsing that barrier. For analytic purposes, responses of “not applicable” were set to missing.
Information on the administration and duration of each next-generation PrEP modality was provided before conducting the survey. The LAI was described as an injectable form of PrEP that is administered every 2–3 months and the RM as a PrEP gel that can be inserted into the rectum before sex. The AI was explained as “a process where antibodies, or particles the body uses to fight viruses such as HIV, are infused directly into a person's blood.” Finally, we described the SI as “a small flexible rod, about the size of a match, that is placed under the skin periodically to deliver a PrEP medication into the bloodstream.” We explained that each of these PrEP forms is still in development and not available for public use at this time.
We asked participants to rank all formulations, including LAIs, RMs, AIs, SIs, and daily oral PrEP, in order of how likely they would be to use each. Product rankings were assessed with the prompt: Please rank each of the following PrEP formulations in order of how likely you would be to use it, where 1 = “most likely to use” and 5 = “least likely to use.” The interviewer then read a list of each next-generation formulation along with daily oral PrEP, and participants assigned each a rank from 1 to 5. Preference for each formulation relative to oral PrEP was assessed with the following questions: (1) “If you had a choice to use a daily pill or a shot every 2–3 months for PrEP, which would you choose?,” (2) “If you had a choice to use a daily pill or a gel applied to the rectum before sex for PrEP, which would you choose?,” (3) “If you had a choice to use a daily pill or an IV antibody infusion treatment every 2 months for PrEP, which would you choose?,” and (4) “If you had a choice to use a daily pill or an implant for PrEP, which would you choose?.” Responses of “neither” or “unsure” were set to missing. We also assessed general interest in each formulation with the question: “How interested would you be in using [each modality] for PrEP if it were available” with a five-point Likert scale of “very interested,” “somewhat interested,” “neutral,” “not very interested,” and “not at all interested.” Following interest assessments, participants were asked: “What is the most important reason you WOULD or WOULD NOT want to use [each formulation] for PrEP?” Short answer responses were recorded in writing by research staff for each modality.
Analytic approach
We restricted the study sample to participants who were currently taking oral PrEP (n = 108), excluding any that were not on PrEP (n = 2) or had missing data on whether they were currently taking PrEP (n = 4). Bivariate analyses were used to present sociodemographic characteristics, sexual behaviors, and self-reported HIV risk by race/ethnicity. Due to our moderate sample size, black and Hispanic MSM were grouped into one category, and chi-square or Fisher's exact tests were used to identify differences in sociodemographic and sexual behaviors. Endorsed barriers to oral PrEP were also examined by race/ethnicity. We calculated the proportion of the study sample who selected each formulation as their first, second, third, fourth, and fifth choice. Three trained research staff members used open coding to identify and refine themes from the short answer responses to the question about the most important reason the participant would or would not use each formulation for PrEP. Each response was assigned the most appropriate theme category by the research team, and frequencies and percentages of these themes are presented for each formulation. Finally, we conducted exploratory analyses using multivariable logistic regressions to identify associations between sociodemographic characteristics, sexual behaviors, and formulation preferences. Models were constructed for each independent variable (exposure) with a panel of confounding variables, which were determined a priori and by directed acyclic graphs. All analyses were performed in Stata (version 15.1; StataCorp LLC).
Results
Sociodemographic and sexual behaviors
In the study sample (n = 108), the median age was 32 years (interquartile range: 16) (Table 1). The majority of participants identified as gay (89%) and single (67%). Most had at least a college education (81%), private health insurance (71%), and an annual income of at least $60,000 (44%). Approximately 13% were students, and 6% reported unstable housing in the past 6 months. In the 6 months before study participation, 40% reported having six or more sex partners, 38% reported STD diagnosis, 70% reported having sex without a condom, and 25% reported sex with a partner who was HIV positive. Most participants self-identified as low risk for HIV transmission (64%). Of 108 study participants, 25% identified as Hispanic or Latino, 19% as black or African American, and 4% as Asian. With a median age of 30.5 years, black and Hispanic MSM were younger, more likely to be a student (23%), have lower educational attainment, lower income, public insurance coverage (53%), and were more likely to report unstable housing in the past 6 months (11%). There were no significant differences in sexual behaviors or self-reported HIV risk between black and Hispanic MSM and other racial/ethnic groups.
Table 1.
Demographic Characteristics, Sexual Behaviors, and Self-Reported HIV Risk by Race/Ethnicity
| Total (n = 108) | Black or Hispanic/Latino (n = 44) | Not black or Hispanic/Latino (n = 64) | p | |
|---|---|---|---|---|
| Age, median (IQR) | 32 (16) | 30.5 (10) | 36 (18) | <0.001 |
| Sexual orientation, n (%) | 0.196 | |||
| Gay | 96 (88.9) | 36 (81.8) | 60 (93.8) | |
| Bisexual | 12 (11.1) | 8 (18.2) | 4 (6.2) | |
| Education, n (%) | 0.102 | |||
| High school or less | 7 (6.5) | 6 (13.7) | 1 (1.6) | |
| Some college | 13 (12) | 7 (15.9) | 6 (9.4) | |
| College | 50 (46.3) | 21 (47.7) | 29 (45.3) | |
| Graduate school | 38 (35.2) | 10 (22.7) | 28 (43.7) | |
| Student, n (%) | 14 (12.9) | 10 (22.7) | 4 (6.2) | 0.005 |
| Income, n (%) | <0.001 | |||
| ≤ $29,999 | 29 (26.9) | 20 (45.5) | 9 (14.1) | |
| $30,000–$59,999 | 31 (28.7) | 15 (34) | 16 (25) | |
| ≥ $60,000 | 48 (44.4) | 9 (20.5) | 39 (60.9) | |
| Relationship status, n (%) | 0.250 | |||
| Single | 67 (62) | 30 (68.2) | 35 (55.2) | |
| Monogamous | 21 (19.5) | 7 (15.9) | 16 (25.4) | |
| Nonmonogamous | 20 (18.5) | 7 (15.9) | 12 (19.4) | |
| Insurance, n (%) | <0.001 | |||
| Private | 76 (71.0) | 20 (46.5) | 56 (87.5) | |
| Public | 31 (29.0) | 23 (53.5) | 8 (12.5) | |
| Unstable housing, n (%) | 6 (5.6) | 5 (11.4) | 1 (1.6) | 0.080 |
| No. of sex partners, past 6 months, n (%) | 0.524 | |||
| 0 partners | 22 (20.4) | 6 (13.6) | 16 (25) | |
| 1–2 partners | 25 (23.1) | 11 (25.0) | 14 (21.9) | |
| 3–5 partners | 19 (17.6) | 8 (18.2) | 11 (17.2) | |
| 6 or more partners | 42 (38.9) | 19 (43.2) | 23 (35.9) | |
| STD diagnosis, n (%) | 0.069 | |||
| No | 37 (34.2) | 16 (36.4) | 21 (32.8) | |
| Yes, ever | 30 (27.8) | 7 (15.9) | 23 (35.9) | |
| Yes, past 6 months | 41 (38.0) | 21 (47.7) | 20 (31.3) | |
| Receptive sex, past 6 months, n (%) | 0.444 | |||
| No | 29 (26.9) | 13 (29.5) | 16 (25) | |
| Yes | 79 (73.1) | 31 (70.5) | 48 (75) | |
| Condomless sex, past 6 months, n (%) | 0.501 | |||
| No | 33 (30.6) | 12 (27.3) | 21 (32.8) | |
| Yes | 75 (69.4) | 32 (72.7) | 43 (67.2) | |
| Sex with HIV positive partner, past 6 months, n (%) | 0.306 | |||
| No | 81 (75) | 34 (77.3) | 47 (73.4) | |
| Yes | 27 (25) | 10 (22.7) | 17 (26.6) | |
| Self-reported HIV risk, n (%) | 0.702 | |||
| No risk | 10 (9.3) | 6 (13.6) | 4 (6.2) | |
| Low risk | 69 (63.9) | 25 (56.8) | 44 (68.8) | |
| Medium risk | 18 (16.7) | 8 (18.2) | 10 (15.6) | |
| High risk | 11 (10.1) | 5 (11.4) | 6 (9.4) |
IQR, interquartile range; STD, sexually transmitted disease.
Barriers to retention and adherence
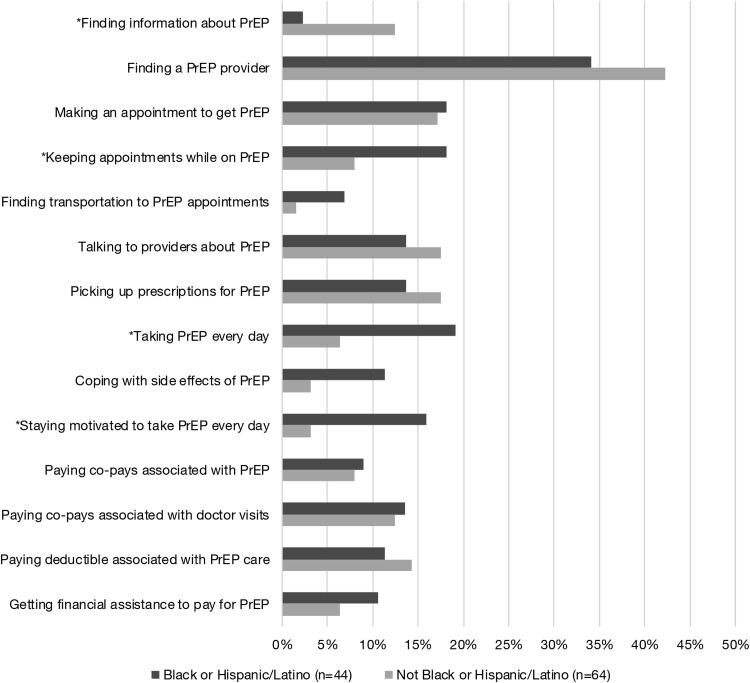
Approximately 65% of all participants reported at least one barrier to using oral PrEP (Fig. 1). The most commonly endorsed barriers were as follows: (1) finding a healthcare provider who could provide PrEP (39%), (2) making an appointment to get PrEP (18%), (3) picking up prescriptions (16%), and (4) talking to healthcare providers about PrEP (16%). We also found significant racial/ethnic differences in barriers. Approximately 19% of black and Hispanic MSM endorsed difficulty taking PrEP every day, 18% reported difficulty keeping appointments, and 16% reported difficulty staying motivated to take PrEP every day (compared to 5%, 8%, and 2% of white patients, respectively). Approximately 13% of white and Asian MSM endorsed difficulty finding information about PrEP, compared to 2% of black and Hispanic MSM. White and Asian men were also more likely to report difficulty coping with side effects and finding information about PrEP, although these differences did not reach statistical significance.
FIG. 1.
Barriers to oral PrEP by race/ethnicity. PrEP, pre-exposure prophylaxis.
Next-generation product rankings
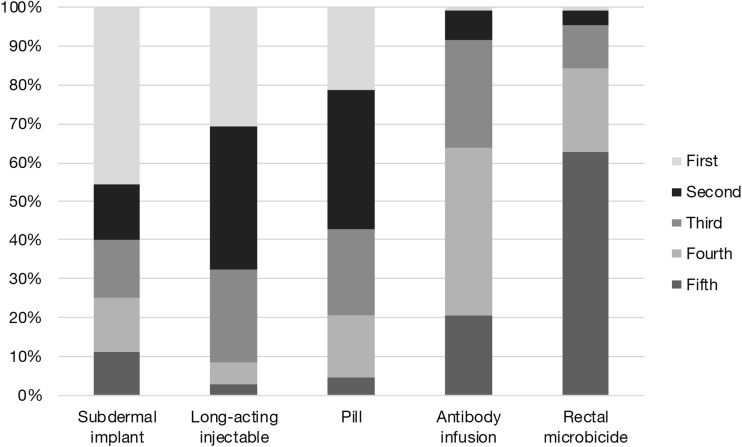
When asked to rank likelihood of using products on a scale from 1 to 5, the SI was most commonly selected as the first choice (45%), followed by the LAI (31%) and daily oral PrEP (21%) (Fig. 2). Both the RM and AI were ranked first by 1% of participants.
FIG. 2.
Ranked preference percentage for each formulation.
Reasons for wanting and not wanting next-generation formulations
Among those who said they were somewhat or very interested in the LAI, the most common reasons cited for wanting to use this modality included: not having to take a daily pill (43%), convenience (30%), and timing or dosage frequency (11%) (Table 2). In response to the prompt of most important reasons for not wanting to use the LAI, participants who said they were not very or not at all interested cited disliking needles (46%), concerns about safety or effectiveness (23%), and logistical difficulties (15%). Convenience (36%), timing or dosage frequency (21%), and perceptions that they would experience fewer side effects (14%) were top reasons for wanting to use the RM among those who expressed interest. Among those not interested in the RM, top reasons for not wanting to use this formulation included concerns about safety or effectiveness (30%), inconvenience (22%), and logistical difficulty (12%). Participants interested in the AI cited convenience (45%), timing or dosage frequency (7%), and not having to take a daily pill (24%) as top reasons. Those not interested in the AI cited timing or dosage frequency (26%), not liking needles (16%), and concerns about physical or psychological discomfort (13%). Convenience was the top reason for wanting to use the SI (49%), followed by timing or dosage frequency (26%) and not having to take a daily pill (10%). Top reasons for not wanting the SI included physical or psychological discomfort (48%), lack of information (19%), and concerns about safety or effectiveness (14%).
Table 2.
Open-Ended Responses to Preference Reasons
| Would (n = 79) | n (%) | Would not (n = 13) | n (%) |
|---|---|---|---|
| Long-acting injectable | |||
| Would not have to take a daily pill | 34 (43) | Does not like needles | 6 (46) |
| Convenience | 24 (30.4) | Safety or effectiveness | 3 (23.1) |
| Timing or dosage frequency | 9 (11.4) | Logistical difficulty | 2 (15.4) |
| Ease of mind | 9 (11.4) | Inconvenience | 2 (15.4) |
| Safety or effectiveness | 3 (3.8) | ||
| Would (n = 14) | n (%) | Would not (n = 73) | n (%) |
|---|---|---|---|
| Rectal microbicide | |||
| Convenience | 5 (35.7) | Safety or effectiveness | 22 (30.1) |
| Timing or dosage frequency | 3 (21.4) | Inconvenience | 16 (21.9) |
| Perceptions of fewer side effects | 2 (14.3) | Logistical difficulty | 9 (12.3) |
| Would not have to take a daily pill | 1 (7.1) | Sexual positioning | 9 (12.3) |
| Logistical ease | 1 (7.1) | Physical or psychological discomfort | 5 (6.9) |
| Initial positive reaction | 1 (7.1) | Initial negative reaction | 4 (5.5) |
| Sexual pleasure | 1 (7.1) | Timing or dosage frequency | 4 (5.5) |
| Sexual pleasure | 3 (4.1) | ||
| Concerns about side effects | 1 (1.4) | ||
| Would (n = 29) | n (%) | Would not (n = 61) | n (%) |
|---|---|---|---|
| Antibody infusion | |||
| Convenience | 13 (44.8) | Timing or dosage frequency | 16 (26.2) |
| Timing or dosage frequency | 7 (24.1) | Does not like needles | 10 (16.4) |
| Would not have to take a daily pill | 7 (24.1) | Physical or psychological discomfort | 8 (13.1) |
| Logistical ease | 1 (3.5) | Inconvenience | 8 (13.1) |
| Safety or effectiveness | 1 (3.5) | Logistical difficulty | 7 (11.5) |
| Initial negative reaction | 5 (8.2) | ||
| Safety or effectiveness | 5 (8.2) | ||
| Concerns about side effects | 1 (1.6) | ||
| Lack of information | 1 (1.6) | ||
| Would (n = 72) | n (%) | Would not (n = 21) | n (%) |
|---|---|---|---|
| Subdermal implant | |||
| Convenience | 35 (48.6) | Physical or psychological discomfort | 10 (47.6) |
| Timing or dosage frequency | 18 (26.4) | Lack of information | 4 (19) |
| Would not have to take a daily pill | 10 (13.9) | Safety or effectiveness | 3 (14.3) |
| Ease of mind | 3 (4.1) | Initial negative reaction | 2 (9.5) |
| Logistical ease | 2 (2.8) | Inconvenience | 1 (4.8) |
| Initial positive reaction | 2 (2.8) | Concerns about side effects | 1 (4.8) |
| Logistical difficulty | 1 (1.4) | ||
Multi-variable analysis for preferences of next-generation PrEP
In multivariate logistic analyses, black and Hispanic MSM were more likely (odds ratio [OR]: 2.45, 95% confidence interval [CI]: 0.86–6.89) to prefer the LAI over daily oral PrEP. MSM with public insurance also had an increased odds (adjusted odds ratio [aOR]: 2.80, 95% CI: 0.71–11.1) of preferring the LAI (Table 3). Preference for the RM was associated with being a current student (aOR: 3.9, 95% CI: 0.61–25.26), having public insurance coverage (aOR: 4.11, 95% CI: 0.87–19.46), and reporting one to two sexual partners in the past 6 months (aOR: 6.68, 95% CI: 0.72–61.52). Participants who reported sex with an HIV-positive partner in the past 6 months were 155% (aOR: 2.55, 95% CI: 0.93–7.03) more likely to prefer the AI. MSM who identified as gay had increased odds (OR: 3.07, 95% CI: 0.86–10.89) of preferring the SI over oral PrEP, as were those with three to five sexual partners in the past 6 months (aOR: 3.15, 95% CI: 0.75–13.17). Preference for the SI was also associated with increased odds of a recent STD diagnosis (aOR: 2.10, 95% CI: 0.78–5.65), reporting receptive anal (aOR: 2.39, 95% CI: 0.97–5.92) or condomless sex in the past 6 months (aOR: 1.93, 95% CI: 0.78–4.78), and low (aOR: 2.71, 95% CI: 0.70–10.53) or medium self-reported HIV risk (aOR: 4.76, 95% CI: 0.80–27.08). Participants with a high school education or less were also 92% (aOR: 0.08, 95% CI: 0.01–0.61) less likely to prefer the SI to daily oral PrEP.
Table 3.
Crude and Adjusted Odds of Preference for Each Formulation Over Oral Pre-Exposure Prophylaxis
| Prefers long-acting injectable (n = 71) | Prefers rectal microbicide (n = 12) | Prefers antibody infusion (n = 25) | Prefers subdermal implant (n = 69) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Age | 1.03 (0.98–1.09) | 1.01 (0.96–1.07) | 0.99 (0.95–1.04) | 1.01 (0.97–1.05) | ||||
| Total barriers to oral PrEPa | 1.0 (0.78–1.28) | 0.99 (0.74–1.32) | 1.02 (0.75–1.37) | 1.14 (0.78–1.65) | 1.16 (0.92–1.46) | 1.16 (0.91–1.49) | 1.22 (0.96–1.56)* | 1.27 (0.97–1.66)* |
| Race/Ethnicity | ||||||||
| Not black or Hispanic/Latino | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black or Hispanic/Latino | 2.45 (0.86–6.98)* | 1.58 (0.47–5.29) | 1.45 (0.58–3.63) | 1.07 (0.45–2.51) | ||||
| Sexual orientation | ||||||||
| Bisexual | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Gay | 0.26 (0.03–2.14) | 0.60 (0.12–3.15) | 0.92 (0.22–3.76) | 3.07 (0.86–10.98)* | ||||
| Educationb | ||||||||
| High school or less | NA | NA | 3.38 (0.59–19.21)* | 3.5 (0.53–23.2) | 0.13 (0.21 to 0.79)** | 0.08 (0.01–0.61)** | ||
| College | 0.94 (0.34–2.55) | 1.1 (0.34–3.59) | 0.56 (0.17–1.90) | 0.54 (0.14–2.09) | 0.52 (0.10–2.59) | 1.9 (0.62–5.87) | 5.83 (1.03–32.91)** | 0.64 (0.23–1.82) |
| Graduate school | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Studentc | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.04 (0.26–4.16) | 1.29 (0.26–6.3) | 2.77 (0.64–11.94) | 3.9 (0.61–25.26)* | 1.33 (0.37–4.78) | 1.19 (0.28–4.98) | 2.75 (0.57–13.23) | 2.97 (0.54–16.31) |
| Incomed | ||||||||
| ≤ $29,999 | 0.85 (0.26–2.79) | 0.67 (0.15–3.02) | 1.22 (0.31–4.76) | 0.98 (0.16–6.11) | 1.69 (0.58–4.97) | 1.21 (0.30–4.79) | 0.71 (0.26–1.91) | 0.79 (0.22–2.84) |
| $30,000–$59,999 | 0.52 (0.16–1.61) | 0.56 (0.13–2.41) | 0.50 (0.09–2.66) | 0.52 (0.06–4.39) | 0.83 (0.27–2.62) | 0.56 (0.14–2.28) | 1.30 (0.44–3.81) | 1.82 (0.48–6.87) |
| ≥ $60,000 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Relationship status | ||||||||
| Single | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Monogamous | 1.86 (0.47–7.29) | 1.17 (0.28–4.87) | 0.98 (0.32–2.94) | 1.79 (0.60–5.37) | ||||
| Nonmonogamous | 1.61 (0.41–6.41) | 0.37 (0.04–3.14) | 0.72 (0.24–2.12) | 0.66 (0.17–2.60) | ||||
| Insurancee | ||||||||
| Private | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Public | 2.5 (0.76–8.21)* | 2.8 (0.71–11.1)* | 3.45 (0.96–12.34)* | 4.11 (0.87–19.46)* | 1.39 (0.51–3.78) | 1.08 (0.34–3.38) | 1.09 (0.43–2.77) | 1.28 (0.42–3.89) |
| Unstable housingf | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.60 (0.10–3.50) | 0.44 (0.59–3.24) | 15.17 (2.23–103.02)** | 13.89 (1.73–111.83)* | 0.96 (0.10–9.66) | 0.7 (0.06–8.47) | 1.85 (0.20–17.23) | 1.75 (0.17–18.44) |
| No. of sex partners, past 6 monthsg | ||||||||
| 0 partners | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–2 partners | 1.63 (0.36–7.33) | 1.57 (0.34–7.12) | 6.63 (0.73–60.22)* | 6.68 (0.72–61.52)* | 2.33 (0.52–10.57) | 2.13 (0.46–9.82) | 2.40 (0.65–8.81) | 2.36 (0.64–8.71) |
| 3–5 partners | 1.25 (0.27–5.73) | 1.31 (0.28–6.1) | 4.20 (0.40–44.40) | 3.85 (0.36–41.35) | 1.62 (0.31–8.48) | 1.56 (0.29–8.31) | 3.15 (0.75–13.17)* | 3.06 (0.72–12.95)* |
| 6 or more partners | 1.20 (0.33–4.29) | 1.32 (0.36–4.9) | 1.11 (0.09–12.92) | 0.96 (0.08–11.48) | 2.60 (0.63–10.74) | 2.45 (0.57–10.5) | 2.18 (0.71–6.69) | 2.09 (0.66–6.622) |
| STD diagnosish | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes, ever | 2.05 (0.54–7.69) | 2.1 (0.43–10.38) | 1.18 (0.16–8.92) | 0.81 (0.08–7.86) | 0.69 (0.17–2.77) | 0.59 (0.122–2.83) | 1.90 (0.63–5.73) | 3.26 (0.75–14.26) |
| Yes, past 6 months | 1.32 (0.45–3.87) | 1.2 (0.37–3.92) | 4.12 (0.81–20.93)* | 3.23 (0.52–20.1) | 2.72 (0.90–8.19)* | 2.85 (0.082–9.9) | 2.10 (0.78–5.65)* | 2.64 (0.85–8.19)* |
| Receptive sex, past 6 months | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 0.96 (0.33–2.79) | 0.70 (0.19–2.52) | 1.13 (0.41–3.10) | 2.39 (0.97–5.92)* | ||||
| Condomless sex, past 6 monthsi | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.84 (0.29–2.42) | 0.41 (0.69–2.42) | 1.43 (0.36–5.66) | 1.2 (0.22–6.74) | 3.15 (0.98–10.16)* | 3.86 (0.6–18.02) | 1.93 (0.78–4.78)* | 1.33 (0.34–5.17) |
| Sex with HIV positive partner, past 6 months | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 0.53 (0.18–1.54) | 1.08 (0.27–4.33) | 2.55 (0.93–7.03)* | 0.80 (0.31–2.07) | ||||
| Self-reported HIV riskj | ||||||||
| No risk | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Low risk | 0.38 (0.04–3.25) | 0.3 (0.03–3.09) | 0.46 (0.08–2.60) | 0.34 (0.39–3) | 0.68 (0.15–3.04) | 0.43 (0.58–2.8) | 2.71 (0.70–10.53)* | 3.56 (0.66–18.75)* |
| Medium risk | 0.50 (0.04–5.70) | 0.52 (0.04–7.4) | 0.53 (0.06–4.53) | 0.42 (0.03–6.14) | 0.73 (0.12–4.39) | 0.25 (0.03–2.4) | 4.67 (0.80–27.08)* | 7.87 (0.95–65.2)* |
| High risk | 0.25 (0.02–3.04) | 0.26 (0.02–3.93) | 0.44 (0.03–5.88) | 0.14 (0.02–2.98) | 0.50 (0.06–4.00) | 0.19 (0.16–2.2) | 0.67 (0.11–3.92) | 0.82 (0.1–6.93) |
Significant at p < 0.1.
Significant at p < 0.05.
Adjusted models include income, education, age, race/ethnicity, insurance, unstable housing.
Adjusted models include age, race/ethnicity, income.
Adjusted models include age, race/ethnicity, education.
Adjusted models include age, race/ethnicity, education, student status.
Adjusted models include income, age, education.
Adjusted models include age, race/ethnicity income, race, education.
Adjusted models include relationship status.
Adjusted models include age, number of sex partners, condomless sex, relationship status, and self-reported HIV risk.
Adjusted models include relationship status, number of sex partners.
Adjusted models include STD diagnosis, number of sex partners, receptive sex, condomless sex, sex with HIV-positive partner in the past 6 months.
aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
Discussion
MSM who participated in this study endorsed a number of barriers to oral PrEP, including finding a healthcare provider, making appointments, and picking up prescriptions. Black and Hispanic MSM were more likely to experience difficulty keeping PrEP appointments, taking PrEP every day, and staying motivated to take PrEP compared to white or Asian MSM. These findings are consistent with research demonstrating higher structural barriers to PrEP care among black/African American and Hispanic/Latino men.31,32 Most research on barriers to oral PrEP has been conducted among MSM who have not yet initiated PrEP. Our focus on barriers among MSM who are currently taking PrEP provides insight into how these men may struggle with adherence and retention in care as opposed to uptake. It is important to note that structural obstacles to care, including provider availability and perceptions, uninsurance, and underinsurance, will persist even after new modalities become available.33,34
Among our study sample, the SI was the most frequently ranked first PrEP formulation choice, followed by the LAI, daily oral pill, RM, and AI. Formulation rankings suggest that both the SI and LAI are preferred over daily oral PrEP. Of all next-generation formulations, most participants preferred the LAI (65.7%) and the SI (63.9%) to daily oral PrEP. This finding is inconsistent with a 2017 study which found that only 30.8% of MSM currently taking daily oral PrEP would prefer the LAI to daily oral PrEP.20 To our knowledge, this is the only other study to date that has assessed the extent to which MSM already using daily oral PrEP would be willing to switch to next-generation formulations.
Responses to reasons for wanting each formulation demonstrate that not having to take a daily oral pill, convenience, and timing or dosage frequency were the most commonly cited across the next-generation modalities examined in this study. Disliking of needles was the top reason most patients did not want the LAI or the AI. Other top reasons for not wanting next-generation modalities included concerns about safety or effectiveness, physical or psychological discomfort, inconvenience, and logistical difficulty. These findings indicate that ease of use (i.e., convenience, inconvenience, timing or dosage frequency, and logistics of getting/taking the formulation) is a primary reason motivating appeal toward next-generation modalities. Concerns about safety/effectiveness are mutable and should be explicitly addressed by providers once these formulations become available.
Different sociodemographic characteristics and sexual behaviors predicted preference for different next-generation formulations. Black and Hispanic MSM were more likely to prefer all next-generation PrEP formulations to daily oral PrEP, although this difference was only significant at p ≤ 0.10 for the LAI. This finding is generally consistent with next-generation acceptability research, some of which demonstrates more interest in the LAI among black and Hispanic MSM.35 In addition, sexual behaviors, including number of total partners and recent STD diagnosis, were most commonly associated with preference for the SI. These associations should be interpreted with caution, especially concerning their implications for person-centered PrEP care. While next-generation formulations may help MSM overcome barriers to uptake and adherence, individual preferences must always supersede provider inclinations toward a particular modality.
This study has several limitations. First, our moderate sample size did not allow us to examine differences between black and Hispanic MSM. Second, participants self-selected to be part of this study and were already taking daily oral PrEP, thus our findings may not generalize to other populations. Because this research was conducted in STD clinics, we were, however, able to demonstrate the acceptability of these formulations among those already taking daily oral PrEP within a clinical setting where most people receive PrEP care. Assessing preferences in this population also has utility for understanding whether and how next-generation nonoral PrEP formulations may help individuals who are motivated to take PrEP overcome barriers to care. Finally, survey data may be subject to recall or social desirability bias. Recent research has identified inconsistencies in hypothetical willingness and actual intentions in PrEP uptake, highlighting limits inherent in acceptability research.36 All MSM in this study were currently taking daily oral PrEP, demonstrating an established willingness to take this medication. By asking participants to rank each formulation and indicate whether or not they prefer each form to daily oral PrEP, our measures of preference may circumvent the “willingness” versus “intentions” disconnect.
To our knowledge, this is the first study to compare the LAI, RM, AI, and SI with daily oral PrEP, and one of the first to examine next-generation preferences among MSM currently taking daily oral PrEP. Reported barriers to daily oral PrEP, along with racial/ethnic differences in reported barriers, indicate a need for interventions to address these barriers at both the individual and structural levels. Our findings suggest that top reasons for interest in alternative PrEP formulations among MSM already taking oral PrEP are most commonly related to ease of use, although participants also cited concerns about safety or effectiveness and discomfort as a primary deterrent. Finally, next-generation modalities may be preferred over daily oral PrEP by MSM with certain sociodemographic characteristics or sexual behaviors. Ultimately, variations in modality preferences highlight a desire for choice and a need for research on how to translate preferences to person-centered clinical practice and public health policy. Partnerships among government agencies, health care workers, researchers, and patients will be essential for doing so.37 Once these formulations are available, ensuring access through comprehensive insurance coverage for the full range of methods and integration into nonspecialized settings will also be necessary for optimal uptake and retention in PrEP care.
Acknowledgments
The authors are thankful to all of the men who participated in this study.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by National Institute of Allergy and Infectious Diseases grant P30AI042853 (PI: Cu-Uvin) through the Providence/Boston Center for AIDS Research (CFAR) awarded as a CFAR Developmental grant.
References
- 1. HIV in the United States and Dependent Areas | Statistics Overview | Statistics Center | HIV/AIDS | CDC. Available at: https://www.cdc.gov/hiv/statistics/overview/ataglance.html (Last accessed March14, 2019)
- 2. Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol 2017;27:238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet Lond Engl 2016;387:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: Estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. MMWR Morb Mortal Wkly Rep 2015;641291–1295 [DOI] [PubMed] [Google Scholar]
- 5. King HL, Keller SB, Giancola MA, et al. Pre-Exposure Prophylaxis Accessibility Research and Evaluation (PrEPARE study). AIDS Behav 2014;18:1722–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu A, Cohen S, Follansbee S, et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med 2014;11:e1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Philbin MM, Parker CM, Parker RG, Wilson PA, Garcia J, Hirsch JS. The promise of pre-exposure prophylaxis for black men who have sex with men: An ecological approach to attitudes, beliefs, and barriers. AIDS Patient Care STDS 2016;30:282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spinner CD, Boesecke C, Zink A, et al. HIV pre-exposure prophylaxis (PrEP): A review of current knowledge of oral systemic HIV PrEP in humans. Infection 2016;44:151–158 [DOI] [PubMed] [Google Scholar]
- 9. Raifman J, Dean LT, Montgomery MC, et al. Racial and ethnic disparities in HIV pre-exposure prophylaxis awareness among men who have sex with men. AIDS Behav 2019. [Epub ahead of print]; DOI: 10.1007/s10461-019-02462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS Lond Engl 2017;31:731–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garnett M, Hirsch-Moverman Y, Franks J, Hayes-Larson E, El-Sadr WM, Mannheimer S. Limited awareness of pre-exposure prophylaxis among black men who have sex with men and transgender women in New York City. AIDS Care 2018;30:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García M, Harris AL. PrEP awareness and decision-making for Latino MSM in San Antonio, Texas. PLoS One 2017;12:e0184014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brooks RA, Allen VC, Regan R, Mutchler MG, Cervantes-Tadeo R, Lee S-J. HIV/AIDS conspiracy beliefs and intention to adopt preexposure prophylaxis among black men who have sex with men in Los Angeles. Int J STD AIDS 2018;29:375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rolle C-P, Rosenberg ES, Siegler AJ, et al. Challenges in translating PrEP interest into uptake in an observational study of young black MSM. J Acquir Immune Defic Syndr 2017;76:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: The implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med 2008;67:478–486 [DOI] [PubMed] [Google Scholar]
- 16. Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol 2015;41:311–330 [Google Scholar]
- 17. Lykins WR, Luecke E, Johengen D, van der Straten A, Desai TA. Long acting systemic HIV pre-exposure prophylaxis: An examination of the field. Drug Deliv Transl Res 2017;7:805–816 [DOI] [PubMed] [Google Scholar]
- 18. Haire BG. Preexposure prophylaxis-related stigma: Strategies to improve uptake and adherence—A narrative review. HIV AIDS (Auckl) 2015;7:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med 2007;22:1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. John SA, Whitfield THF, Rendina HJ, Parsons JT, Grov C. Will gay and bisexual men taking oral pre-exposure prophylaxis (PrEP) switch to long-acting injectable PrEP should it become available? AIDS Behav 2018;22:1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beymer MR, Gildner JL, Holloway IW, Landovitz RJ. Acceptability of injectable and on-demand pre-exposure prophylaxis among an online sample of young men who have sex with men in California. LGBT Health 2018;5:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parsons JT, Rendina HJ, Whitfield THF, Grov C. Familiarity with and preferences for oral and long-acting injectable HIV pre-exposure prophylaxis (PrEP) in a national sample of gay and bisexual men in the U.S. AIDS Behav 2016;20:1390–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyers K, Wu Y, Brill A, Sandfort T, Golub SA. To switch or not to switch: Intentions to switch to injectable PrEP among gay and bisexual men with at least twelve months oral PrEP experience. PLoS One 2018;13:e0200296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carballo-Diéguez A, Dolezal C, Bauermeister JA, O'Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: Results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect 2008;84:483–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nodin DN, Carballo-Diéguez A, Ventuneac AM, Balan IC, Remien R. Knowledge and acceptability of alternative HIV prevention bio-medical products among MSM who bareback. AIDS Care 2008;20:106–115 [DOI] [PubMed] [Google Scholar]
- 26. Greene GJ, Swann G, Fought AJ, et al. Preferences for long-acting pre-exposure prophylaxis (PrEP), daily oral PrEP, or condoms for HIV prevention among U.S. men who have sex with men. AIDS Behav 2017;21:1336–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koblin BA, Chesney MA, Husnik MJ, et al. High-risk behaviors among men who have sex with men in 6 US cities: Baseline data from the EXPLORE study. Am J Public Health 2003;93:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold T, Brinkley-Rubinstein L, Chan PA, et al. Social, structural, behavioral and clinical factors influencing retention in pre-exposure prophylaxis (PrEP) care in Mississippi. PLoS One 2017;12:e0172354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan PA, Glynn TR, Oldenburg CE, et al. Implementation of preexposure prophylaxis for human immunodeficiency virus prevention among men who have sex with men at a New England sexually transmitted diseases clinic. Sex Transm Dis 2016;43:717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc 2016;19:20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eaton LA, Driffin DD, Smith H, Conway-Washington C, White D, Cherry C. Psychosocial factors related to willingness to use pre-exposure prophylaxis for HIV prevention among black men who have sex with men attending a community event. Sex Health 2014;11:244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lelutiu-Weinberger C, Golub SA. Enhancing PrEP access for black and Latino men who have sex with men. J Acquir Immune Defic Syndr 2016;73:547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mullins TLK, Zimet G, Lally M, Xu J, Thornton S, Kahn JA. HIV care providers' intentions to prescribe and actual prescription of pre-exposure prophylaxis to at-risk adolescents and adults. AIDS Patient Care STDS 2017:31:504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marks SJ, Merchant RC, Clark MA, et al. Potential healthcare insurance and provider barriers to pre-exposure prophylaxis utilization among young men who have sex with men. AIDS Patient Care STDS 2017;31:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levy ME, Patrick R, Gamble J, et al. Willingness of community-recruited men who have sex with men in Washington, DC to use long-acting injectable HIV pre-exposure prophylaxis. PLoS One 2017;12:e0183521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rendina HJ, Whitfield THF, Grov C, Starks TJ, Parsons JT. Distinguishing hypothetical willingness from behavioral intentions to initiate HIV pre-exposure prophylaxis (PrEP): Findings from a large cohort of gay and bisexual men in the U.S. Soc Sci Med 2017;172:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DiStefano AS, Takeda M. HIV Pre-exposure prophylaxis and postexposure prophylaxis in Japan: Context of use and directions for future research and action. AIDS Patient Care STDS 2017;31:60–77 [DOI] [PubMed] [Google Scholar]