Abstract
Introduction
Stunting is a key issue for adolescents with perinatally acquired HIV (APH) that needs to be better understood. As part of the IeDEA multiregional consortium, we described growth evolution during adolescence for APH on antiretroviral therapy (ART).
Methods
We included data from sub‐Saharan Africa, the Asia‐Pacific, and the Caribbean, Central and South America regions collected between 2003 and 2016. Adolescents on ART, reporting perinatally acquired infection or entering HIV care before 10 years of age, with at least one height measurement between 10 and 16 years of age, and followed in care until at least 14 years of age were included. Characteristics at ART initiation and at 10 years of age were compared by sex. Correlates of growth defined by height‐for‐age z‐scores (HAZ) between ages 10 and 19 years were studied separately for males and females, using linear mixed models.
Results
Overall, 8737 APH were included, with 46% from Southern Africa. Median age at ART initiation was 8.1 years (interquartile range (IQR) 6.1 to 9.6), 50% were females, and 41% were stunted (HAZ<−2 SD) at ART initiation. Males and females did not differ by age and stunting at ART initiation, CD4 count over time or retention in care. At 10 years of age, 34% of males were stunted versus 39% of females (p < 0.001). Females had better subsequent growth, resulting in a higher prevalence of stunting for males compared to females by age 15 (48% vs. 25%) and 18 years (31% vs. 15%). In linear mixed models, older age at ART initiation and low CD4 count were associated with poor growth over time (p < 0.001). Those stunted at 10 years of age or at ART initiation had the greatest growth improvement during adolescence.
Conclusions
Prevalence of stunting is high among APH worldwide. Substantial sex‐based differences in growth evolution during adolescence were observed in this global cohort, which were not explained by differences in age of access to HIV care, degree of immunosuppression or region. Other factors influencing growth differences in APH, such as differences in pubertal development, should be better documented, to guide further research and inform interventions to optimize growth and health outcomes among APH.
Keywords: HIV, adolescent, growth, stunting, cohort studies, developing countries
1. Introduction
Adolescence, defined by the World Health Organization (WHO) as between 10 and 19 years of age 1, is a critical transition period in life, accompanied by significant biological and psychosocial changes 2. In the context of HIV, major improvements in access to antiretroviral therapy (ART) for children with perinatally acquired HIV infection have led to reductions in HIV‐related mortality, resulting in a growing population of adolescents living with perinatally acquired HIV (APH). In 2016, it was estimated that 2.1 million adolescents were living with HIV worldwide, with 80% in sub‐Saharan Africa 3. AIDS‐related deaths are one of the leading causes of mortality among adolescents in this region 4. Adolescents living with HIV face specific challenges in terms of HIV care and outcomes 5, including HIV disclosure issues 6, access to sexual and reproductive health services 7 and transition from paediatric to adult care 8. Adolescents are reported to have the lowest adherence to ART 9 and high rates of lost‐to‐follow‐up (LTFU) 10.
Growth deficiency is a key concern for children and adolescents living with HIV 11, 12. There are overlapping effects on growth resulting from a vicious cycle between malnutrition and HIV infection. Malnutrition impairs the immune system and weakens the body’s defences, leading to HIV disease progression 13; while HIV infection, related opportunistic infections and chronic inflammation may weaken nutritional status 14, 15. Repeated episodes of malnutrition during infancy and childhood may have occurred frequently among APH, and lifelong HIV infection can lead to chronic malnutrition and growth retardation as APH age into adulthood. Adolescence is the second most important period of growth after the first year of life 16, hence these factors may hinder pubertal growth spurt for APH. Chronic diseases, malnutrition and HIV infection during childhood could also delayed puberty, worsening growth retardation during adolescence for APH 17, 18, 19, 20.
While data show that nearly half of adolescents suffer from stunting in some developing countries 21, data on growth and stunting are limited among APH. Several studies have shown that growth improves after ART initiation, with larger increases for children initiated earlier on ART 22, 23, but most of these studies were conducted among children younger than 10 years of age or during the first two years on ART. Little is known about growth during adolescence among APH and those on long‐term ART. As part of the IeDEA (International epidemiology Databases to Evaluate AIDS) multiregional consortium, we described growth evolution and its associated factors during adolescence for males and females with perinatally acquired HIV.
2. Methods
2.1. Study population
The IeDEA global paediatric collaboration is part of the global IeDEA research consortium (https://www.iedea.org/), supported by the US National Institutes of Health since 2006, to describe HIV epidemiology and evaluate HIV outcomes using large patient‐level observational databases. HIV care cohorts from sub‐Saharan Africa (West, Central, Eastern and Southern Africa regions), the Asia‐Pacific, and the Caribbean, Central and South America (CCASAnet) regions contributed data for this analysis. We merged data collected from HIV clinics in partnership with IeDEA from these six sub‐regions between 2003 and 2016. Among patients who had at least one visit available during adolescence, with at least one height measurement during this period (i.e. age 10 to 19 years), inclusion criteria to assess growth throughout adolescence were as follows: reported perinatally acquired infection or entering HIV care before 10 years of age (proxy for perinatally acquired HIV when not documented); having a documented date of ART initiation, at least one height measurement between 10 and 16 years of age; and followed in care until at least 14 years of age. APH were thus excluded if known to have acquired HIV non‐perinatally or if not known, be enrolled in care after 10 years of age. They were then excluded if no ART initiation (excluded group A) and no HAZ measurements between 10 and 16 years of age (excluded group B) were documented, and if they were not followed after 14 years of age (excluded group C).
2.2. Variables and data management
We studied growth using height‐for‐age, defined by the WHO child growth standards 24, 25, expressed in z‐scores (HAZ). Stunting was defined as HAZ lower than − 2 standard deviations (SD), with moderate stunting between − 3 and − 2 SD and severe stunting lower than − 3 SD. Z‐scores lower than − 10 or greater than + 10 were viewed as outliers and removed. Growth velocity defined as height gain in centimetre (cm) per year was compared to the reference population from the WHO child growth standards for a subset of adolescents having at least two height measurements. We calculated growth velocity per year by dividing the height gain in cm between two clinical visits by the time in years between these two visits. Velocities greater than 20 cm/year and below − 1 cm/year were excluded as outliers. Wasting was defined using weight‐for‐height z‐score (WHZ) for children under five years of age, and body‐mass‐index‐for‐age z‐score (BAZ) for children aged more than five years. Immunodeficiency for age at ART initiation was defined following the 2006 WHO guidelines 26. The location of the clinic site (urban or mostly urban, rural or mostly rural) was also recorded. Follow‐up for patients was analysed on a six‐monthly basis, with data on immunologic, clinical, anthropometric status and ART regimen collected. Loss to follow‐up was defined as having a last contact longer than six months before database closure in children not known to have died or transferred out, and transfer was documented when adolescents were transferred to another paediatric clinic or to adult care.
2.3. Ethics approval
Each participating IeDEA region obtained local institutional review boards’ approvals to participate. Consent requirements were deferred to the local institutional review boards. The analysis only used anonymized data that had been collected as part of routine clinical care.
2.4. Statistical analysis
Patient characteristics were described at ART initiation and at age 10 years, and compared by sex using Chi‐square tests for categorical variables and Kruskal‐Wallis tests for continuous variables. Characteristics of the study population were also compared to those of excluded adolescents. Prevalence of stunting was measured at ART initiation and each year between 10 and 19 years of age, stratified by sex. Mean HAZ, together with 95% confidence intervals, was plotted stratified by age and sex.
The analysis and inclusion to the study started at age 10 years (baseline). Growth, modelled as mean HAZ between ages 10 and 19 years, was studied using linear mixed models, with an unstructured variance‐covariance matrix and random intercept. All participants with at least one HAZ were able to contribute to the model 27. The model contained time‐varying age (=10 years plus follow‐up time) as a covariate which was included non‐linearly using (penalized) splines 28. Age at ART initiation, stunting and wasting at ART initiation and at age 10 years, current CD4 count, CD4 count at age 10 years, CD4 count at ART initiation and at first visit as well as region and location of clinic site were considered as potential adjustment variables. To take into account missing data, especially at baseline, we conducted multiple imputation for stunting and wasting at age 10 years and ART initiation as well as CD4 count and location, using the Amelia II package in R 29. The imputation model contained all measured variables, as well as splines of time and lagged variables of CD4 count. Variable selection using the AIC criteria was applied to the imputed data sets 30, 31. The final mixed model containing the selected variables was fitted in all five imputed data sets and results were combined according to Rubin’s rules 32. The penalized spline for the association of age with HAZ is displayed for the first imputed data set (see Figure S1 for the other imputed data sets).
3. Results
3.1. Selection and characteristics of the study population
Between 2003 and 2016, 68,461 patients receiving care in the six IeDEA regional cohorts had at least one visit during adolescence. Of these, 50,469 (74%) had one available HAZ measurement during the same period, and 21,038 (42%) of them met criteria for perinatally acquired infection. Among APH on ART, the majority had at least one available HAZ measurement between 10 and 16 years of age. The median age at last follow‐up was 14.2 (interquartile range (IQR) 12.1 to 16.5 years), with 43% followed up until at least 14 years of age. Among the APH excluded for not being followed after 14 years of age, 73% had not yet reached age 14 years by the time the database was closed. The final study population included 8737 APH (Figure 1).
Figure 1.
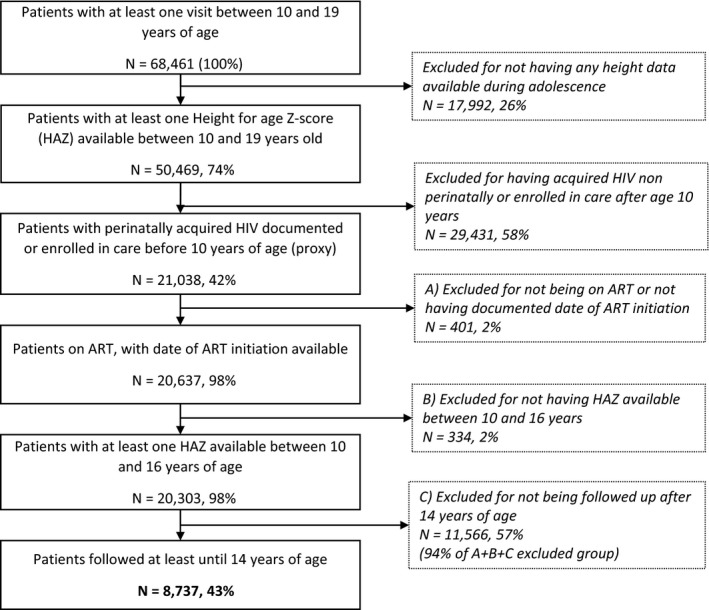
Flowchart of the study population, IeDEA global paediatric collaboration, 2003 to 2016.
APH excluded for three criteria (no information on ART initiation (A), no HAZ measurements between 10 and 16 years of age (B), no follow‐up after 14 years of age (C), Figure 1) were compared to the final study population. Those from the exclusion group A (n = 401) were more frequently LTFU (22% vs. 8%) and less stunted (18% vs. 29%, p < 0.001). The exclusion group B (n = 334) initiated ART mostly after age 10 years (63% vs. 17%, p < 0.001), with a higher rate of death (4% vs. 2%, p = 0.003) and LTFU (39% vs. 8%, p < 0.001). Finally, the exclusion group C (n = 11,566) was more likely to have started ART below five years of age compared to the study population (34% vs. 15%, p < 0.001), they more frequently had missing data for assessing stunting, wasting and CD4 count at 10 years (p < 0.001) and had slightly higher rates of LTFU (10% vs. 8%, p < 0.001) but mortality was similar. The latter group comprised 94% of those excluded for these three reasons and are mostly children who had not yet reached the age of 14 years as they had started ART more recently and at younger ages and could not contribute to examining growth throughout adolescence (Table S1).
Overall, 50.5% were females, 45.6% were living in Southern Africa, and 76.7% were followed in an urban or mostly urban clinical care centre. Median age at ART initiation was 8.1 years (IQR 6.1 to 9.6), with 84.8% initiating ART after age five years. The prevalence of stunting at ART initiation was 49.6% among those with available HAZ measurement (n = 6560); of these, 45.9% were severely stunted. Among those with available WHZ/BAZ data (n = 7103), 18% were wasted at ART initiation and 10% at age 10 years. Overall, characteristics at ART initiation did not differ by sex, with similar age groups (p = 0.186), CD4 count (p = 0.063) and stunting (p = 0.101). Males were slightly more wasted than females at ART initiation (p = 0.010). The absolute differences in median age at ART initiation (males vs. females: 8.02 vs. 8.19, p = 0.007) and median HAZ (males vs. females: −2.05 vs. −1.96, p = 0.020) were minimal (Table 1). At age 10 years, the prevalence of stunting was 34.4% among males and 39.5% among females (p < 0.001), with about one‐third severely stunted in each group. There were no observed differences by sex in CD4 count groups or the severity of wasting (Table 1).
Table 1.
Characteristics at ART initiation and at 10 years of age for males and females, N = 8737, IeDEA global paediatric collaboration, 2003 to 2016
| Variables | Males (N = 4329) | Females (N = 4408) | p‐valuea | Total | |||
|---|---|---|---|---|---|---|---|
| Characteristics at ART initiation | |||||||
| Age | |||||||
| Median, IQR (years) | 8.0 [6.1 to 9.5] | 8.2 [6.2 to 9.6] | 0.007 | 8.1 [6.1 to 9.6] | |||
| Age groups | 0.186 | ||||||
| 0 to 2 years | 146 | 3.4 | 166 | 3.8 | 312 | 3.6 | |
| 2 to 5 years | 527 | 12.2 | 488 | 11.1 | 1015 | 11.6 | |
| 5 to 10 years | 2950 | 68.1 | 2959 | 67.1 | 5909 | 67.6 | |
| >10 years | 706 | 16.3 | 795 | 18.0 | 1501 | 17.2 | |
| CD4 count | |||||||
| Median, IQR (cell/mm3) | 294 [130 to 517] | 307 [148 to 516] | 301 [139 to 516] | ||||
| Immunodeficiency for ageb | 0.148 | ||||||
| No | 604 | 13.9 | 643 | 14.6 | 1247 | 14.3 | |
| Moderate | 1008 | 23.3 | 1087 | 24.7 | 2095 | 24.0 | |
| Severe | 1598 | 36.9 | 1620 | 36.7 | 3218 | 36.8 | |
| Missing | 1119 | 25.9 | 1058 | 24.0 | 2177 | 24.9 | |
| HAZ | |||||||
| Median, IQR (z‐score) | −2.05 [−2.95 to −1.16] | −1.96 [−2.85 to −1.05] | 0.020 | −1.99 [−2.90 to −1.11] | |||
| Stunting groups | 0.101 | ||||||
| No stunting | 1742 | 40.2 | 1873 | 42.5 | 3615 | 41.4 | |
| Moderately stunted | 949 | 21.9 | 974 | 22.1 | 1923 | 22.0 | |
| Severely stunted | 837 | 19.3 | 795 | 18.0 | 1632 | 18.7 | |
| Missing | 801 | 18.5 | 766 | 17.4 | 1567 | 17.9 | |
| WHZ/BAZ | |||||||
| Wasting groups | 0.010 | ||||||
| No wasting | 2828 | 65.3 | 3010 | 68.3 | 5838 | 66.8 | |
| Moderately wasted | 362 | 8.4 | 346 | 7.8 | 708 | 8.1 | |
| Severely wasted | 306 | 7.1 | 251 | 5.7 | 557 | 6.4 | |
| Missing | 833 | 19.2 | 801 | 18.2 | 1634 | 18.7 | |
| Characteristics at 10 years | |||||||
| CD4 count | |||||||
| Median, IQR (cell/mm3) | 652 [410 to 920] | 674 [434 to 952] | 0.008 | 664 [423 to 938] | |||
| CD4 count groups | 0.133 | ||||||
| <250 | 297 | 6.9 | 272 | 6.2 | 569 | 6.5 | |
| >250 | 2292 | 52.9 | 2420 | 54.9 | 4712 | 53.9 | |
| Missing | 1740 | 40.2 | 1716 | 38.9 | 3456 | 39.6 | |
| HAZ | |||||||
| Median, IQR (Z‐score) | −1.59 [−2.32 to −0.87] | −1.70 [−2.48 to −0.90] | <0.001 | −1.64 [−2.41 to −0.89] | |||
| Stunting groups | <0.001 | ||||||
| No stunting | 2221 | 51.3 | 2079 | 47.2 | 4300 | 49.2 | |
| Moderately stunted | 854 | 19.7 | 900 | 20.4 | 1754 | 20.1 | |
| Severely stunted | 310 | 7.2 | 460 | 10.4 | 770 | 8.8 | |
| Missing | 944 | 21.8 | 969 | 22.0 | 1913 | 21.9 | |
| WHZ/BAZ | |||||||
| Wasting groups | 0.233 | ||||||
| No wasting | 3109 | 71.8 | 3179 | 72.1 | 6288 | 72.0 | |
| Moderately wasted | 259 | 6.0 | 257 | 5.8 | 516 | 5.9 | |
| Severely wasted | 122 | 2.8 | 95 | 2.2 | 217 | 2.5 | |
| Missing | 839 | 19.4 | 877 | 19.9 | 1716 | 19.6 | |
| Context | |||||||
| Location of clinical centres | |||||||
| Urban or mostly urban | 3321 | 76.7 | 3438 | 78.0 | 0.121 | 6759 | 77.4 |
| Rural or mostly rural | 947 | 21.9 | 895 | 20.3 | 1842 | 21.1 | |
| Missing | 61 | 1.4 | 75 | 1.7 | 136 | 1.6 | |
| Regions | 0.022 | ||||||
| West Africa | 361 | 8.3 | 345 | 7.8 | 706 | 8.1 | |
| Central Africa | 200 | 4.6 | 200 | 4.5 | 400 | 4.6 | |
| East Africa | 741 | 17.1 | 752 | 17.1 | 1493 | 17.1 | |
| Southern Africa | 2028 | 46.9 | 1956 | 44.4 | 3984 | 45.6 | |
| Asia‐Pacific | 724 | 16.7 | 817 | 18.5 | 1541 | 17.6 | |
| CCASAnet | 275 | 6.4 | 338 | 7.7 | 613 | 7.0 | |
BAZ, BMI‐for‐Age Z‐score, WHO Child Growth Standards; HAZ, Height‐for‐Age Z‐score; IQR, interquartile range; WHZ, Weight‐for‐Height Z‐score.
Comparison tests between males and females using chi square and Kruskal‐Wallis tests.
WHO 2006 guidelines.
Median number of height measurements was 21 (IQR 11 to 33). Males and females had similar retention, with a median age at enrolment (clinic entry) of 7.1 years (IQR 5.1 to 8.6, p = 0.425) and median age at last visit of 16.1 years (IQR 14.9 to 17.7, p = 0.160); 16.7% were transferred (p = 0.435), 7.6% were LTFU (p = 0.966) and 1.9% died during adolescence (p = 0.283) (data not shown).
3.2. Growth development during adolescence
3.2.1. Growth velocity curves
In patients with at least two height measurements (n = 6397), growth velocity during adolescence was highest at age 13 years for APH males (mean value: 7.2 cm per year) while the peak growth velocity was reached at age 11 for APH females (mean value: 6.4 cm per year). Overall, growth velocity among males was similar to the reference population at age 10 but then diverged with relative reductions among APH until age 15 (mean value at age 13 years: 5.5 cm per year). After this period, while growth velocity for the reference population decreased, it stayed at a higher level for APH throughout late adolescence (mean values: 4.7 vs. 3.5 cm per year at 16 years, 1.9 vs. 0.3 cm per years at 19 years). For females, growth velocity was slightly lower than reference values for APH until age 12 years (mean value at 11 years: 6.1 cm per year) and then stayed consistently higher (means values: 3.2 vs. 1.6 cm per year at 15 years, 1.7 vs. 0.3 cm per year at 17 years, 1.2 vs. 0.1 cm per year at 19 years) (Figure 2).
Figure 2.
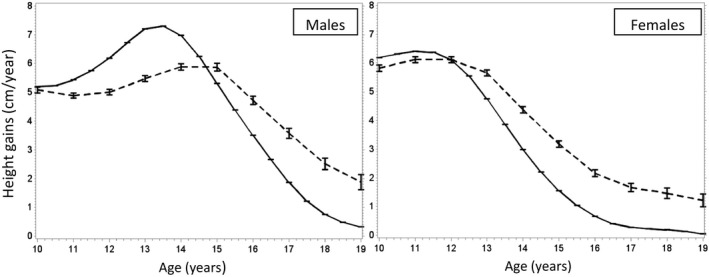
Mean height gain in cm per year for males (left) and females (right) during adolescence (dashed line), compared to the reference population of the WHO child growth standards (solid line). IeDEA global paediatric collaboration, 2003 to 2016.
3.2.2. Prevalence of stunting and HAZ curves
HAZ evolution during adolescence differed by sex and was in line with the deviations observed in growth velocity. For males, the growth deficit observed in early adolescence resulted in a decrease of mean HAZ between 10 and 14 years of age (mean values: −1.6 SD at 10 years, −2.1 SD at 14 years), followed by a constant increase until age 19 years, reaching similar values to those at age 10 (mean value: −1.43 SD at 19 years). For females, mean HAZ increased continuously during adolescence, especially after age 12 years (mean values: −1.7 SD at 12 years, −1.3 SD at 15 years, −1.0 SD at 19 years). HAZ was higher for females than males after age 12 years, leading to a higher prevalence of stunting for males (51% vs. 35% at 13 years, p < 0.001; 31% vs. 15% at 18 years, p < 0.001) (Figure 3, left‐hand side). The imputed mixed model resulted in adjusted HAZ estimates close to the crude values (Figure 3, right‐hand side). Trends in growth evolution by sex were similar by region (Figure S2).
Figure 3.
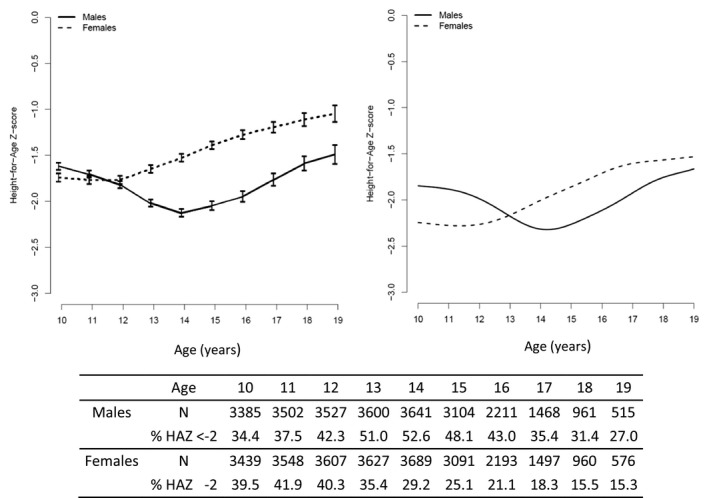
Mean Height‐for‐Age Z‐score (HAZ) evolution during adolescence with sample size and prevalence of stunting at each age (N = 8737). Crude results (left) and adjusted estimates of the first imputed mixed model (right) using the following reference population: ART start at age 5, current CD4 = 100, CD4 at age 10 = 100, not rural, moderate stunting at age 10 and ART start, region Asia‐Pacific. IeDEA global paediatric collaboration, 2003 to 2016.
3.2.3. Factors associated with HAZ evolution
Multivariable imputed linear mixed models are shown in Table 2. Most of the factors associated with HAZ evolution were common for both males and females. Older age at ART initiation was associated with a lower mean HAZ over time (example for males: mean difference of −0.149 SD for each year over time, p < 0.001). Higher CD4 count over time was associated with higher HAZ (example for females: +0.019 SD per 100 cells/mm3). Those with moderate or severe stunting at ART initiation had lower mean HAZ at baseline (p < 0.001) but also higher HAZ increase between 10 and 19 years of age compared to those not stunted (p < 0.001). For males, growth evolution also depended on CD4 count at baseline (+0.003 SD per 100 cells/mm3 over time). Males who were followed in rural HIV clinic centres had lower HAZ increase compared to those followed in urban centers (−0.047 SD per year, p < 0.001), while mean HAZ was similar at 10 years of age (p = 0.289).
Table 2.
Factors associated with HAZ evolution between 10 and 19 years of age for males and females
| Variables | Males | p‐value | Differences in yearly mean (SD) HAZ increase | p‐value | Females | p‐value | Differences in yearly mean (SD) HAZ increase | p‐value |
|---|---|---|---|---|---|---|---|---|
| Differences in mean (SD) HAZ at age 10 or over time | Differences in mean (SD) HAZ at age 10 or over time | |||||||
| Age at ART initiation (years) | −0.149 (−0.019) | <0.001 | −0.113 (0.017) | <0.001 | ||||
| Current CD4 count (per 100 cells/mm3) | 0.014 (0.001) | <0.001 | 0.019 (0.004) | 0.004 | ||||
| CD4 count at 10 years (per 100 cells/mm3) | 0.003 (0.001) | 0.005 | – | – | ||||
| Stunting at ART initiation | ||||||||
| Moderate versus no | −0.322 (0.022) | <0.001 | 0.018 (0.038) | <0.001 | −0.307 (0.024) | <0.001 | 0.023 (0.003) | <0.001 |
| Severe versus no | −0.229 (0.018) | <0.001 | 0.013 (0.002) | <0.001 | −0.255 (0.018) | <0.001 | 0.013 (0.002) | <0.001 |
| Stunting at 10 years of age | ||||||||
| Moderate versus no | −0.543 (0.028) | <0.001 | 0.007 (0.009) | <0.001 | −0.909 (0.023) | <0.001 | 0.127 (0.003) | <0.001 |
| Severe versus no | −0.306 (0.018) | <0.001 | −0.007 (0.006) | 0.234 | −0.639 (0.021) | <0.001 | 0.088 (0.002) | <0.001 |
| Located in rural versus urban area | 0.059 (0.045) | 0.289 | −0.047 (0.003) | <0.001 | −0.010 (3.831) | 0.795 | – | – |
Moderate stunting = (−3; −2 (SD, Severe stunting <−3 SD)). Multivariable linear mixed models. N = 8737, IeDEA global paediatric collaboration, 2003 to 2016. Adjusted on IeDEA regions. Interaction with time was added for the variables “stunting” for both males and females and location for males. Difference in estimates were thus expressed in mean HAZ at baseline (10 years of age) and in yearly mean HAZ increase (second column) for these variables. The other variables (age at ART initiation, current CD4 count and CD4 count at 10 years for both males and females and location for females) were expressed in difference in estimates over time (first column). Estimates for time variables: Males: t = −0.203, t2 = −0.014, t3 = −0.001; females: t = −0.149, t2 = 0.053, t3 = −0.004.
4. Discussion
Growth retardation represents a major concern for APH in IeDEA cohorts; half of APH were stunted at ART initiation, and the prevalence of stunting remained high during adolescence. While males and females had similar baseline and follow‐up characteristics, their growth patterns during adolescence diverged considerably. Despite males experiencing greater height gains later in adolescence, these were not sufficient to compensate for the smaller than expected height gains in early adolescence, resulting in persistently high rates of stunting until 19 years of age. Females had substantial height gains in mid‐ to late adolescence, above the population reference, allowing them to have a slow but constant HAZ increase throughout adolescence; resulting in a much lower prevalence of stunting by 19 years of age.
Few studies on growth have been conducted among APH, most of them focusing on the first years of ART 17. Some cross‐sectional studies have described stunting among adolescents with HIV. In West and Central Africa, among an ART‐treated population, the prevalence of stunting was 34% among patients aged 10 to 19 years 33 and 42% among patients aged 10 to 16 years in Senegal 34. In El Salvador, 48% of children with HIV over 12 years of age were stunted 35. In the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) including cohorts of APH from IeDEA and other networks in low‐, middle‐ and high‐income countries, median HAZ at ART initiation and at 10 years of age were similar to our study; however, APH living in high‐income countries had higher HAZ than in low‐and‐middle‐income countries (LMICs) 36.
In our cohort, late age at ART initiation was strongly associated with stunting and poor HAZ evolution. This finding has been observed in many other studies, but mostly among children younger than age 10 years 22, 23. Children who spend their first years of life with HIV without ART may have chronic inflammation due to uncontrolled HIV, which can affect growth. The longer this exposure to uncontrolled HIV, the more adversely growth might be affected. Repeated opportunistic infections like pneumonia, chronic diarrhoea and tuberculosis during this period can also result in stunting. It is concerning that despite the WHO recommendations for immediate ART initiation, age at ART initiation was high, approximately a year later than age at enrolment. This population likely had poor access to ART, as already highlighted by the IeDEA collaboration 37. While reasons for delayed ART initiation should be examined, most of these children would have first enrolled before the WHO recommendation for immediate ART in all children < 15 years of age, and hence would have had to meet disease severity or CD4 criteria before initiating ART. With better access to paediatric care and earlier ART initiation than during the study period for children living with HIV, we could expect to observe lower rates of stunting at ART initiation and better catch‐up growth during adolescence in the future, decreasing the burden of stunting for APH. However, stunting is still highly prevalent in most of the study settings in the general population and access to early ART initiation is not yet a reality in some sub‐regions such as in West and Central Africa.
Similarly, adolescents with low CD4 count over time had poorer growth in our cohort. Even on ART, chronic immunodeficiency could alter growth 13. We observed a trend of poorer growth evolution among males attending rural clinics, which suggests higher vulnerability of APH living in rural settings that could be related to poverty and food access as well as the general challenges of ART adherence and retention in HIV care observed during adolescence 37.
The growth differences observed between males and females in this study were not fully explained by the available data. The pubertal development of APH could be different for males and females and have varying effects on growth, but the literature on this topic is scarce 17, 19, 38, especially in LMICs 18. In the US, compared to HIV‐exposed but uninfected adolescents, APH had delayed pubertal onset, from six to eight months later in females and 10 to 11 months later in males. In that study, however, the mean HAZ value was normal and there were no apparent links between pubertal development and low HAZ 17. Ugandan and Zimbabwean APH have been reported to have substantial pubertal delays, more pronounced for those who were stunted and initiated ART late, but similar between males and females, although males faced more height disadvantage throughout adolescence, as in our study 18.
Another possible explanation for the growth differences by sex was found in a US study where APH males had lower bone mineral density (BMD) compared to males without HIV in late puberty, while there were no differences for females. However, there were no height differences between those with and without HIV in this study 39. To the best of our knowledge, the effect of BMD on growth among adolescents living in LMICs has not been explored 40.
Delayed pubertal onset is also observed in the general population in LMICs 41, and may reflect poor nutrition during the first 1000 days of life and in later childhood 42, which is often the case for children with HIV 22. In rural villages of The Gambia, HAZ evolution was described from birth to 30 years and showed similar patterns to our study, with an apparent decline at the start of adolescence, especially for males, which could be explained by high rates of stunting during infancy and a later entry into puberty compared to the UK reference population 43. Without further data on the timing of puberty onset and hormonal pathways in children with HIV, it is difficult to determine the links and the causal relationships between delayed pubertal onset and growth retardation during adolescence, for males and females.
The trajectory of growth evolution was similar across the IeDEA regions, with differences between males and females. The hypothesis that in some socio‐economic and cultural contexts males could be disadvantaged compared to females regarding nutrition and access to food is therefore unlikely to explain our results. Nutritional habits among adolescents are however insufficiently documented in the general population in our contexts, and even less well documented for adolescents with HIV 43.
Our study has some limitations. Besides lacking puberty data like Tanner staging or age at first menstruation, we also did not have detailed anthropometric data around birth and the first 1000 days of life to explain some aspects of future growth 21. There were insufficient data on HIV viral load to assess the potential association with HAZ. A study in Botswana showed that severe short stature among children and adolescents with HIV was associated with virologic failure 44. In a review on growth after ART initiation among children, the ART regimen used was not associated with growth evolution 22. Also, pregnancy data were not recorded in all regions and then not included. Metabolic data were also not available, but would have been valuable to better assess the effect of long‐term ART on growth 45, 46
Our results could have been affected by selection bias, as they were driven by the large proportion of children from Southern Africa, which may not be representative of growth among APH worldwide. Excluding children who were not followed‐up until at least 14 years of age substantially restricted our sample size and adolescents who were LTFU or died before age 14 were not included, leading to survivor bias, with the risk of underestimating the prevalence of stunting in this population. Indeed, some studies tracing children after being LTFU have shown that this population was at higher risk of being severely immunodeficient and malnourished 47, 48. Furthermore, perinatal infection was defined using a threshold of enrolment in HIV care before age 10 years as a proxy, which excludes the subset of APH who may enter care after age 10 years and would be at even higher risk of stunting due to the delay in diagnosis, HIV care and ART initiation. Thus, the growth pattern described here may underestimate the full extent of stunting among APH.
We did not analyse outcomes beyond 19 years of age in order to focus on the period of adolescence. Additional height gains after age 19 might be expected, and thus this analysis might have concluded before we knew the final height attainments of all patients included. In rural non‐HIV populations in the Gambia, similar to our results, growth continued in late adolescence, and extended to the age of 22 to 24 years in males and 18 to 19 years in girls 43. This could be due to population‐level maturational delays, caused by delayed puberty onset, allowing stunted children and adolescents to catch‐up on their growth by prolonging pubertal growth 43, 49, with different timing in males and females 50. This mechanism highlights adolescence as a second critical window for growth after the first year of life, and the opportunity for catch‐up growth in stunted children living in LMICs, regardless of HIV status 42.
5. Conclusions
This study is, to our knowledge, the first multiregional study assessing growth among a large sample size of APH in LMICs. The high burden of stunting we observed could have serious consequences for social and cognitive development 51. Overall, APH are a vulnerable group, with multiple and interrelated HIV‐specific and general health needs 52. While males seem even more affected by stunting, it is important to ensure that all APH receive comprehensive support that addresses growth beyond childhood and into adolescence. Improved documentation of growth, nutritional status and pubertal phase among APH would provide key data to guide further research and inform interventions to optimize growth and health outcomes.
Competing interest
Authors have no conflict of interest to declare.
Funding
Research reported in this publication was supported by the US National Institutes of Health. Asia‐Pacific: The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, and National Institute on Drug Abuse as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). Caribbean, Central and South America network for HIV epidemiology (CCASAnet) is a member cohort of the International Epidemiology Databases to Evaluate AIDS (leDEA) (U01AI069923; CMG is a PI). Central Africa research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI096299. East African Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Drug Abuse (NIDA), National Cancer Institute (NCI), and the National Institute of Mental Health (NIMH), in accordance with the regulatory requirements of the National Institutes of Health under Award Number U01AI069911 (KWK is a PI), East Africa IeDEA Consortium. Southern African research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number U01AI069924 (MAD is a PI). West African Research reported in this publication was supported by the US National Institutes of Health (NIAID, NICHD, NCI and NIMH) under Award Number U01AI069919. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Julie Jesson was funded by Sidaction.
Authorship
JJ and M Schomaker conducted the analysis. JJ wrote the paper. MD, VL and M Schomaker supervised the analysis and the writing of the paper. KM contributed to the data management. DW and AK contributed and represent the cohort data from the Asia‐Pacific region, SS and MM for the Southern Africa region, M Sylla and KK for the Western Africa region, SA and RV from the Eastern Africa region, CM for CCASAnet and MY for Central Africa region. All authors have read and approved the final manuscript.
Supporting information
Table S1. Comparison of characteristics between the study population and the excluded population according to three criteria.
Figure S1. Penalized splines for the association of age with HAZ for the 2 to 5 imputed data sets.
Figure S2. Mean Height‐for‐Age Z‐score (HAZ) evolution for males (blue) and females (red) during adolescence, trends by regions using raw data. IeDEA global pediatric collaboration, 2003 to 2016
Acknowledgments
We acknowledge all of the children and their families followed up in the participating paediatric centres. We also thank the staff from all participating paediatric centres. We warmly thank all the investigators and paediatric coordinators from the IeDEA regions contributing to the project: Asia‐Pacific (Annette Sohn), CCASAnet–Latin America (Jorge Pinto and Catherine McGowan), Central Africa (Marcel Yotebieng and Andrew Edmonds), East Africa (Kara Wools‐Kaloustian), Southern Africa (Mary‐Ann Davies), West Africa (François Dabis and Valériane Leroy), the IeDEA Pediatric Working Group (Rachel Vreeman, Chair).
Jesson, J. , Schomaker, M. , Malasteste, K. , Wati, D. K. , Kariminia, A. , Sylla, M. , Kouadio, K. , Sawry, S. , Mubiana‐Mbewer, M. , Ayaya, S. , Vreeman, R. , McGowan, C. C. , Yotebieng, M. , Leroy, V. and Davies, M.‐A. ; on behalf of the IeDEA global cohort consortium . Stunting and growth velocity of adolescents with perinatally acquired HIV: differential evolution for males and females. A multiregional analysis from the IeDEA global paediatric collaboration. J Intern AIDS Soc. 2019; 22:e25412
References
- 1. WHO Expert Committee on Health Needs of Adolescents & World Health Organization . Health needs of adolescents: report of a WHO expert committee [meeting held in Geneva from 28 September to 4 October 1976]. World Health Organization. Available from: https://apps.who.int/iris/handle/10665/41252
- 2. Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387(10036):2423–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS . 2017 Estimates. Geneva, Switzerland: UNAIDS; 2017. [cited 2018 Sep 5]. Available from: http://aidsinfo.unaids.org/ [Google Scholar]
- 4. UNAIDS . All In to #EndAdolescentAIDS. Geneva, Switzerland: UNAIDS; 2015. [cited 2018 Sep 5]. Available from: http://www.unaids.org/sites/default/files/media_asset/20150217_ALL_IN_brochure.pdf [Google Scholar]
- 5. Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. J Int AIDS Soc. 2017;20 Suppl 3:21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahourou D, Raynaud J‐P, Leroy V. The challenges of timely and safe HIV disclosure among perinatally HIV‐infected adolescents in sub‐Saharan Africa. Curr Opin HIV AIDS. 2018;13(3):220–9. [DOI] [PubMed] [Google Scholar]
- 7. Hamzah L, Hamlyn E. Sexual and reproductive health in HIV‐positive adolescents. Curr Opin HIV AIDS. 2018;13(3):230–5. [DOI] [PubMed] [Google Scholar]
- 8. Judd A, Davies M‐A. Adolescent transition among young people with perinatal HIV in high‐income and low‐income settings. Curr Opin HIV AIDS. 2018;13(3):236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV‐infected adolescents in sub‐Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18:20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kranzer K, Bradley J, Musaazi J, Nyathi M, Gunguwo H, Ndebele W, et al. Loss to follow‐up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc. 2017;20(1):21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams PL, Jesson J. Growth and pubertal development in HIV‐infected adolescents. Curr Opin HIV AIDS. 2018;13(3):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jesson J, Leroy V. Challenges of malnutrition care among HIV‐infected children on antiretroviral treatment in Africa. Med Mal Infect. 2015;45(5):149–56. [DOI] [PubMed] [Google Scholar]
- 13. Rytter MJH, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition–a systematic review. PLoS ONE. 2014;9:e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cervia JS, Chantry CJ, Hughes MD, Alvero C, Meyer WA, Hodge J, et al. Associations of proinflammatory cytokine levels with lipid profiles, growth, and body composition in HIV‐infected children initiating or changing antiretroviral therapy. Pediatr Infect Dis J. 2010;29(12):1118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johann‐Liang R, O’Neill L, Cervia J, Haller I, Giunta Y, Licholai T, et al. Energy balance, viral burden, insulin‐like growth factor‐1, interleukin‐6 and growth impairment in children infected with human immunodeficiency virus. AIDS. 2000;14(6):683–90. [DOI] [PubMed] [Google Scholar]
- 16. Akseer N, Al‐Gashm S, Mehta S, Mokdad A, Bhutta ZA. Global and regional trends in the nutritional status of young people: a critical and neglected age group. Ann N Y Acad Sci. 2017;1393(1):3–20. [DOI] [PubMed] [Google Scholar]
- 17. Williams PL, Abzug MJ, Jacobson DL, Wang J, Van Dyke RB, Hazra R, et al. Pubertal onset in children with perinatal HIV infection in the era of combination antiretroviral treatment. AIDS. 2013;27(12):1959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szubert AJ, Musiime V, Bwakura‐Dangarembizi M, Nahirya‐Ntege P, Kekitiinwa A, Gibb DM, et al. Pubertal development in HIV‐infected African children on first‐line antiretroviral therapy. AIDS. 2015;29(5):609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellavia A, Williams PL, DiMeglio LA, Hazra R, Abzug MJ, Patel K, et al. Delay in sexual maturation in perinatally HIV‐infected youths is mediated by poor growth. AIDS. 2017;31(9):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villamor E, Jansen EC. Nutritional determinants of the timing of puberty. Annu Rev Public Health. 2016;37:33–46. [DOI] [PubMed] [Google Scholar]
- 21. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet. 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 22. McGrath CJ, Diener L, Richardson BA, Peacock‐Chambers E, John‐Stewart GC. Growth reconstitution following antiretroviral therapy and nutritional supplementation: systematic review and meta‐analysis. AIDS. 2015;29(15):2009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jesson J, Koumakpaï S, Diagne NR, Amorissani‐Folquet M, Kouéta F, Aka A, et al. Effect of age at antiretroviral therapy initiation on catch‐up growth within the first 24 months among HIV‐infected children in the IeDEA West African pediatric cohort. Pediatr Infect Dis J. 2015;34(7):e159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WHO Multicentre Growth Reference Study Group , editor. WHO child growth standards: length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age ; methods and development. Geneva, Switzerland: WHO; 2006. 312 p. [Google Scholar]
- 25. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO . WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV‐related disease in adults and children. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 27. Thiébaut R, Walker S. When it is better to estimate a slope with only one point. QJM. 2008;101(10):821–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wood SN. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall, 2006. 392 p. [Google Scholar]
- 29. Honaker J, King G, Blackwell M. Amelia II: a program for missing data. journal of statistical software. J Stat Softw. 2011;45(7):1–47. [Google Scholar]
- 30. Schomaker M MAMI: Model Averaging (and Model Selection) after Multiple Imputation ‐ R package Version 0.9.10. 2017. [cited 2019 Nov 1]. Available from http://mami.r-forge.r-project.org
- 31. Schomaker M, Heumann C. Model selection and model averaging after multiple imputation. Comput Stat Data Anal. 2014;71:758–70. [Google Scholar]
- 32. Rubin DB. Multiple Imputation after 18+ Years. J Am Stat Assoc. 1996;91(434):473–89. [Google Scholar]
- 33. Jesson J, Masson D, Adonon A, Tran C, Habarugira C, Zio R, et al. Prevalence of malnutrition among HIV‐infected children in Central and West‐African HIV‐care programmes supported by the Growing Up Programme in 2011: a cross‐sectional study. BMC Infect Dis. 2015;15:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cames C, Pascal L, Diack A, Mbodj H, Ouattara B, Diagne NR, et al. Risk factors for growth retardation in HIV‐infected senegalese children on antiretroviral treatment: the ANRS 12279 MAGGSEN pediatric cohort study. Pediatr Infect Dis J. 2017;36(4):e87–92. [DOI] [PubMed] [Google Scholar]
- 35. Martín‐Cañavate R, Sonego M, Sagrado MJ, Escobar G, Rivas E, Ayala S, et al. Dietary patterns and nutritional status of HIV‐infected children and adolescents in El Salvador: a cross‐sectional study. PLoS ONE. 2018;13:e0196380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration , Slogrove AL, Schomaker M, Davies M‐A, Williams P, Balkan S, et al. The epidemiology of adolescents living with perinatally acquired HIV: a cross‐region global cohort analysis. PLoS Med. 2018;15:e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Desmonde S, Tanser F, Vreeman R, Takassi E, Edmonds A, Lumbiganon P. Access to antiretroviral therapy in HIV‐infected children aged 0–19 years in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Global Cohort Consortium, 2004–2015: a prospective cohort study. PLoS Med. 2018; 15(5):2004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nabukeera‐Barungi N, Elyanu P, Asire B, Katureebe C, Lukabwe I, Namusoke E, et al. Adherence to antiretroviral therapy and retention in care for adolescents living with HIV from 10 districts in Uganda. BMC Infect Dis. 2015;15:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacobson DL, Lindsey JC, Gordon CM, Moye J, Hardin DS, Mulligan K, et al. Total body and spinal bone mineral density across Tanner stage in perinatally HIV‐infected and uninfected children and youth in PACTG 1045. AIDS. 2010;24(5):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arpadi SM, Shiau S, Marx‐Arpadi C, Yin MT. Bone health in HIV‐infected children, adolescents and young adults: a systematic review. J AIDS Clin Res. 2014;5(11):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sommer M. An overlooked priority: puberty in sub‐Saharan Africa. Am J Public Health. 2011;101(6):979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spear BA. Adolescent growth and development. J Am Diet Assoc. 2002;102 3 Suppl:S23–9. [DOI] [PubMed] [Google Scholar]
- 43. Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, et al. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97(5):911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Joel DR, Mabikwa V, Makhanda J, Tolle MA, Anabwani GM, Ahmed SF. The prevalence and determinants of short stature in HIV‐infected children. J Int Assoc Provid AIDS Care. 2014;13(6):529–33. [DOI] [PubMed] [Google Scholar]
- 45. Blázquez D, Ramos‐Amador JT, Saínz T, Mellado MJ, García‐Ascaso M, De José MI, et al. Lipid and glucose alterations in perinatally‐acquired HIV‐infected adolescents and young adults. BMC Infect Dis. 2015;15:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arrive E, Viard J‐P, Salanave B, Dollfus C, Matheron S, Reliquet V, et al. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS ONE. 2018;13:e0206745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ardura‐Garcia C, Feldacker C, Tweya H, Chaweza T, Kalulu M, Phiri S, et al. Implementation and operational research: early tracing of children lost to follow‐up from antiretroviral treatment: true outcomes and future risks. J Acquir Immune Defic Syndr. 2015;70(5):e160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sengayi M, Dwane N, Marinda E, Sipambo N, Fairlie L, Moultrie H. Predictors of loss to follow‐up among children in the first and second years of antiretroviral treatment in Johannesburg, South Africa. Glob Health Action. 2013;6:19248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parent A‐S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon J‐P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–93. [DOI] [PubMed] [Google Scholar]
- 50. Lwambo NJ, Brooker S, Siza JE, Bundy DA, Guyatt H. Age patterns in stunting and anaemia in African schoolchildren: a cross‐sectional study in Tanzania. Eur J Clin Nutr. 2000;54(1):36–40. [DOI] [PubMed] [Google Scholar]
- 51. Reinhardt K, Fanzo J. Addressing chronic malnutrition through multi‐sectoral, sustainable approaches: a review of the causes and consequences. Front Nutr. 2014;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Slogrove AL, Sohn AH. The global epidemiology of adolescents living with HIV: time for more granular data to improve adolescent health outcomes. Curr Opin HIV AIDS. 2018;13(3):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of characteristics between the study population and the excluded population according to three criteria.
Figure S1. Penalized splines for the association of age with HAZ for the 2 to 5 imputed data sets.
Figure S2. Mean Height‐for‐Age Z‐score (HAZ) evolution for males (blue) and females (red) during adolescence, trends by regions using raw data. IeDEA global pediatric collaboration, 2003 to 2016


