Abstract
Climate change can have a pronounced impact on the physiology and behaviour of fishes. Notably, many climate change stressors, such as global warming, hypoxia and ocean acidification (OA), have been shown to alter the kinematics of predator–prey interactions in fishes, with potential effects at ecological levels. Here, we review the main effects of each of these stressors on fish escape responses using an integrative approach that encompasses behavioural and kinematic variables. Elevated temperature was shown to affect many components of the escape response, including escape latencies, kinematics and maximum swimming performance, while the main effect of hypoxia was on escape responsiveness and directionality. OA had a negative effect on the escape response of juvenile fish by decreasing their directionality, responsiveness and locomotor performance, although some studies show no effect of acidification. The few studies that have explored the effects of multiple stressors show that temperature tends to have a stronger effect on escape performance than OA. Overall, the effects of climate change on escape responses may occur through decreased muscle performance and/or an interference with brain and sensory functions. In all of these cases, since the escape response is a behaviour directly related to survival, these effects are likely to be fundamental drivers of changes in marine communities. The overall future impact of these stressors is discussed by including their potential effects on predator attack behaviour, thereby allowing the development of potential future scenarios for predator–prey interactions.
Keywords: Escape response, fish, global warming, hypoxia, ocean acidification, predator–prey interactions
Lay summary: Climate change in the aquatic environment is leading to global warming, OA and an increase in hypoxic areas and events. Here, we review the effects of these three major stressors of climate change on the escape response of fishes and their implications for predator–prey relationships.
Introduction
A rapid increase in atmospheric carbon dioxide combined with anthropogenic pollutants is causing major changes in the physical and chemical properties of the ocean, with major impacts on its inhabitants. Climate change models project that ocean pCO2 will exceed 900 ppm by 2100 (Meinshausen et al., 2011) from current levels of ~ 400 ppm, leading to a decrease in ocean pH. Elevation of this primary greenhouse gas will also lead to a predicted 3–5°C increase in ocean temperatures over the same time period. The temperature dependence of chemical reactions means that levels of available oxygen within the shallow ocean will decrease, particularly when combined with increased levels of nutrient discharge along the shores and eutrophication (Breitburg et al., 2018), and these have already led to an increase in the size and number of hypoxic zones in coastal and oceanic waters. In the aquatic environment, global warming, ocean acidification (OA) and hypoxia are intrinsically linked with each other and with an array of other stressors (e.g. heavy metals, plastics, petrol chemicals) that are predicted to increase over time due to accelerating global economies (Halpern et al., 2007). Global warming can increase the release of CO2 from terrestrial and marine sinks, thereby creating a positive feedback causing a further increase in temperatures (Doney et al., 2009). Similarly, global warming is contributing to deoxygenation, as a result of a decrease in oxygen solubility and an increase in oxygen consumption in marine organisms (Breitburg et al., 2018). OA is predicted to be amplified by hypoxia in coastal areas, where much higher pCO2 values than predicted for other areas of the ocean can be expected, because of the production of CO2 related to heterotrophic degradation of organic material (Melzner et al., 2013). Furthermore, although the levels of each of these three fundamental stressors is known to show a natural daily and seasonal variation (Crabbe, 2008; Frölicher et al., 2009; Hofmann et al., 2011), the extent of their variation is predicted to increase, particularly for CO2 (Schulz and Riebesell, 2013; Shaw et al., 2013; Pacella et al., 2018) and temperature (Wang and Dillon, 2014).
Increased environmental fluctuations and climate change are posing major challenges to marine organisms. Climate change can affect their relative abundance and distribution as a result of their different physiological tolerances (Pörtner and Farrell, 2008). As a consequence, latitudinal shifts as well as mortality events of several marine species have been observed during the last few decades (Perry et al., 2005; Coma et al., 2009; Sunday et al., 2012; Poloczanska et al., 2013). However, recent work has suggested that the effect of climate change on marine organisms will be more complex than suggested by the relationship between each stressor and individual performance (Harley et al., 2006). This is largely because in addition to affecting the physiology and performance of a given species, climate change is also likely to affect the relationship between species (Harley et al., 2006; Gilman et al., 2010), particularly the interactions between predators and their prey (Wilmers et al., 2007; Nagelkerken and Munday, 2016; Draper and Weissburg, 2019). It is therefore of fundamental importance to study the effect of climate change on predator–prey interactions in order to increase our ability to predict how climate change will affect marine communities (Hunsicker et al., 2011).
The outcome of predator–prey interactions is determined by their relative sensorimotor performance (Cooper and Blumstein, 2015). Although some research has been carried out on encounters between real predators and prey, in fishes, the escape response has typically been the focus of most studies on predator–prey interactions, largely because of the ease of the experimental approach (Domenici et al., 2011). Fish escape responses typically consist of two muscular contractions followed by a glide or continuous swimming (Weihs, 1973; Domenici and Blake, 1997). The response is usually controlled by a pair of giant neurons located in the hindbrain of most fish species, the Mauthner cells, which receive multimodal sensory input and ensure short latency responses (Eaton et al., 2001; Kohashi and Oda, 2008). Previous work has shown that the early stages of an escape response are fundamental in avoiding predation (Walker et al., 2005; Catania, 2009; McCormick et al., 2018a). In fishes, escape performance can be assessed by multiple traits that include both locomotor (e.g. speed, acceleration) and non-locomotor components, primarily related to behavioural and sensory performance such as the timing, the distance and the directionality of the response (Domenici, 2010a). Traits such as speed and acceleration of the escape responses are related to locomotor performance, hence muscle power and to some extent also neural control (Wakeling, 2006; Domenici, 2010b). On the other hand, predator and prey detection, reaction distances (i.e. the distance between the predator and prey at the time an escape is initiated, also called Flight Initiation Distance, FID, Cooper and Blumstein, 2015), the apparent looming threshold [ALT, i.e. the threshold looming rate of the approaching predator that triggers a response in the prey (Dill, 1974)], response latencies and the directionality of escape are related to neural and sensory capacity (Eaton et al., 2001; Domenici, 2010a). The relative importance of each trait in determining the outcome of a given predator–prey interaction is likely to be context and species dependent (Domenici, 2010a; Domenici and Hale, 2019). For example, while speed was found to be the main determinant of escape success of guppies evading their natural cichlid predator (Walker et al., 2005), in other predator–prey pairs, the main factor was found to be reaction distance (Scharf et al., 2003; Nair et al., 2017) or responsiveness (Fuiman et al., 2006; McCormick et al., 2018a).
Given the importance of predator–prey interactions in governing the distribution and abundance of marine fishes (Hunsicker et al., 2011), it is fundamental to gain an understanding of how climate change can affect their escape and attack performance. This review focuses primarily on the effect of the ‘deadly trio’ of stressors (Bijma et al., 2013), i.e. warming, acidification and hypoxia, on escape kinematics and performance, because prey have been the focus of most predator–prey studies carried out during the last couple of decades. Typically, prey responses have been studied using an artificial startling stimulus and their performance has been assessed under various levels of a given stressor. While this is a simplistic and reductionist approach, its advantage resides in assessing prey behaviour without the effect of the variability in the predator behaviour. The review will also discuss recent work based on more complex and realistic settings, in which a real predator attacking a prey was used, under various combinations of multiple stressors and, in some cases, using fluctuating levels mirroring those found in nature. Finally, we will discuss future avenues for research in which we advocate an increase in the complexity of the experiments, in terms of multiple stressors, environmental fluctuations, realistic predator–prey settings and trans-generational experiments, as well as the development of a modelling approach, in order to increase our ability to make predictions on how climate change will affect the ecology of predator–prey interactions.
The effect of temperature
Global warming is having a major effect on the structure and functionality of aquatic communities, affecting the abundance and geographical distribution of aquatic organisms (Daufresne et al., 2009; Sunday et al., 2012; Poloczanska et al., 2013). Ecological effects at the population, community and ecosystem level have been claimed to be largely related to the physiological responses of aquatic organisms to temperature (Helmuth et al., 2005; Pörtner and Knust, 2007; Pörtner and Farrell, 2008; Chown et al., 2010). Fishes, like all other ectotherms, are particularly vulnerable to temperature changes (Pörtner and Farrell, 2008; Isaak and Rieman, 2013) and therefore, it is important to understand how temperature affects their overall performance as well as their inter-specific interactions, in order to allow predictions of how climate change will affect aquatic communities.
Many organismal functions are temperature dependent, and thermal response curves have been derived that illustrate the relationship between various fundamental biological rates (such as growth, metabolic scope, metabolic rate, growth, activity and reproduction) and temperature (Wood et al., 1997; Fenkes et al., 2016). In addition to such basic physiological traits, swimming performance and kinematics are known to be affected by temperature due to alterations in aerobic activity and endurance (Claireaux et al., 2000; McKenzie and Claireaux, 2010; Johansen and Jones, 2011; Lea et al., 2016), cardiac output (Eliason et al., 2011), muscle development (Hanel and Wieser, 1996) as well as power output for anaerobic swimming (burst) through changes in the contractile properties of the swimming muscles (Wakeling, 2006). Here, we discuss how temperature may specifically affect escape swimming performance and kinematics, which are relevant for predator–prey interactions. In addition, temperature can affect other traits that are key in predator–prey interactions, such as those related to brain and sensory functions, e.g. the timing of the response (Preuss and Faber, 2003). As a result, temperature is likely to change the balance of predator and prey performance, which can have a major effect on aquatic communities at large.
Temperature effects on escape locomotion
Temperature can have major direct or indirect effects on swimming performance and consequently on predator–prey interactions. The indirect effects are related to the well-known relationship between temperature and the general condition of the fishes, i.e. growth rate (i.e. size), condition factor, muscle content, development and reproductive status (Pauly, 1980; Pankhurst et al., 1996; Pörtner, 2002; Garcia de la serrana et al., 2012; Ackerly and Ward, 2016). All of these factors can affect the overall performance of fishes including its swimming kinematics (James and Johnston, 1998a,b). Importantly, an increased size, due to temperature-induced faster growth rate, can cause a higher speed because of the positive relationship between size and burst speed (James and Johnston, 1998a; Domenici, 2001). Therefore, the effect of temperature on factors such as development, body size and condition can indirectly cause changes in burst swimming, such that warming may also indirectly increase burst swimming performance (Martínez et al., 2004; Álvarez and Metcalfe, 2007; Ackerly and Ward, 2016; Watson et al., 2018). On the other hand, the direct effects of temperature on escape swimming performance are mainly due to the effect of temperature on muscle performance and power output, which tend to increase at higher temperatures (Wakeling, 2006; Wilson et al., 2010; James, 2013). Acute decreases in temperature can result in a decrease in body bend and muscle strain rate during escape responses, while an acute increase in temperature increases muscle shortening speed, causing greater power output and higher escape swimming performance (Wakeling et al., 2000).
It is likely that alterations in performance will be most apparent when temperature changes occur over a short time period (i.e. acute), while acclimation can compensate for the effect of temperature on escape swimming to a large degree when temperatures change slowly, such as during seasonal temperature shifts in mean temperature (Beddow et al., 1995; Johnson and Bennett, 1995; Temple and Johnston, 1998; Wilson et al., 2010). The extent of this compensation depends on the duration of the acclimation, is largely species and habitat specific, and is related to thermal tolerance ranges, such that species with a wide thermal range (e.g. goldfish Carassius auratus) tend to show greater compensation in escape swimming (e.g. speed, distance travelled, turning rates) than species with a narrow thermal range (e.g. killifish Fundulus heteroclitus) after 4 weeks of acclimation (Johnson and Bennett, 1995). Similarly, in Trinidad guppies (Poecilia reticulata) and Mediterranean grey mullets (Liza aurata), the escape speeds of fish acclimated (for 70 days and 1 month, respectively) to each temperature did not differ, as a result of complete compensation (Muñoz et al., 2012; Killen et al., 2015). In common carp (Cyprinus carpio), 2 weeks of acclimation can induce changes at the anaerobic muscle fibre level and extend the range of temperature for escape swimming but do not result in full compensation (Wakeling et al., 2000). In golden perch (Macquaria ambigua) tested within a range of 10–25°C after 3 weeks of acclimation, escape swimming performance decreased significantly at temperatures below approximately 15°C but was relatively constant between 15 and 25°C (Lyon et al., 2008). The strength of the acclimation response can also be associated with seasonal variation in temperature, especially during development (Wakeling et al., 2000). In mosquitofish, (Gambusia holbrooki) escape speeds did not differ between spring and summer fish at their natural water temperatures of 15 and 25°C, respectively (Seebacher et al., 2014). While temperature acclimation in adults and juveniles is reversible, temperature effects during the early developmental stages can have more long-term effects on physical traits (Johnston and Temple, 2002) and can be reflected in burst swimming performance (Batty et al., 1993).
Furthermore, increasing temperature can directly affect escape response by reducing the viscosity of water. In order to partition the effect of temperature into those due to change in viscosity and those due to effect on fish physiology, experiments on escape responses have been carried out by changing the viscosity of the water. Work on various species has shown that the effect of viscosity may be relevant for the escape swimming of small larvae at lower temperatures, while for larger larvae and higher temperatures, the effect of temperature on fish physiology is the main factor driving changes in swimming performance (Herbing, 2002). The escape speeds in large juvenile goldfish (C. auratus) (77 mm in body length) were not affected by increases in viscosity at constant temperature, while escape speeds in smaller guppies (Poecilia reticulata) (22 mm in body length) were reduced (Johnson et al., 1998). Viscosity was found to have an overall effect on the escape response kinematics of larval zebrafish (Brachydanio rerio) (3.3 mm in body length), by significantly decreasing displacement, maximum velocity and acceleration during stage 1 (i.e. the first muscle contraction) of the escape response; however, the viscosity values used were outside the range of viscosity variation caused by temperature changes in nature (Danos and Lauder, 2012).
Temperature effects on non-locomotor variables of the escape response
Temperature affects various non-locomotor components of the escape response, such as responsiveness, escape latency, reaction distance and directionality from the threat. Temperature has been known to affect escape latency, mainly as a result of the changes in the neural conduction speed, with the highest conduction speed occurring at high temperatures. Fish acclimated for 2 weeks at different temperatures, i.e. 5–25°C (Webb, 1978) and 15–25°C (Penghan et al., 2016), showed shorter escape latencies at higher temperature. Szabo and colleagues acclimated goldfish (C. auratus) for 4 weeks at 5, 15 or 25°C and found that the higher temperature yielded the shorter latencies, suggesting that these effects were due, at least in part, to higher conduction velocity in the Mauthner axons of warm-acclimated populations (Szabo et al., 2008). Furthermore, acclimation to high temperature was found to increase responsiveness, likely because fish became hyper-excitable as a result of a change in the balance between excitatory and inhibitory synaptic transmission (Szabo et al., 2008). Although higher temperature may increase both responsiveness and the latency performance of fish, other escape traits such as directionality (the proportion of escape responses away from the threat) may be impaired (Szabo et al., 2008). Szabo et al. (2008) suggest that decreased directionality was due to the higher conduction velocity resulting from high temperature in warm-acclimated population, which may reduce the temporal separation of inputs to the two Mauthner cells, causing reduced right–left discrimination. In contrast, work based on acute cooling found that low temperatures increase responsiveness and decreased directionality (i.e. by increasing the proportion of responses towards the threat) in goldfish (C. auratus) (Preuss and Faber, 2003). These studies show that acute cooling lowered the behavioural threshold for spike initiation in the Mauthner cell, presumably because of changes in the intrinsic properties of the Mauthner cells (Preuss and Faber, 2003). Similarly, acute warming (34–39°C) was found to decrease the reaction distance in goldfish (C. auratus) to an approaching threat compared to the control level (15°C), likely as a result of the malfunctioning of the neurosensory system (Webb and Zhang, 1994).
Each species’ capacity to acclimate to temperature change is likely to be dependent on the relative position of an individual on its thermal reaction norm, on the evolutionary history and geographical range of the species, the latitudinal/geographic location and on the thermal history of the test population. For example, while a short-term (4 days) exposure to high temperature was found to decrease directionality away from the threat in two species of coral reef fishes (Pomacentrus moluccensis and Pomacentrus amboinensis), directionality returned to control levels after a longer term (90 days) acclimation only in P. moluccensis (Warren et al., 2017). However, escape latency was not found to be affected by temperature within the range of temperatures used (29–31°C) (Warren et al., 2017). Seasonal variation in temperature was also found to affect responsiveness and reaction distance, since performance in these traits decreases towards the end of the spawning season (September to November) over a period of temperature decline (Ojanguren and Fuiman, 2010). Thermal conditions during development (i.e. higher incubation temperature) are also likely to have a positive effect on burst swimming performance (i.e. burst speed) and therefore on vulnerability to predation (Seebacher and Grigaltchik, 2015).
Temperature effects on predator–prey encounters
Given all the effects that temperature can have on escape locomotion and behaviour, temperature is expected to modulate the outcome of predator–prey interactions. Species-specific differences, the duration of temperature acclimation, as well as the differences in temperature between the experimental treatments may modulate these effects. Because predators and prey may respond differently to climate change, the consequent impact on their interactions may have profound effects on population dynamics (Dell et al., 2014). Temperature changes may not only alter predator attack and prey escape performance, for example through an effect of temperature on the muscle or sensory performance of the prey as discussed above, but also prey capture kinematics (Wintzer and Motta, 2004; Devries and Wainwright, 2006; Higham et al., 2015). They may also indirectly affect the outcome of predator–prey encounters because temperature has a well-known effect on metabolism of the predators, with metabolic scope increasing with temperature up to an optimum, beyond which a decline is observed (Pörtner and Farrell, 2008). Temperature changes can, therefore, potentially alter predator activity and hunger level, hence their motivation to forage.
Studies on the effect of temperature on predator–prey interactions have used different acclimation periods, species and experimental temperatures. Different acclimation periods are ecologically relevant because of the temporally hierarchical nature of temperature fluctuations in aquatic systems. However, this diversity in the experimental approach is likely to be the main cause of some differences in the overall results, with longer acclimation periods typically showing a higher degree of compensation in performance. A week exposure to high temperature was found to increase the capture success of the fish predator (the dottyback, Pseudochromis fuscus) on fish prey in coral reef species (Pomacentrus wardi) (Allan et al., 2015). This was likely due to a combination of effects on predators and prey. In term of the prey performance, reaction distance to the approaching predator and escape swimming decreased at high temperature. Conversely, attack rate and attack speed of the predator increased at high temperature. Allan et al. (2015) suggest that the effect on predator performance was likely due to an effect of temperature on motivation, since high temperature increases metabolic rate and thus the hunger level.
Other studies also suggest that temperature can affect not only locomotor performance but also the predator’s motivation to attack prey. Using 1 month of acclimation, Grigaltchik et al. (2012) showed that warm acclimated bass (Macquaria novemaculeata) had higher attack rates on mosquitofish (Gambusia holbrooki) than cold acclimated ones, and higher prey capture success. Interestingly, laboratory trials showed that the burst swimming performances of acclimated seabass did not differ between warm and cold temperatures, indicating that muscle function was maintained through acclimation, thus low capture rates at low temperature were likely to be due to decreased motivation to catch prey as a result of lower food requirement (Grigaltchik et al., 2012). Furthermore, when tested at a temperature higher than the acclimation range, attack rates declined, while the escape swimming performance of the prey was higher than the attack speed of the predators. Therefore, in some predator–prey pairs, predator pressure may increase with temperature up to a point, and then it may decline again (Grigaltchik et al., 2012), although this pattern will be modulated by acclimation time. A similar pattern was found in the relationship between temperature and strike rate of common coral trout (Plectropomus leopardus), from the Great Barrier Reef (Scott et al., 2017). Hence, differences in the thermal reaction norms of performance and physiology between prey and predator will affect the outcome of interactions in response to temperature change.
Further research has suggested a decrease in predator pressure at low temperatures. This is in line with Dell et al. (2014), who suggest that in ectotherm pairs of predators and prey at low temperatures, the escape speed of prey tends to remain close to peak levels and thus higher than the attack speeds of the predator. Work on pike (Esox lucius)—brown trout (Salmo trutta) interactions suggests that changes in relative speed performance were at the basis of the effect of long-term temperature exposure (3 months) on the outcome of the interactions (Öhlund et al., 2015). Attack rates decreased sharply at low temperatures (below 11°C) and mirrored a decline in the attack speed of the predator, while escape speeds remained relatively constant (Fig. 1). Two potential explanations were put forward: (i) low temperature affects the neural performance of fish, which is likely to have a stronger effect on the predator than on the prey, because catching a fast-moving prey is a complex task that requires synchronization of motor and cognitive processes, while escaping mainly requires rapid random motion and (ii) low temperature reduces motivation, e.g. because of lower hunger and prey availability (Öhlund et al., 2015). Overall, these studies suggest that at lower temperatures, predator–prey interactions involving fish as prey and predators tend to decrease in frequency (Fig. 2). In the case of endotherm predators (e.g. birds and marine mammals) attacking fish, the effects of temperature are more complex and do not usually result in a reduction of hunger and food intake in the same way as for ectotherms. For example, birds (cormorants Phalacrocorax carbo carbo) feeding in the winter economize energy expenditure by halving the time spent at sea, and halving the number but doubling the mass of each prey fish taken (Johansen et al., 2001). Bailleul and colleagues suggest that after migrating to the Antarctic continent, elephant seal (Mirounga leonina) may target cold waters to facilitate the capture of fish prey that are less active at low temperatures (Bailleul et al., 2007). It is therefore plausible that low-temperature conditions may increase the predation success rate of piscivorous endotherms (Fig. 2).
Figure 1.
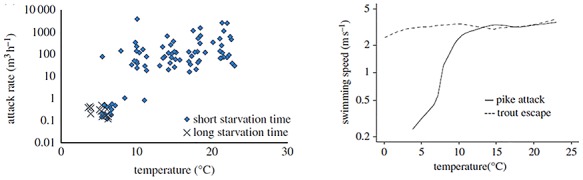
The differential effect of temperature on predators and prey. (A) The effect of temperature on the attack rate of northern pike (Esox lucius) attacking brown trout (Salmo trutta). Attack rates are expressed as m3 h−1, since they are measured as 1/Pt, where P is predator density and t is time to capture. (B) The effect of temperature on pike attacking speed and brown trout escape speed. From Öhlund et al. (2015).
Figure 2.
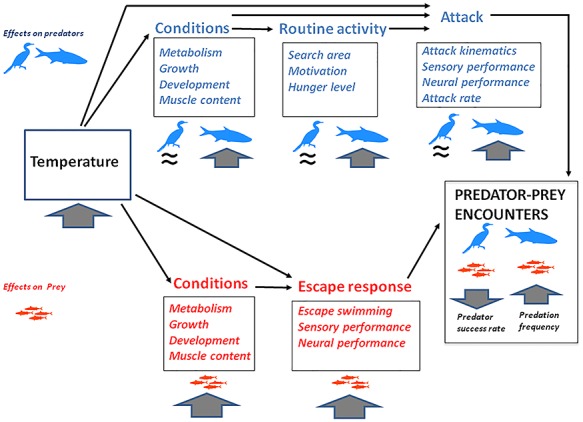
The potential effects of changes in temperature on predator–prey interactions, based on fish as prey, and fish or an endotherm (e.g. a bird) as predators, based on a scenario of future global warming. Downwards and upwards arrows indicate a decrease and an increase, respectively; while a ≈ sign indicates no significant change. At high temperatures, prey conditions (growth and metabolism) increase and so does the escape performance, also because of the direct effect of temperature. Temperature has similar positive effects on fish predator attacks (via direct effects and because of decreased body conditions). In addition, because of their higher routine activity and higher hunger level (due to higher metabolism), the frequency of predator–prey interactions increases (though they may decline at high temperatures that exceed acclimation temperatures; see main text). Temperature has little effect on performance and hunger level on endotherms (compared to the effect on ectotherms), and therefore predator success rate is expected to decrease at high temperature because of the positive effects of temperature on prey escape performance.
Accordingly, marine endotherms were suggested to be competitively favoured over ectothermic predators as water temperatures decline and this may be the basis for why predators such as marine mammals and birds show high phylogenetic diversity in cold, temperate latitudes while their diversity is low in warmer regions (Cairns et al., 2008; Grady et al., 2019). This pattern may be related to the asymmetric response in a number of traits (e.g. metabolic, sensory and locomotory rates), which is constant in endotherms while decreases with temperature in ectotherms (Cairns et al., 2008; Dell et al., 2014; Grady et al., 2019). As a result, colder water is favourable to endothermic predators attacking slower ectothermic prey (Cairns et al., 2008; Grady et al., 2019). Arguably, the overall effects of global warming are bound to be more complex and beyond the scope of the current review, since they can have a number of other direct and indirect effects on aquatic organisms that would impact on predator–prey interactions, such as changes in the distributional ranges of both predators and preys (Learmonth et al., 2006).
Overall, these studies suggest that temperature is a major factor determining the outcome of predator–prey interactions with fish as prey, although the type of change, and therefore, the potential future scenario will depend largely on whether the predators involved are endotherm or ectotherm (Fig. 2). Acclimation may in part compensate for the effect of temperature on some aspects of performance, although motivational components on the predator’s part appear to be a key element of the interactions. In addition, such complex interactions, in which motivational, sensory and locomotor factors can play a role, are unlikely to be driven by a single temperature effect. Regardless of the functional explanation for the effect on predator–prey interactions and the extent to which it is due to changes in escape or attack swimming, sensory performance, differences in predator hunger levels or a combination of all of these factors, studies of predator–prey interactions can be fundamental for predicting tipping points in the responses of ecosystems to global warming (Öhlund et al., 2015).
In addition, while previous work shows that climate change stressors can have a number of effects on escape performance and therefore on prey vulnerability, further important effects of climate change (e.g. warming) may impact other aspects of swimming (e.g. aerobic swimming), which can trade off with anaerobic swimming (Reidy et al., 2000), (but see Marras et al., 2013), with potential consequences of sustained activity such as that used for migration. Therefore, the overall ecological effects of climate change on organisms need to take into account many interacting levels of physiological impacts that may result in a number of trade-offs.
The effect of hypoxia
The increasing frequency of hypoxia events during the last decades makes hypoxia one of the most threatening stressors for aquatic organisms (Diaz, 2001; Breitburg et al., 2018). While anoxia can be lethal for most aquatic organisms, hypoxia is known to induce many non-lethal effects. For example, hypoxia has a negative impact on fish growth (Chabot and Dutil, 1999), development (Shang and Wu, 2004), activity levels (Schurmann and Steffensen, 1994), muscle composition (Rees et al., 2012), aerobic scope (Claireaux and Chabot, 2016) and aerobic swimming performance (Vagner et al., 2008). In addition, as a result of hypoxia exposure, various short- and long-term adaptations can occur in fish, such as modifications in body and gill morphology (Sollid et al., 2003; Crispo and Chapman, 2011). Since body morphology can affect escape locomotion (Domenici et al.,, 2008; Langerhans and Reznick, 2010), such modifications may potentially cause an indirect effect of hypoxia on predator–prey interactions.
Fish escape locomotion relies on fast anaerobic muscle fibres; therefore, hypoxia was originally hypothesized to have no effect on a single burst swimming event (Beamish, 1978). However, hypoxia is known to reduce the sensory performance in various vertebrate taxa, such as amphibians (Sitko and Honrubia, 1986), fishes (Robinson et al., 2013), mammals, and other vertebrates (Fowler and Prlic, 1995). As a consequence, any effect of hypoxia on the sensory performance and neural control of escape response may also be reflected in diminished escape performance. In addition, hypoxia may limit the ability of fish to recover from anaerobic exercise, thereby limiting their potential to engage in repetitive bursts of activity. Here, we discuss how hypoxia may affect escape responses by considering both locomotor (e.g. speed and displacement) and non-locomotor traits (e.g. responsiveness and escape latency), as well as potential strategies employed by fish to minimize the negative effect of hypoxia, such as aquatic surface respiration, and the related behavioural trade-offs.
The effect of hypoxia on escape locomotion
In escaping golden grey mullet (Liza aurata), cumulative distance and maximum swimming speeds were found to be reduced following a few hours of exposure to acute hypoxia (Lefrançois et al., 2005). This decreased escape performance was observed in severe hypoxia (i.e. 10% of air saturation) only when access to the surface was impeded experimentally. On the other hand, when L. aurata had access to the surface, they performed aquatic surface respiration (ASR, which allows fish to breath at the oxygenated layer near the air–water interface) and their performance did not differ from that of fish in normoxia (Lefrançois et al., 2005). From a kinematic perspective, decreased escape swimming performance in this species was related to a decreased proportion of double bend responses, i.e. escape responses consisting of stages 1 and 2 (i.e. the first two body bends in an escape response, Domenici and Blake, 1997), as opposed to single bend responses in which only one body bend was observed and therefore lower speed (Fig. 3). Work on other species, however, did not find a significant effect of similarly acute hypoxia exposure on escape locomotion in European seabass (Dicentrarchus labrax) (Lefrancois and Domenici, 2006) or crucian carp (C. carassius) (Penghan et al., 2014).
Figure 3.
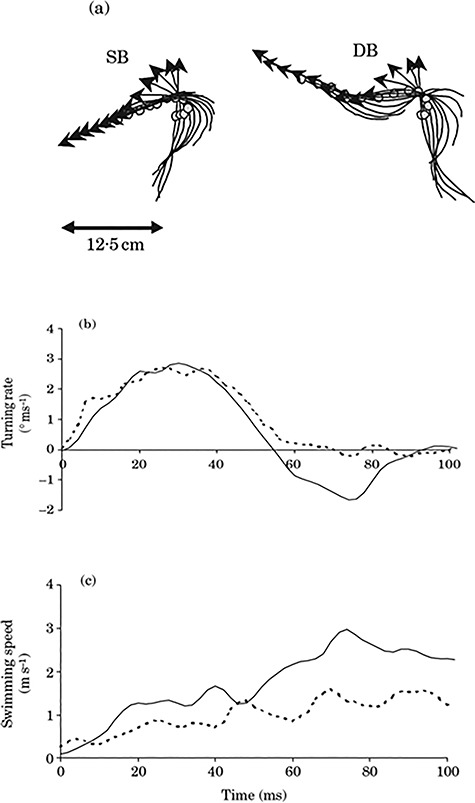
The escape response of golden grey mullets (Liza aurata). (a) Tracing of the midline and centre of mass (open circle) of golden grey mullets, shown at 10 ms of intervals. Top left, a single bend (SB) response in which fish glides after the first body bend (stage 1). Top right, a double bend (DB) response, in which fish performs two consecutive body bends. (b) the turning rate vs. time curve shows that DB responses (continuous line) reverse the turn (i.e. negative values), while the turning rate of SB response (dotted line) is around zero after stage 1 (i.e. after around 60 ms). (c) The curve of escape speed vs. time shows that speed is higher in DB (continuous line) than in SB responses (dotted line). DB responses are more common in normoxia than in hypoxia, in golden grey mullet. From Lefrancois et al. (2005).
Zhang et al. (2013) and Ackerly et al. (2017) suggest that the duration of the exposure to hypoxia (i.e. chronic versus acute stress) may have a role in determining the behavioural response of fishes, although this is species specific. For example, grass carp (Ctenopharyngodon idellus) showed a decrease in maximum escape velocity only in normoxia-acclimated fish exposed to hypoxia, while no change was observed when fish were reared in similar hypoxic conditions (Zhang et al., 2013). Ackerly and colleagues acclimated the African mormyrid (Marcusenius victoria) for 8 weeks to high and low dissolved oxygen (i.e. 20% of air saturation) before their escape swimming was tested under both these normoxic and hypoxic conditions. The results showed that the hypoxia-acclimated fish had shorter escape displacement when tested under hypoxia than in normoxia, while displacement of normoxia-acclimated fish was not affected by the test condition (Ackerly et al., 2017). Gotanda and colleagues investigated the effect of long-term exposure to hypoxia in the African cichlid (Pseudocrenilabrus multicolor victoriae) (Gotanda et al., 2012). Fish reared during at least 10 months in 15% air saturation-hypoxia developed larger gills, deeper bodies, and larger, wider heads than fish reared in normoxia. While different body morphologies could potentially have an effect on swimming performance, hypoxia-reared fish showed no difference in maximum velocity or acceleration compared to normoxia-reared fish. Gotanda et al. (2012) suggest that hypoxia-reared fish might compensate for morphological differences by using a high proportion of double-bend responses, to achieve similar performance as normoxia-reared fish. Overall, these results show that the effect of hypoxia on fish escape locomotion (i) is species specific, with some species showing decreased performance in hypoxia while other species show no effect; (ii) depends on acclimation and rearing conditions, with acclimation potentially allowing fish to partially compensate for exposure to hypoxic conditions in some cases; and (iii) may be affected by access to the water surface.
Hypoxia effects on non-locomotor variables of the escape response
In fish escape responses, non-locomotor variables such as responsiveness, timing and response distances are known to affect vulnerability to predation (Domenici, 2010a). This is particularly relevant for the effect of hypoxia, because of the decreased brain and sensory performance in hypoxia observed in fish and other vertebrates (Fay and Ream, 1992; Fowler and Prlic, 1995; Robinson et al., 2013). Hypoxia was found to reduce the responsiveness to a startling stimulus in both D. labrax and L. aurata exposed to an acute decrease of oxygen whether this latter species had access to the surface or not (Lefrançois et al., 2005; Lefrancois and Domenici, 2006). A similar pattern was observed in the common sole (Solea solea) facing a progressive decrease in oxygen down to 15% air saturation over c. 1.5 h (Cannas et al., 2012), as well as in grass carp (Ctenopharyngodon idellus) (Zhang et al., 2013) and white grunts (Haemulon plumieri), even if hypoxic conditions were less severe (Sánchez-García et al., 2017). Because responsiveness is a fundamental component of escape performance (Fuiman et al., 2006), a decrease in responsiveness such as that observed in hypoxia may increase the risk of mortality and is expected to influence the outcome of predator–prey interactions.
Functionally, the hypoxia-related reduction in responsiveness may be due to an increase in response threshold, and/or decreased motivation to escape related to stress and exhaustion (Lefrançois et al., 2005). Although most species investigated showed a decrease in responsiveness when exposed to hypoxia, the African mormyrid (Marcusenius victoria) showed no effect when exposed to acute or chronic hypoxia, suggesting that the species-specific differences in hypoxia tolerance are an important factor (Ackerly et al., 2017). Species-specific difference were also found in the latency of the escape response; escape latency was not affected by hypoxia in L. aurata and D. labrax, while it increased in C. idellus (Zhang et al., 2013) and decreased in P.m. victoriae (Gotanda et al., 2012).
Directionality (i.e. whether a response is directed away or towards the threat, Domenici, 2010a) was found to be impacted by hypoxia in the two species in which directionality was tested [L. aurata (Lefrançois et al., 2005) and D. labrax (Lefrancois and Domenici, 2006)]. Here, fish were exposed for a few hours to oxygen conditions lower than 20 and 50% of air saturation, respectively. In both cases, hypoxia-exposed fish showed a higher proportion of responses towards the threat. This is likely to result from an inaccurate left–right discrimination of the startle direction, possibly related to sensory disorientation (Lefrançois et al., 2005). Because the early stages of an escape response are crucial for survival (Walker et al., 2005; McCormick et al., 2018a), an early mistake directing the prey towards the danger may increase its vulnerability.
Effects of hypoxia on fish behaviour and predator–prey interactions
Aquatic surface respiration (ASR) and air breathing (AB) are performed by some fish species in order to avoid hypoxic conditions in the water column (Domenici et al., 2007b). While ASR can reduce the negative effects of hypoxia on escape response as discussed above, it may also cause additional costs and risks (Domenici et al., 2007b). Fish performing ASR and AB are at higher risk of predation owing to increased visibility to aerial predators (Kramer, 1987; Domenici et al., 2007b), and the presence of predators can delay the onset of ASR (Shingles et al., 2005). However, when performing ASR and AB, fish can decrease the risk of predation by surfacing with irregular frequency or in synchrony with others (Kramer and Graham, 1976). Irregularity reduces the predictability of the ASR and AB event, and therefore, the vulnerability to predation and synchronous surfacing was suggested to create sensory confusion in the predator (Kramer and Graham, 1976; Gee, 1980; Chapman and Chapman, 1994). Hypoxia is also known to increase the risk of predation by altering efficiency of anti-predator behaviours, such as school cohesiveness in pelagic species (Domenici et al., 2002; Domenici et al., 2007b) and crypsis in benthic species via changes in conspicuousness due to hypoxia-dependent increase in ventilation rates (Cannas et al., 2012).
Similar to temperature, the effect of hypoxia on predator–prey interactions are taxon specific. Arguably, the effect of hypoxia on fish–fish interactions are likely to differ from those on predator–prey interactions involving fish as prey, and predators that are not affected by hypoxia, such as birds, aquatic mammals, or other hypoxia-tolerant taxa (Fig. 4). If the predators are fish, they are likely to be less hungry and feed less frequently than in normoxia (Poulin et al., 1987; Chabot and Dutil, 1999; Thetmeyer et al., 1999; Engström-Öst and Isaksson, 2006; Ripley and Foran, 2007), because of the reduced metabolism (Claireaux and Chabot, 2016) and possibly also decreased sensory performance (Robinson et al., 2013). On the other hand, water hypoxia will have no effect on birds or marine mammals. As a result, predator pressure is likely to decrease in hypoxia when the predators are fish, while it should not change if the predators are unaffected by hypoxia. While there is both species-specific and methodology-specific (acute versus chronic) differences in the results of the effect of hypoxia on escape performance, most studies show some negative behavioural effects, particularly on directionality and responsiveness, while escape locomotion is affected only in some species (e.g. grey mullet; Lefrancois et al., 2005). As a result, escape performance may decrease largely because of a decreased non-locomotor performance. Therefore, a potential scenario for fish–fish interactions is that the frequency of predation events should decrease in hypoxia (Fig. 4), with the exception of fish predators that are highly hypoxia tolerant such as certain air-breathing species (Wolf and Kramer, 1987). This is in line with work based on fish–fish interactions, which shows that the number of fish prey larvae consumed by Spanish mackerel (Scomberomorus niphonius) (Shoji et al., 2005), as well as by juvenile striped bass (Morone saxatilis) and adult naked goby (Gobiosoma bosc) (Breitburg et al., 1994) decreased in hypoxia. Conversely, predation by species that are hypoxia independent or highly hypoxia tolerant is expected to increase in low oxygen conditions, because prey escape performance in fish tends to decrease, while hunger in these predators will not be affected (Fig. 4). Indeed, works using hypoxia-tolerant predator species such as sea nettle (Chrysaora quinquecirrha) (Breitburg et al., 1994) or moon jellyfish (Aurelia aurita) (Shoji et al., 2005) show that predation on fish increases in hypoxia. Furthermore, aquatic birds such as little egrets (Egretta garzetta) were observed to take advantage of the ASR behaviour some lagoon fish undertake in the morning, when their surfacing in hypoxic waters (i.e. due to daily fluctuation in oxygen levels) makes them easy prey for birds (Kersten et al., 1991).
Figure 4.
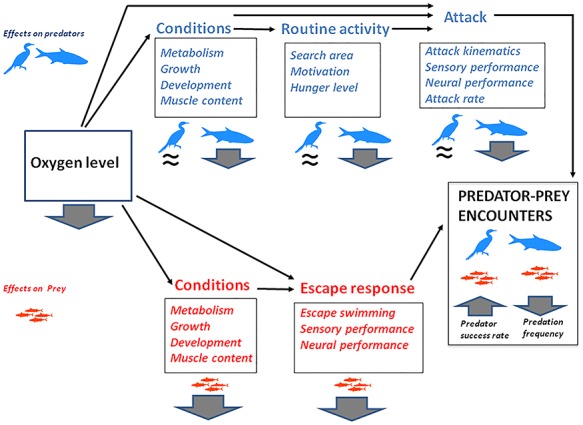
The potential effects of changes in oxygen level on predator–prey interactions, based on fish as prey, and fish or an endotherm (air breathing) as predators (e.g. bird), based on a scenario of future hypoxia. Downwards and upwards arrow indicate a decrease and an increase, respectively; while a ≈ sign indicates no significant change. When oxygen decreases, fish prey decreases their metabolism and growth, and their general conditions. This can have a negative impact on their locomotor and sensory performance. In addition to this indirect effect, hypoxia can also decrease escape performance directly (particularly non-locomotor performance). Hypoxia can also affect the conditions (growth and metabolism) of fish predator, but have no effect on endotherm, air-breathing predators such as birds. Similarly to fish prey, decreased conditions can impact the attack performance of fish predators, in addition to the direct (potential) effect of hypoxia on their performance in general. In addition, hypoxia-dependant decrease in metabolism decreases hunger level (thus motivation to feed and search activity) in fish predators, but not in birds. A possible scenario in fish–fish interactions is that the performance of both predators and prey decreases in hypoxia, and so does hunger level in predators. This could result in a decreased frequency of predator–prey events. On the other hand, birds could take advantage of the lower performance of fish prey in hypoxia, thus increasing their success rate in capturing a prey.
While these potential scenarios are generally supported by previous work on predator–prey interactions in hypoxia, it is worth noting that hypoxia can also be associated to some behavioural strategies by the predators or the prey to circumvent the constraints imposed by hypoxia. For example, while predation by fish tends to decrease in hypoxia, certain fish species living in stratified waters routinely undertake brief foraging forays into hypoxic habitats to catch their invertebrate prey, suggesting that foraging in hypoxic waters may be common in fish when prey abundance is low in surface waters (Rahel and Nutzman, 1994; Taylor et al., 2007; Roberts et al., 2012). In addition, even within fish species, there can be differences in hypoxia tolerance (Bell and Eggleston, 2005), and it has been suggested that small fish tend to be more hypoxia tolerant that large ones, which may imply that fish prey tend to be more hypoxia tolerant than their fish predators (Robb and Abrahams, 2002; Robb and Abrahams, 2003; Pan et al., 2016). Indeed, certain species of small fish were suggested to use deep hypoxic waters as a refuge from large predators (Chapman et al., 1996a,b; Parker-Stetter and Horne, 2009; Ekau et al., 2010; Hedges and Abrahams, 2015; Vejřík et al., 2016). Clearly, hypoxia affects both the feeding and escape behaviour of predators and prey, as well as their habitat selection. Therefore, the complexity of the system is largely dictated by the relative hypoxia tolerances of each species involved and a modelling approach could be useful to improve our predictive ability of the effect of hypoxia on predator–prey interactions (de Mutsert et al., 2016). Future studies may benefit from emphasizing such aspects, and incorporating the different capacities that prey and predators may have for acclimating and/or adapting to the more frequent and severe hypoxia episodes that are predicted.
The effect of acidification
Climate change models project that ocean pCO2 will exceed 900 ppm by 2100 (Meinshausen et al., 2011) from current levels of ~ 400 ppm, leading to a decrease in ocean pH, which occurs via the dissolution of CO2 as it is absorbed into the world’s oceans, where it is hydrated to form carbonic acid. Elevation of this primary greenhouse gas will also lead to a predicted 3–5°C increase in ocean temperatures over the same time period. The temperature dependence of chemical reactions means that levels of available oxygen within the shallow ocean will decrease. OA is predicted to affect interspecific interactions through changes in behaviour, locomotor and sensory performance (Wittmann and Pörtner, 2013; Nagelkerken and Munday, 2016). While early work on the effects of OA was mainly focused on calcifying organisms (Orr et al., 2005), research in the last decade has shown that a number of behavioural disruptions in fish and marine invertebrates can occur as a consequence of exposure to high CO2 levels (Nagelkerken and Munday, 2016). Recent meta-analytical work has found various effects of exposure of high CO2 effects on calcification, metabolic rates and behavioural performances in fishes, in addition to increased predation risk and decreased foraging, particularly for fish larvae (Cattano et al., 2018). With respect to predator–prey interactions, one of the most relevant effects is the disruption of the ability to avoid predator odours, as it was found in coral reef fish larvae (Dixson et al., 2010), with potential negative consequences in terms of their survival (Munday et al., 2010). Recently, the kinematics and behaviour that underpin complex predator–prey interactions have also been used to characterize OA-related impairment (Allan et al., 2013; Munday et al., 2016; Allan et al., 2017; McCormick et al., 2018b). In the following section, we discuss the chronic and acute effects of OA on the kinematics and behaviour of fish escape responses, with considerations on predator–prey behaviours and their potential ecological consequences, while pointing out species-specific differences found in the responses. Research on the effect of OA on fish escape behaviour is relatively new compared to, for example, the effects of temperature. As a consequence, little is understood about the mechanisms underlying reported responses and the behavioural results are not always consistent among different species. This lack of consistency does not allow the development of hypothetical general scenarios of the effect of OA on predator–prey interactions, unlike the case of temperature and hypoxia.
The effect of acidification on escape locomotion
Similar to temperature and hypoxia effects, exposure to both chronic and acute high CO2 levels can affect locomotor performance, but whether the effect is negative or positive depends largely on the underlying physiology of the animal being tested. In addition, elevated CO2 levels can cause changes in aerobic scope (Couturier et al., 2013; Rummer et al., 2013) with direct implications on the functioning of predator–prey interactions (Rosa and Seibel, 2008; Couturier et al., 2013). The way in which elevated CO2 affects aerobic scope has yielded contrasting results with some species showing an increase (Rummer et al., 2013) and other species a decrease in aerobic scope (Munday et al., 2009). The results of the effect of high CO2 on aerobic scope are potentially relevant for burst swimming, despite the fact that escape locomotion is powered anaerobically. This is because any effect of high CO2 conditions on aerobic scope may indirectly affect escape performance since the energy debt created by anaerobic activity has to be paid off by post-exercise oxygen consumption (Moyes et al., 1993). However, in the Ambon damselfish (P. amboinensis), despite the finding of an increase in aerobic scope with increased CO2 (Couturier et al., 2013), 4 days of exposure to high CO2 (~900 μatm) decreased escape locomotor performance (in terms of escape distance within a fixed time) (Allan et al., 2013; Fig. 5). Similarly, a 4-day (~900 μatm) exposure to high CO2 negatively affected the escape swimming (mean and maximum speed) of the cinnamon clownfish (Amphiprion melanopus) (Allan et al., 2014) and decreased the escape distance moved and average escape speed in the yellowtail kingfish (Seriola lalandi) [exposure period of 21 days post-hatch (~1000 μatm)] (Watson et al., 2018). In contrast, Allan et al. (2017) found that there was no effect of high CO2 exposure (~900 μatm for 7 days) on escape distance, but there was a significant effect on escape speed in the Ward’s damselfish (P. wardi) (Allan et al., 2017). There was also no effect of high CO2 exposure on the maximum bending angle and the duration of stage 1 in the escape response of marine medaka (Oryzias melastigma) following a 8–16-day embryonic exposure to two CO2 levels (~1100 and 1800 μatm) (Wang et al., 2017). Therefore, although the escape swimming of some species appears to be affected by high CO2, the results are inconsistent among species, even when they belong to the same genus.
Figure 5.
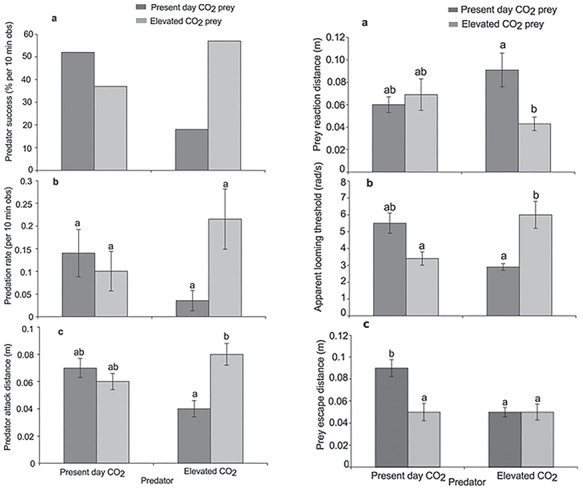
The effect of elevated CO2 on predator attack behaviour [left panel, (a) predator success, (b) predation rate, (c) predator attack distance] and prey escape response [right panel, (a) prey reaction distance, (b) apparent looming threshold, (c) prey escape distance]. Predators and prey were exposed to elevated CO2 (880 μatm) or a present-day control (440 μatm). The prey is the planktivorous damselfish P. amboinensis and the predator is the piscivorous dottyback P. fuscus, from Allan et al. (2013).
The effects of acidification on non-locomotor variables of the escape response
The most significant impact of high CO2 exposure on escape responses appears to be related to the sensory performance that underpins successful escapes. If we consider the initial phase of a predator–prey interaction, which is often triggered by a visual cue, any process that alters this can alter the outcome of the interaction. High CO2 is known to affect the performance of various sensory channels including vision (Draper and Weissburg, 2019). For example, Chung and colleagues found that retinal flicker frequency was disrupted in the spiny damselfish (Acanthochromis polyacanthus) after a 6–7-day exposure to high CO2 (~900 μatm) (Chung et al., 2014), which could have an impact on the ability of prey to visually detect approaching threats and the distance at which it responds to a high-speed predatory attack. Once an attack is perceived, the most important trait associated with a successful escape response is the reaction distance of the prey. Allan et al. (2013) showed that high CO2 exposure appears to reduce this distance as well as cause changes in ALT (in sensuDill, 1974) in P. amboinensis attacked by real predators, leading to increased mortality (Fig. 5), although work based on predator–prey encounters using a different species from the same genus (P. wardi) showed no effect of high CO2 on either reaction distance or ALT (Allan et al., 2017). Work using a mechanical stimulus showed that exposure to high CO2 negatively affected two fundamental traits of escape response, directionality and responsiveness, but had no effect on escape latency (Allan et al. 2014). Marine medaka (Oryzias melastigma) larvae showed no effect on escape latency but did exhibit a lower responsiveness to a mechano-sensory stimuli when reared in high CO2 compared to larvae reared under control conditions (Wang et al., 2017). Wang and colleagues suggest that lower responsiveness in high CO2 may be due to an effect on the development of the ear (e.g. otoliths), the afferent connections in the VIIIth nerve and the inputs to the Mauthner cells.
The neural basis of high CO2 impairment of the escape response as found in some of the species investigated is unknown. It has been hypothesized that OA may induce changes in the sensory responsiveness of the Mauthner cell or other reticulospinal cells responsible for escapes (Allan et al., 2013; Wang et al., 2017). Previous work has demonstrated that the mechanism underlying behavioural malfunction in fishes exposed to elevated CO2 is due to disrupted neurotransmitter function, which is attributed to changes in the ion gradients over neuronal membranes (Nilsson et al., 2012). This disruption has been suggested to lead to the excitation of GABA-A receptors (Nilsson et al., 2012). Interestingly, Mauthner cells have GABA receptors distributed throughout them (Korn and Faber, 2005). GABA, acting primarily through interaction with GABA-A receptors, may cause Mauthner cell inhibition through membrane hyperpolarization and increased membrane conductance, which could potentially alter the response of the Mauthner cell (Lee et al., 1993). Therefore, it is possible that the sensorimotor performance and the timing of the Mauthner cell’s firing in the more sensitive fish species are negatively affected by high-CO2 exposure, resulting in the misfiring and/or changes in the timing of the action potentials that generate escape response. This hypothesis is yet to be tested and would involve complex electrophysiology but presents an exciting avenue for future research. Allan and colleagues found that parental exposure to high CO2 (11 months at ~1000 μatm) reduced the negative effects of increased CO2 exposure on fish escape responses (i.e. both locomotor and non-locomotor traits), suggesting that trans-generational acclimation may occur. However, acclimation was not complete in escape speed and absent in directionality, suggesting that parental exposure does not completely compensate for the effects of high CO2 on escape performance (Allan et al., 2014).
Acidification effects on predator–prey encounters
To date, there are only a few studies that have examined the effects of CO2 exposure on both predator and prey attack and escape responses. Allan et al. (2013) found that although elevated CO2 had no effect on predator attack speed, the distance at which the predators started to strike at the prey was affected following high CO2 exposure (Fig. 5), suggesting an effect of high CO2 on motivation to feed. However, this effect differed depending on whether the prey had also been exposed to high CO2. A functional interpretation of the effects of CO2 exposure on predators is challenging because their response is a combination of their motivation to attack and the behaviour of the prey and because the neural mechanisms underpinning predatory attacks have not been studied as extensively as those for escape responses (Schriefer and Hale, 2004).
The dynamics and outcome of predator–prey encounters are likely to be dependent on the different sensitivity of individual species (predators and prey) to elevated CO2. For example, studies involving predators and prey exposed to increased CO2 have paired coral reef fish recruits with reef-based predators (Allan et al., 2013). If we consider the differences between the pelagic environment inhabited by the recruits, and the reef environment, inhabited by the predators, these environments have very different levels of dissolved CO2. Coral reefs are dynamic shallow water habitats that experience diel cycles in CO2 due to the photosynthesis and respiration of corals. Therefore, it is possible that reef-based predators have already been exposed to high CO2 levels and may be displaying compensatory acclimation. This hypothesis is further supported by Jarrold and colleagues who showed that experimental exposure to diel CO2 cycles (42 dph at 1000 μatm ± 300 and 1000 ± 500 μatm) can reduce associated behavioural impairments in coral reef fish (Jarrold et al., 2017). In addition to the effects on the kinematics underlying predator–prey interactions that occur at least in some species, one of the most consistent impairments due to increased CO2 levels is in the olfactory behaviour of species from multiple taxa (fish and invertebrates), across multiple ecosystems, responding maladaptively to predator odours and chemical alarm cues (for review, see Nagelkerken and Munday, 2016). A change in the olfactory landscape is a reliable indicator of a potential predatory attack. Therefore, misidentifying these important cues can lead to increased mortality rates (Ferrari et al., 2011).
The results on the few predator–prey interactions presented here make it difficult to generalize across species, let alone across families; therefore, caution must be exercised when interpreting the ecological significance of these effects. Therefore, arguably, the effects of OA on fish escape responses and predator strikes, metabolism and hunger level are not sufficiently robust and the mechanistic basis not sufficiently understood, to be used for suggesting future scenarios at this time. More works on the mechanisms underpinning the effects of high CO2 and on a large number of species and species interactions are needed in order to increase our predictive power of the effect of high CO2 on predator–prey interactions. Increasing our understanding of the effects of high CO2 on predator–prey interactions is fundamental because these effects can cascade and result in changes in the species composition of communities, potentially disrupting the stability of the ecosystem (McCormick et al., 2013; Nagelkerken et al., 2018). Given that OA presents a significant and persistent environmental stressor, OA-induced maladaptive behaviours may cause future imbalances in predator–prey interactions. Therefore, knowing the physiological and neural basis of the effects of OA on predators and prey, and their relative adaptive potential, will allow us to better predict which species and which predator–prey interactions may be more sensitive than others.
Multiple stressors
Single stressor experiments are typical of the early stages in the development of a research field, but these are soon followed by increasingly complex, multi-factorial experiments that attempt to incorporate additional layers of reality into the artificiality of manipulative experiments. Such experiments allow us to explore whether the stressors combine to yield additive, antagonistic or synergistic effects on the variables measured. To date, climate change experiments in the context of escape responses have been rare and involved binary combinations of stressors to mimic future environmental scenarios. The most commonly used combination of stressors has been water temperature and elevated CO2 levels. Previous meta-analyses have found that the interactions of elevated CO2 or hypoxia with elevated water temperature lead to synergistic effects on a range of physiological and life history characters (Harvey et al., 2013; McBryan et al., 2013), but studies have seldom examined the impacts of multiple stressors on escape responses. All meta-analyses conclude that we must be cautious in making inferences from single-stressor studies.
To date, only two studies have looked at the effect of elevated CO2 and temperature on the response of a fish to an artificial startle stimulus. Nasuchon and colleagues examined the escape response under elevated CO2 (400/1000 ppm) and temperature (15 and 19°C) of adult Japanese anchovy Engraulis japonicus acclimated for 1 month (Nasuchon et al., 2016). Neither CO2 nor temperature affected the kinematic parameters analyzed (i.e. the escape trajectory, swimming velocity, acceleration, escape direction and frequency of bends), with the exception of the turning rate that was significantly higher at 19°C than at 15°C. Watson et al. (2018) used a fully factorial design (27 and 30°C and 405 and 930 ppm CO2) to test the combined effects of projected climate change on the escape response of larval kingfish (Seriola lalandi). Here, embryos and larvae were reared under the different environmental conditions until testing at 21 days post-hatch. High temperature improved performance by increasing responsiveness and maximum speed and decreasing escape latency. However, these effects were largely driven by a temperature-related increase in body mass and thus a more developmentally advanced stage for those individuals exposed to the higher temperature. On the other hand, high CO2 decreased distance covered and average speed during the escape but had no effect on responsiveness nor escape latency (Watson et al., 2018). There was no interaction between CO2 and temperature for any of the variables measured. These studies suggest there are likely to be species-specific differences in the response of fishes to the interacting effects of CO2 and temperature. Clearly, further examples are required before we can determine whether there is any phylogenetic pattern to the impact of these factors on escape performance.
An important step towards understanding the impact of climate change at the community level is to explore predator–prey interactions from the perspective of both participants at the same time. Only one study has examined both the responses of the prey and the predator to combined future stressors. Allan and colleagues exposed newly settled damselfish (P. wardi) and predatory dottybacks (P. fuscus) to control or elevated CO2 (~405 and 930 μatm) and temperature (27 and 30°C) conditions for 7 days in a 2 × 2 design. Detailed examination of the interaction between predator and prey found that high temperature had an overwhelming effect on the escape behaviour of the prey (i.e. on reaction distance, ALT, escape distance, but not escape speed) compared with the combined exposure to elevated CO2 and high temperature (significant interaction effect on escape speed), or the independent effect of elevated CO2 (which affected only escape speed) (Fig. 6). Exposure to high temperatures led to increased capture success and predation rate. Interestingly, there was little influence of elevated CO2 on the behaviour of the predator, emphasizing the species specificity of the response to elevated CO2 and the pressing requirement to examine fish escape responses in an ecological context within future climate scenarios (Allan et al., 2017).
Figure 6.
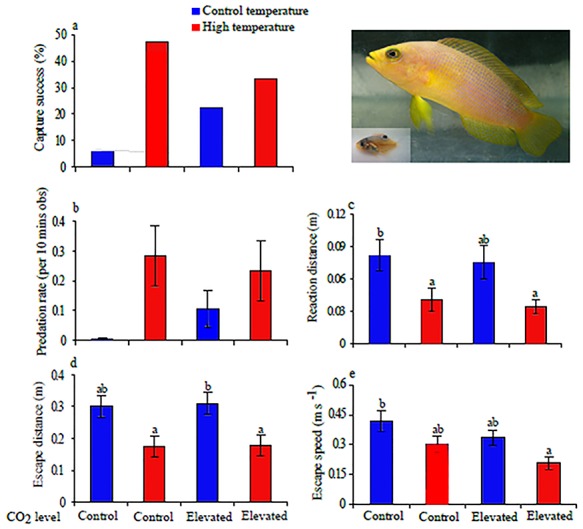
The effect of temperature (27 and 30°C) and elevated CO2 (405 and 930 μatm) on the interaction between dottyback predator (P. fuscus) and its damselfish prey (P. wardi); (a) capture success, (b) predation rate, (c) prey reaction distance, (d) escape distance and (e) escape speed [modified version from Allan et al. (2017)].
Recently, studies have begun to incorporate natural cycles within climate variables, such as O2, CO2 or temperature. In the only study to date to attempt to investigate the effect of diel cycles of CO2 on fish anti-predator performance (Jarrold and Munday, 2018), the escape response to a startle stimulus of juvenile damselfish (Acanthochromis polyacanthus) was examined. Fish were reared for 11 weeks in two stable (450 and 1000 μatm) and two diel-cycling elevated CO2 treatments (1000 ± 300 and 1000 ± 500 μatm) at both current-day (29°C) and projected future temperatures (31°C). No interaction between CO2 and temperature for any performance characteristics was found, though survival was affected. Survival was lower in the two cycling CO2 treatments at 31°C compared with 29°C, but did not differ between temperatures in the two stable CO2 treatments. While temperature had significant independent effects on escape performance traits, there was no effect of stable or cycling CO2 levels nor was there an interaction between temperature and CO2.These findings support the idea that water temperature, confined within realistic near-future projections, has the greatest impact on escape performance compared to elevated CO2 (Allan et al., 2017, Jarrold and Munday, 2018). Other stressors, such as dissolved oxygen can also display major diel fluctuations (e.g. Domenici et al., 2007a) and these are yet to be incorporated into experiments. Although experiments that incorporate cycles in more than one stressor have yet to be undertaken, these will be important as diel temperature cycles peak during the daylight hours, while diel CO2 cycles peak at night, and oxygen levels tend to be lowest at dawn. These diel patterns may complicate predictions about how they interact to affect fish performance.
Conclusions and suggestions for further research
To date, experiments have been overly simplistic, which hampers the predictions of future community dynamics. With few exceptions, levels of experimental treatments have been static rather than naturally dynamic. Experiments will benefit from incorporating the interactive effects of two or more stressors, each with a minimum of three levels. Ideally, choice of levels for each stressor will be informed by information on species’ stressor-specific reaction norms for performance as this will enhance interpretation. While studies are becoming increasingly complex, researchers are yet to incorporate parental effects into studies of the interactive effects of climate stressors on escape performance. The way parents respond to their environment can shape offspring phenotypes, and these may help or hinder escape performance. Trans-generational experiments examining various traits including growth and metabolism have shown how parental exposure to high CO2 (Miller et al., 2012; Allan et al., 2014; Welch et al., 2014; McMahon et al., 2018) and temperature (Salinas and Munch, 2012; Donelson et al., 2014; Shama et al., 2014; Bernal et al., 2018) affect offspring performance in isolation, but it is not known how interactions between these stressors may modify parental effects and offspring responses. Clearly, experiments that span multiple generations and incorporate two or more stressors are needed to provide an understanding of the role of parental inputs on fishes under climate change. We need to understand the mechanisms whereby performance is actually being affected by the stressors and their interactions if we are to understand the species capacity to adapt or acclimate to these conditions.
Where possible, studies should ideally make a conscious and argued decision on the duration of exposure to the various stressors and findings and this should be put in relation to the magnitude of natural fluctuations in the environmental stressors within the study system, and their predicted changes in the future. For instance, temperatures change naturally in a hierarchy of temporal scales, with acute changes (maybe tidal or daily) within a more slowly changing average (seasonal) temperature signal. The capacity of an organism to cope with and respond to acute changes in environmental variables may then be just as important as their capacity to adjust through acclimation to seasonally driven cycles.
Empirical studies on various taxa that have been sampled across a species geographic range have shown that sensitivity to elevated CO2 conditions is linked to the local conditions experienced (Kelly et al., 2013; Pespeni et al., 2013; Thomsen et al., 2017; Vargas et al., 2017). Similarly, temperature and hypoxia tolerances are species and site specific (Bickler and Buck, 2007; Payne et al., 2016). A meta-analysis of the impact of multiple stressors found that synergistic interactions may be quite common in nature, particularly where two or more stressors co-occur (Crain et al., 2008). Their analysis found that when a third stressor was added to an experiment, it changed interaction effects in two-thirds of the experiments and doubled the number of synergistic interactions. It is likely that future meta-analyses of the influence of stressors on escape response and predator–prey interactions in general will find similar levels of complex interactive effects.
Modelling the effects of changes in temperature, oxygen and CO2 levels on predator–prey interactions is a challenging but a much needed task in order to increase our predictive ability of the ecological effects of climate change. The importance of these effects in shaping marine communities is highlighted by the differential outcomes that climate change may have on various species and taxa of predators and prey (Domenici et al., 2007a), which can be modelled conceptually (Öhlund et al., 2015) and metabolically (Dell et al., 2014; Grady et al., 2019). A promising avenue to gain predictive power on the ecological effects of climate change is that of focusing on the more obvious differences between various taxa of predators and prey. Endotherms and ectotherms will respond differently to climate change, and whether they are predators or prey will have consequences for the outcome of interactions that will affect the patterns of species abundance and community composition, with high relevance for conservation and mitigation strategies (Dell et al., 2014; Grady et al., 2019). Similarly, how body size may modulate the effect of climate change (e.g. small fish may be more hypoxia tolerant than large fish) (Pan et al., 2016) is an interesting area for future research. Furthering our understanding of how climate change stressors affect escape responses in fish of different species and sizes is a fundamental step to scale up to predator–prey interactions and their consequences at the community level.
Acknowledgements
P.D. was supported by an Eranet-Lac Grant (Project CLIMAR). M.I.M. was supported by ARC Centre of Excellence for Coral Reef Studies (EI140100117).
References
- Ackerly KL, Chapman LJ, Krahe R (2017) Hypoxia acclimation increases novelty response strength during fast-starts in the African mormyrid, Marcusenius victoriae. Comp Biochem Physiol A Mol Integr Physiol 213: 36–45. [DOI] [PubMed] [Google Scholar]
- Ackerly KL, Ward AB (2016) How temperature-induced variation in musculoskeletal anatomy affects escape performance and survival of zebrafish (Danio rerio). J Exp Zool A Ecol Genet Physiol 325: 25–40. [DOI] [PubMed] [Google Scholar]
- Allan BJM, Domenici P, McCormick MI, Watson SA, Munday PL (2013) Elevated CO2 affects predator-prey interactions through altered performance. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BJM, Domenici P, Munday PL, McCormick MI (2015) Feeling the heat: the effect of acute temperature changes on predator–prey interactions in coral reef fish. Conservation Physiol 3: cov011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BJM, Domenici P, Watson SA, Munday PL, McCormick MI (2017) Warming has a greater effect than elevated CO2 on predator–prey interactions in coral reef fish. Proc R Soc B Biol Sci 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BJM, Miller GM, McCormick MI, Domenici P, Munday PL (2014) Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc R Soc B Biol Sci 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez D, Metcalfe NB (2007) The tradeoff between catch-up growth and escape speed: variation between habitats in the cost of compensation. Oikos 116: 1144–1151. [Google Scholar]
- Bailleul F, Charrassin J-B, Monestiez P, Roquet F, Biuw M, Guinet C (2007) Successful foraging zones of southern elephant seals from the Kerguelen Islands in relation to oceanographic conditions. Philos Trans R Soc B Biol Sci 362: 2169–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty RS, Blaxter JHS, Fretwell K (1993) Effect of temperature on the escape responses of larval herring, Clupea harengus. Mar Biol 115: 523–528. [Google Scholar]
- Beamish FWH. (1978) Swimming capacity In Hoar WS, Randall DJ, eds, Fish Physiology, EdVII Academic Press, New York, pp. 101–187 [Google Scholar]
- Beddow TA, Leeuwen JL, Johnston IA (1995) Swimming kinematics of fast starts are altered by temperature acclimation in the marine fish Myoxocephalus scorpius. J Exp Biol 198: 203–208. [DOI] [PubMed] [Google Scholar]
- Bell GW, Eggleston DB (2005) Species-specific avoidance responses by blue crabs and fish to chronic and episodic hypoxia. Mar Biol 146: 761–770. [Google Scholar]
- Bernal MA, Donelson JM, Veilleux HD, Ryu T, Munday PL, Ravasi T (2018) Phenotypic and molecular consequences of stepwise temperature increase across generations in a coral reef fish. Mol Ecol 27: 4516–4528. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Buck LT (2007) Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol 69: 145–170. [DOI] [PubMed] [Google Scholar]
- Bijma J, Pörtner H-O, Yesson C, Rogers AD (2013) Climate change and the oceans—what does the future hold? Mar Pollut Bull 74: 495–505. [DOI] [PubMed] [Google Scholar]
- Breitburg D, et al. (2018) Declining oxygen in the global ocean and coastal waters. Science 359: eaam7240. [DOI] [PubMed] [Google Scholar]
- Breitburg DL, Steinberg N, DuBeau S, Cooksey C, Houde ED (1994) Effects of low dissolved oxygen on predation on estuarine fish larvae. Mar Ecol Prog Ser 104: 235–246. [Google Scholar]
- Cairns DK, Gaston AJ, Huettmann F (2008) Endothermy, ectothermy and the global structure of marine vertebrate communities. Mar Ecol Prog Ser 356: 239–250. [Google Scholar]
- Cannas M, Domenici P, Lefrançois C (2012) The effect of hypoxia on ventilation frequency in startled common sole Solea solea. J Fish Biol 80: 2636–2642. [DOI] [PubMed] [Google Scholar]
- Catania KC. (2009) Tentacled snakes turn C-starts to their advantage and predict future prey behavior. Proc Natl Acad Sci USA 106: 11183–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattano C, Claudet J, Domenici P, Milazzo M (2018) Living in a high CO2 world: a global meta-analysis shows multiple trait-mediated fish responses to ocean acidification. Ecol Monogr 88: 320–335. [Google Scholar]
- Chabot D, Dutil J-D (1999) Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J Fish Biol 55: 472–491. [Google Scholar]
- Chapman LJ, Chapman CA (1994) Observations on synchronous air breathing in Clarias liocephalus. Copeia 1994: 246–249. [Google Scholar]
- Chapman LJ, Chapman CA, Chandler M (1996a) Wetland ecotones as refugia for endangered fishes. Biol Conserv 78: 263–270. [Google Scholar]
- Chapman LJ, Chapman CA, Ogutu-Ohwayo R, Chandler M, Kaufman L, Keiter AE (1996b) Refugia for endangered fishes from an introduced predator in Lake Nabugabo, Uganda. Conserv Biol 10: 554–561. [Google Scholar]
- Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ Jr, Stenseth NC, Pertoldi C (2010) Adapting to climate change: a perspective from evolutionary physiology. Clim Res 43: 3–15. [Google Scholar]
- Chung W-S, Marshall NJ, Watson S-A, Munday PL, Nilsson GE (2014) Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. J Exp Biol 217: 323–326. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through Fry's concept of aerobic metabolic scope. J Fish Biol 88: 232–251. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Webber DM, Lagardère JP, Kerr SR (2000) Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J Sea Res 44: 257–265. [Google Scholar]
- Coma R, Ribes M, Serrano E, Jiménez E, Salat J, Pascual J (2009) Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc Natl Acad Sci 106: 6176–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WE, Blumstein DT (2015) Escaping From Predators: An Integrative View of Escape Decisions. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Couturier CS, Stecyk JAW, Rummer JL, Munday PL, Nilsson GE (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe MJC. (2008) Climate change, global warming and coral reefs: modelling the effects of temperature. Comput Biol Chem 32: 311–314. [DOI] [PubMed] [Google Scholar]
- Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11: 1304–1315. [DOI] [PubMed] [Google Scholar]
- Crispo E, Chapman LJ (2011) Hypoxia drives plastic divergence in cichlid body shape. Evol Ecol 25: 949–964. [Google Scholar]
- Danos N, Lauder GV (2012) Challenging zebrafish escape responses by increasing water viscosity. J Exp Biol 215: 1854–1862. [DOI] [PubMed] [Google Scholar]
- Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci 106: 12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsert K, Steenbeek J, Lewis K, Buszowski J, Cowan JH, Christensen V (2016) Exploring effects of hypoxia on fish and fisheries in the northern Gulf of Mexico using a dynamic spatially explicit ecosystem model. Ecol Model 331: 142–150. [Google Scholar]
- Dell AI, Pawar S, Savage VM (2014) Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J Anim Ecol 83: 70–84. [DOI] [PubMed] [Google Scholar]
- Devries MS, Wainwright PC (2006) The effects of acute temperature change on prey capture kinematics in largemouth bass, Micropterus salmoides. Copeia 2006: 437–444. [Google Scholar]
- Diaz RJ. (2001) Overview of hypoxia around the world. J Environ Qual 30: 275–281. [DOI] [PubMed] [Google Scholar]
- Dill LM. (1974) Escape response of zebra Danio (Brachydanio rerio). Part 1—stimulus for escape. Anim Behav 22: 711–722. [Google Scholar]
- Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13: 68–75. [DOI] [PubMed] [Google Scholar]
- Domenici P. (2001) The scaling of locomotor performance in predator-prey encounters: from fish to killer whales. Comp Biochem Physiol A Comp Physiol 131: 169–182. [DOI] [PubMed] [Google Scholar]
- Domenici P. (2010a) Context-dependent variability in the components of fish escape response: integrating locomotor performance and behavior. J Exp Zool 313A: 59–79. [DOI] [PubMed] [Google Scholar]
- Domenici P. (2010b) Escape responses in fish: Kinematics, performance and behavior In Domenici P, Kapoor BG, eds, Fish Locomotion: An Eco-ethological Perspective. 123–170. Enfield Scientific Publishers [Google Scholar]
- Domenici P, Blagburn JM, Bacon J (2011) Animal escapology II: review of escape trajectory case studies. J Exp Biol 214: 2474–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P, Blake R (1997) The kinematics and performance of fish fast-start swimming. J Exp Biol 200: 1165–1178. [DOI] [PubMed] [Google Scholar]
- Domenici P, Claireaux G, McKenzie DJ (2007a) Environmental constraints upon locomotion and predator-prey interactions in aquatic organisms: an introduction. Philos Trans R Soc B Biol Sci 362: 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P, Ferrari RS, Steffensen JF, Batty RS (2002) The effect of progressive hypoxia on school structure and dynamics in Atlantic herring Clupea harengus. Proc R Soc Lond B Biol Sci 269: 2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P, Lefrançois C, Shingles A (2007b) Hypoxia and the anti-predator behaviour of fishes. Philos Trans R Soc B 362: 2105–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P, Turesson H, Brodersen J, Brönmark C (2008) Predator-induced morphology enhances escape locomotion in crucian carp. Proc R Soc Biol Sci B 275: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P, Hale ME (2019) Escape responses of fish: a review of the diversity in motor control, kinematics and behaviour. J Exp Biol 222: jeb166009. doi: 10.1242/jeb.166009. [DOI] [PubMed] [Google Scholar]
- Donelson JM, McCormick MI, Booth DJ, Munday PL (2014) Reproductive acclimation to increased water temperature in a tropical reef fish. PLoS One 9: e97223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1: 169–192. [DOI] [PubMed] [Google Scholar]
- Draper AM, Weissburg MJ (2019) Impacts of global warming and elevated CO2 on sensory behavior in predator-prey interactions: a review and synthesis. Front Ecol Evol 7, 72. [Google Scholar]
- Eaton RC, Lee RKK, Foreman MB (2001) The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol 63: 467–485. [DOI] [PubMed] [Google Scholar]
- Ekau W, Auel H, Pörtner HO, Gilbert D (2010) Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 7: 1669–1699. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye Salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Engström-Öst J, Isaksson I (2006) Effects of macroalgal exudates and oxygen deficiency on survival and behaviour of fish larvae. J Exp Mar Biol Ecol 335: 227–234. [Google Scholar]
- Fay RR, Ream TJ (1992) The effects of temperature change and transient hypoxia on auditory nerve fiber response in the goldfish (Carassius auratus). Hear Res 58: 9–18. [DOI] [PubMed] [Google Scholar]
- Fenkes M, Shiels HA, Fitzpatrick JL, Nudds RL (2016) The potential impacts of migratory difficulty, including warmer waters and altered flow conditions, on the reproductive success of salmonid fishes. Comp Biochem Physiol A Mol Integr Physiol 193: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2011) Intrageneric variation in antipredator responses of coral reef fishes affected by ocean acidification: implications for climate change projections on marine communities. Glob Chang Biol 17: 2980–2986. [Google Scholar]
- Fowler B, Prlic H (1995) A comparison of visual and auditory reaction-time and P300 latency thresholds to acute-hypoxia. Aviat Space Environ Med 66: 645–650. [PubMed] [Google Scholar]
- Frölicher TL, Joos F, Plattner G-K, Steinacher M, Doney SC (2009) Natural variability and anthropogenic trends in oceanic oxygen in a coupled carbon cycle–climate model ensemble. Glob Biogeochem Cycles 23, GB1003. [Google Scholar]
- Fuiman LA, Rose KA, Cowan JH, Smith EP (2006) Survival skills required for predator evasion by fish larvae and their relation to laboratory measures of performance. Anim Behav 71: 1389–1399. [Google Scholar]
- Garcia de la D, VLA V, Andree KB, Darias M, Estévez A, Gisbert E, Johnston IA (2012) Development temperature has persistent effects on muscle growth responses in Gilthead Sea bream. PLoS One 7: e51884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JH. (1980) Respiratory patterns and antipredator responses in the central mudminnow, Umbra limi, a continuous, facultative, air-breathing fish. Can J Zool 58: 819–827. [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25: 325–331. [DOI] [PubMed] [Google Scholar]
- Gotanda KM, Reardon EE, Murphy SMC, Chapman LJ (2012) Critical swim speed and fast-start response in the African cichlid Pseudocrenilabrus multicolor victoriae: convergent performance in divergent oxygen regimes. Can J Zool 90: 545–554. [Google Scholar]
- Grady JM, et al. (2019) Metabolic asymmetry and the global diversity of marine predators. Science 363: eaat4220. [DOI] [PubMed] [Google Scholar]
- Grigaltchik VS, Ward AJW, Seebacher F (2012) Thermal acclimation of interactions: differential responses to temperature change alter predator–prey relationship. Proc R Soc B Biol Sci 279: 4058–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BS, Selkoe KA, Micheli F, Kappel CV (2007) Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv Biol 21: 1301–1315. [DOI] [PubMed] [Google Scholar]
- Hanel R, Wieser W (1996) Growth of swimming muscles and its metabolic cost in larvae of whitefish at different temperatures. J Fish Biol 48: 937–951. [Google Scholar]
- Harley CDG, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9: 228–241. [DOI] [PubMed] [Google Scholar]
- Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3: 1016–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges KJ, Abrahams MV (2015) Hypoxic refuges, predator–prey interactions and habitat selection by fishes. J Fish Biol 86: 288–303. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Kingsolver JG, Carrington E (2005) Biophysics, physiological ecology, and climate change: does mechanism matter? Annu Rev Physiol 67: 177–201. [DOI] [PubMed] [Google Scholar]
- Herbing I. (2002) Effects of temperature on larval fish swimming performance: the importance of physics to physiology. J Fish Biol 61: 865–876. [Google Scholar]
- Higham TE, Stewart WJ, Wainwright PC (2015) Turbulence, temperature, and turbidity: the Ecomechanics of predator–prey interactions in fishes. Integr Comp Biol 55: 6–20. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, et al. (2011) High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS One 6: e28983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsicker ME, et al. (2011) Functional responses and scaling in predator–prey interactions of marine fishes: contemporary issues and emerging concepts. Ecol Lett 14: 1288–1299. [DOI] [PubMed] [Google Scholar]
- Isaak DJ, Rieman BE (2013) Stream isotherm shifts from climate change and implications for distributions of ectothermic organisms. Glob Chang Biol 19: 742–751. [DOI] [PubMed] [Google Scholar]
- James R, Johnston IA (1998a) Scaling of muscle performance during escape responses in the fish Myoxocephalus scorpius L. J Exp Biol 201: 913–923. [DOI] [PubMed] [Google Scholar]
- James RS. (2013) A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J Comp Physiol B 183: 723–733. [DOI] [PubMed] [Google Scholar]
- James RS, Johnston IA (1998b) Influence of spawning on swimming performance and muscle contractile properties in the short-horn sculpin. J Fish Biol 53: 485–501. [Google Scholar]
- Jarrold MD, Humphrey C, McCormick MI, Munday PL (2017) Diel CO2 cycles reduce severity of behavioural abnormalities in coral reef fish under ocean acidification. Sci Rep 7: 10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold MD, Munday PL (2018) Elevated temperature does not substantially modify the interactive effects between elevated CO2 and Diel CO2 cycles on the survival, growth and behavior of a coral reef fish. Front Mar Sci 5. [Google Scholar]
- Johansen JL, Jones GP (2011) Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob Chang Biol 17: 2971–2979. [Google Scholar]
- Johansen R, Barrett RT, Pedersen T (2001) Foraging strategies of great cormorants Phalacrocorax carbo carbo wintering north of the Arctic circle. Bird Study 48: 59–67. [Google Scholar]
- Johnson T, Cullum A, Bennett A (1998) Partitioning the effects of temperature and kinematic viscosity on the C-start performance of adult fishes. J Exp Biol 201: 2045–2051. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Bennett AF (1995) The thermal-acclimation of burst escape performance in fish—an integrated study of molecular and cellular physiology and organismal performance. J Exp Biol 198: 2165–2175. [DOI] [PubMed] [Google Scholar]
- Johnston IA, Temple GK (2002) Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J Exp Biol 205: 2305–2322. [DOI] [PubMed] [Google Scholar]
- Kelly MW, Padilla-Gamiño JL, Hofmann GE (2013) Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob Chang Biol 19: 2536–2546. [DOI] [PubMed] [Google Scholar]
- Kersten M, Britton RH, Dugan PJ, Hafner H (1991) Flock feeding and food intake in Little egrets: the effects of prey distribution and behaviour. J Anim Ecol 60: 241–252. [Google Scholar]
- Killen SS, Reid D, Marras S, Domenici P (2015) The interplay between aerobic metabolism and antipredator performance: vigilance is related to recovery rate after exercise. Front Physiol 6, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi T, Oda Y (2008) Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci 28: 10641–10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS (2005) The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47: 13–28. [DOI] [PubMed] [Google Scholar]
- Kramer DL. (1987) Dissolved oxygen and fish behavior. Environ Biol Fish 18: 81–92. [Google Scholar]
- Kramer DL, Graham JB (1976) Synchronous air breathing, a social component of respiration in fishes. Copeia 1976: 689–697. [Google Scholar]
- Langerhans RB, Reznick DN (2010) Ecology and evolution of swimming performance in fishes: predicting evolution with biomechanics In Fish Locomotion: An Eco-ethological Perspective, eds. Domenici P and Kapoor BG), pp. 200–248: Enfield Scientific Publishers. [Google Scholar]
- Lea JMD, Keen AN, Nudds RL, Shiels HA (2016) Kinematics and energetics of swimming performance during acute warming in brown trout Salmo trutta. J Fish Biol 88: 403–417. [DOI] [PubMed] [Google Scholar]
- Learmonth JA, MacLeod CD, Santos MB, Pierce GJ, Crick HQP, Robinson RA (2006) Potential effects of climate change on marine mammals In Gibson RN, Atkinson RJA, Gordon JDM, eds, Oceanography and Marine Biology: An Annual Review Vol 44, pp. 431–464
- Lee RKK, Finger TE, Eaton RC (1993) GABAergic innervation of the Mauthner cell and other reticulospinal neurons in the goldfish. J Comp Neurol 338: 601–611. [DOI] [PubMed] [Google Scholar]
- Lefrancois C, Domenici P (2006) Locomotor kinematics and behaviour in the escape response of European sea bass, Dicentrarchus labrax L., exposed to hypoxia. Marine Biol (Berlin) 149: 969–977. [Google Scholar]
- Lefrançois C, Shingles A, Domenici P (2005) The effect of hypoxia on locomotor performance and behaviour during escape in Liza aurata. J Fish Biol 67: 1712–1730. [Google Scholar]
- Lyon JP, Ryan TJ, Scroggie MP (2008) Effects of temperature on the fast-start swimming performance of an Australian freshwater fish. Ecol Freshw Fish 17: 184–188. [Google Scholar]
- Marras S, Killen SS, Domenici P, Claireaux G, McKenzie DJ (2013) Relationships among traits of aerobic and anaerobic swimming performance in individual European Sea bass Dicentrarchus labrax. PLoS One 8: e72815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M, Bédard M, Dutil J-D, Guderley H (2004) Does condition of Atlantic cod (Gadus morhua) have a greater impact upon swimming performance at Ucrit or sprint speeds? J Exp Biol 207: 2979–2990. [DOI] [PubMed] [Google Scholar]
- McBryan TL, Anttila K, Healy TM, Schulte PM (2013) Responses to temperature and hypoxia as interacting stressors in fish: implications for adaptation to environmental change. Integr Comp Biol 53: 648–659. [DOI] [PubMed] [Google Scholar]
- McCormick MI, Fakan E, Allan BJM (2018a) Behavioural measures determine survivorship within the hierarchy of whole-organism phenotypic traits. Funct Ecol 32: 958–969. [Google Scholar]
- McCormick MI, Watson S-A, Munday PL (2013) Ocean acidification reverses competition for space as habitats degrade. Sci Rep 3: 3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MI, Watson S-A, Simpson SD, Allan BJM (2018b) Effect of elevated CO2 and small boat noise on the kinematics of predator–prey interactions. Proc R Soc B Biol Sci 285: 20172650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie DJ, Claireaux G (2010) Effects of environmental factors on the physiology of sustained aerobic exercise In Domenici P, Kapoor BG, eds, Fish Locomotion—An Eco-ethological Perspective. Science Publishers, Enfield, New Hampshire, pp. 296–332. [Google Scholar]
- McMahon SJ, Donelson JM, Munday PL (2018) Food ration does not influence the effect of elevated CO2 on antipredator behaviour of a reef fish. Mar Ecol Prog Ser 586: 155–165. [Google Scholar]
- Meinshausen M, et al. (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Chang 109: 213–241. [Google Scholar]
- Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2013) Future Ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160: 1875–1888. [Google Scholar]
- Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Chang 2: 858. [Google Scholar]
- Moyes CD, Schulte PM, Andwest TG (1993) Burst Exercise Recovery Metabolism in Fish White Muscle. CRC Press, Boca Raton, FL [Google Scholar]
- Munday PL, Crawley NE, Nilsson GE (2009) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388: 235–242. [Google Scholar]
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP (2010) Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA 107: 12930–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Welch MJ, Allan BJM, Watson S-A, McMahon SJ, McCormick MI (2016) Effects of elevated CO2 on predator avoidance behaviour by reef fishes is not altered by experimental test water. PeerJ 4: e2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz NJ, Breckels RD, Neff BD (2012) The metabolic, locomotor and sex-dependent effects of elevated temperature on Trinidadian guppies: limited capacity for acclimation. J Exp Biol 215: 3436–3441. [DOI] [PubMed] [Google Scholar]
- Nagelkerken I, Goldenberg SU, Coni EOC, Connell SD (2018) Microhabitat change alters abundances of competing species and decreases species richness under ocean acidification. Sci Total Environ 645: 615–622. [DOI] [PubMed] [Google Scholar]
- Nagelkerken I, Munday PL (2016) Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Chang Biol 22: 974–989. [DOI] [PubMed] [Google Scholar]
- Nair A, Nguyen C, McHenry MJ (2017) A faster escape does not enhance survival in zebrafish larvae. Proc R Soc B Biol Sci 284, 20170359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuchon N, Yagi M, Kawabata Y, Gao K, Ishimatsu A (2016) Escape responses of the Japanese anchovy Engraulis japonicus under elevated temperature and CO2 conditions. Fish Sci 82: 435–444. [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sorensen C, Watson SA, Munday PL (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang 2: 201–204. [Google Scholar]
- Öhlund G, Hedström P, Norman S, Hein CL, Englund G (2015) Temperature dependence of predation depends on the relative performance of predators and prey. Proc R Soc B Biol Sci 282: 20142254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojanguren AF, Fuiman LA (2010) Seasonal variability in antipredator performance of red drum larvae. Mar Ecol Prog Ser 413: 117–123. [Google Scholar]
- Orr JC, et al. (2005) Anthropogenic Ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681. [DOI] [PubMed] [Google Scholar]
- Pacella SR, Brown CA, Waldbusser GG, Labiosa RG, Hales B (2018) Seagrass habitat metabolism increases short-term extremes and long-term offset of CO2 under future ocean acidification. Proc Natl Acad Sci 115: 3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YK, Ern R, Esbaugh AJ (2016) Hypoxia tolerance decreases with body size in red drum Sciaenops ocellatus. J Fish Biol 89: 1488–1493. [DOI] [PubMed] [Google Scholar]
- Pankhurst NW, Purser GJ, Van Der Kraak G, Thomas PM, Forteath GNR (1996) Effect of holding temperature on ovulation, egg fertility, plasma levels of reproductive hormones and in vitro ovarian steroidogenesis in the rainbow trout Oncorhynchus mykiss. Aquaculture 146: 277–290. [Google Scholar]
- Parker-Stetter SL, Horne JK (2009) Nekton distribution and midwater hypoxia: a seasonal, diel prey refuge? Estuar Coast Shelf Sci 81: 13–18. [Google Scholar]
- Pauly D. (1980) On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J Mar Sci 39: 175–192. [Google Scholar]
- Payne NL, Smith JA, Meulen DE, Taylor MD, Watanabe YY, Takahashi A, Marzullo TA, Gray CA, Cadiou G, Suthers IM (2016) Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Funct Ecol 30: 903–912. [Google Scholar]
- Penghan L-Y, Pang X, Fu S-J (2016) The effects of starvation on fast-start escape and constant acceleration swimming performance in rose bitterling (Rhodeus ocellatus) at two acclimation temperatures. Fish Physiol Biochem 42: 909–918. [DOI] [PubMed] [Google Scholar]
- Penghan LY, Cao ZD, Fu SJ (2014) Effect of temperature and dissolved oxygen on swimming performance in crucian carp. Aquat Biol 21: 57–65. [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1915. [DOI] [PubMed] [Google Scholar]
- Pespeni MH, Chan F, Menge BA, Palumbi SR (2013) Signs of adaptation to local pH conditions across an environmental mosaic in the California current ecosystem. Integr Comp Biol 53: 857–870. [DOI] [PubMed] [Google Scholar]
- Poloczanska ES, et al. (2013) Global imprint of climate change on marine life. Nat Clim Chang 3: 919. [Google Scholar]
- Pörtner HO. (2002) Physiological basis of temperature-dependent biogeography: trade-offs in muscle design and performance in polar ectotherms. J Exp Biol 205: 2217–2230. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP (2008) Ecology physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315: 95–97. [DOI] [PubMed] [Google Scholar]
- Poulin R, Wolf NG, Kramer DL (1987) The effect of hypoxia on the vulnerability of guppies (Poecilia reticulata, Poeciliidae) to an aquatic predator (Astronotus ocellatus, Cichlidae). Environ Biol Fish 20: 285–292. [Google Scholar]
- Preuss T, Faber DS (2003) Central cellular mechanisms underlying temperature-dependent changes in the goldfish startle-escape behavior. J Neurosci 23: 5617–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahel FJ, Nutzman JW (1994) Foraging in a lethal environment: fish predation in hypoxic waters of a stratified Lake. Ecology 75: 1246–1253. [Google Scholar]
- Rees BB, Targett TE, Ciotti BJ, Tolman CA, Akkina SS, Gallaty AM (2012) Temporal dynamics in growth and white skeletal muscle composition of the mummichog Fundulus heteroclitus during chronic hypoxia and hyperoxia. J Fish Biol 81: 148–164. [DOI] [PubMed] [Google Scholar]
- Reidy S, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203: 347–357. [DOI] [PubMed] [Google Scholar]
- Ripley JL, Foran CM (2007) Influence of estuarine hypoxia on feeding and sound production by two sympatric pipefish species (Syngnathidae). Mar Environ Res 63: 350–367. [DOI] [PubMed] [Google Scholar]
- Robb T, Abrahams MV (2002) The influence of hypoxia on risk of predation and habitat choice by the fathead minnow, Pimephales promelas. Behav Ecol Sociobiol 52: 25–30. [Google Scholar]
- Robb T, Abrahams MV (2003) Variation in tolerance to hypoxia in a predator and prey species: an ecological advantage of being small? J Fish Biol 62: 1067–1081. [Google Scholar]
- Roberts JJ, Grecay PA, Ludsin SA, Pothoven SA, Vanderploeg HA, Hook TO (2012) Evidence of hypoxic foraging forays by yellow perch (Perca flavescens) and potential consequences for prey consumption. Freshw Biol 57: 922–937. [Google Scholar]
- Robinson E, Jerrett A, Black S, Davison W (2013) Hypoxia impairs visual acuity in snapper (Pagrus auratus). J Comp Physiol A 199: 611–617. [DOI] [PubMed] [Google Scholar]
- Rosa R, Seibel BA (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci 105: 20776–20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummer JL, Munday PL, Watson S-A, Couturier CS, Nilsson GE, Stecyk JAW (2013) Elevated CO2 enhances aerobic scope of a coral reef fish. Conservation Physiol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S, Munch SB (2012) Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett 15: 159–163. [DOI] [PubMed] [Google Scholar]
- Sánchez-García MA, Zottoli SJ, Roberson LM (2017) Hypoxia has lasting effects on fast startle behavior of a tropical fish (Haemulon plumieri). bioRxiv 109066. [DOI] [PubMed] [Google Scholar]
- Scharf FS, Buckel JA, McGinn PA, Juanes F (2003) Vulnerability of marine forage fishes to piscivory: effects of prey behavior on susceptibility to attack and capture. J Exp Mar Biol Ecol 294: 41–59. [Google Scholar]
- Schriefer JE, Hale ME (2004) Strikes and startles of northern pike (Esox lucius): a comparison of muscle activity and kinematics between S-start behaviors. J Exp Biol 207: 535–544. [DOI] [PubMed] [Google Scholar]
- Schulz KG, Riebesell U (2013) Diurnal changes in seawater carbonate chemistry speciation at increasing atmospheric carbon dioxide. Mar Biol 160: 1889–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann H, Steffensen J (1994) Spontaneous swimming activity of Atlantic cod Gadus morhua exposed to graded hypoxia at three temperatures. J Exp Biol 197: 129–142. [DOI] [PubMed] [Google Scholar]
- Scott M, Heupel M, Tobin A, Pratchett M (2017) A large predatory reef fish species moderates feeding and activity patterns in response to seasonal and latitudinal temperature variation. Sci Rep 7: 12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Beaman J, Little AG (2014) Regulation of thermal acclimation varies between generations of the short-lived mosquitofish that developed in different environmental conditions. Funct Ecol 28: 137–148. [Google Scholar]
- Seebacher F, Grigaltchik VS (2015) Developmental thermal plasticity of prey modifies the impact of predation. J Exp Biol 218: 1402–1409. [DOI] [PubMed] [Google Scholar]
- Shama LNS, Strobel A, Mark FC, Wegner KM (2014) Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct Ecol 28: 1482–1493. [Google Scholar]
- Shang EHH, Wu RSS (2004) Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ Sci Technol 38: 4763–4767. [DOI] [PubMed] [Google Scholar]
- Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML (2013) Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob Chang Biol 19: 1632–1641. [DOI] [PubMed] [Google Scholar]
- Shingles A, McKenzie DJ, Claireaux G, Domenici P (2005) Reflex cardioventilatory responses to hypoxia in the flathead gray mullet (Mugil cephalus) and their behavioral modulation by perceived threat of predation and water turbidity. Physiol Biochem Zool 78: 744–755. [DOI] [PubMed] [Google Scholar]
- Shoji J, Masuda R, Yamashita Y, Tanaka M (2005) Effect of low dissolved oxygen concentrations on behavior and predation rates on red sea bream Pagrus major larvae by the jellyfish Aurelia aurita and by juvenile Spanish mackerel Scomberomorus niphonius. Mar Biol 147: 863–868. [Google Scholar]
- Sitko S, Honrubia V (1986) Differential effect of ischemia on spontaneous and sinusoidal-evoked activity in semicircular canal afferents in the bullfrog. Acta Otolaryngol 102: 179–185. [DOI] [PubMed] [Google Scholar]
- Sollid J, De Angelis P, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206: 3667–3673. [DOI] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Chang 2: 686. [Google Scholar]
- Szabo TM, Brookings T, Preuss T, Faber DS (2008) Effects of temperature acclimation on a central neural circuit and its behavioral output. J Neurophysiol 100: 2997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JC, Rand PS, Jenkins J (2007) Swimming behavior of juvenile anchovies (Anchoa spp.) in an episodically hypoxic estuary: implications for individual energetics and trophic dynamics. Mar Biol 152: 939–957. [Google Scholar]
- Temple GK, Johnston IA (1998) Testing hypotheses concerning the phenotypic plasticity of escape performance in fish of the family Cottidae. J Exp Biol 201: 317–331. [DOI] [PubMed] [Google Scholar]
- Thetmeyer H, Waller U, Black KD, Inselmann S, Rosenthal H (1999) Growth of European sea bass (Dicentrarchus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture 174: 355–367. [Google Scholar]
- Thomsen J, Stapp LS, Haynert K, Schade H, Danelli M, Lannig G, Wegner KM, Melzner F (2017) Naturally acidified habitat selects for ocean acidification–tolerant mussels. Sci Adv 3: e1602411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner M, Lefrançois C, Ferrari RS, Satta A, Domenici P (2008) The effect of acute hypoxia on swimming stamina at optimal swimming speed in flathead grey mullet Mugil cephalus. Mar Biol 155: 183–190. [Google Scholar]
- Vargas CA, Lagos NA, Lardies MA, Duarte C, Manríquez PH, Aguilera VM, Broitman B, Widdicombe S, Dupont S (2017) Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat Ecol Evol 1: 0084. [DOI] [PubMed] [Google Scholar]
- Vejřík L, et al. (2016) Small fish use the hypoxic pelagic zone as a refuge from predators. Freshw Biol 61: 899–913. [Google Scholar]
- Wakeling JA. (2006) Fast-start mechanics In Shadwick RE, Lauder GV, eds, Fish Biomechanics. Academic Press, San Diego, pp. 333–368. [Google Scholar]
- Wakeling JM, Cole NJ, Kemp KM, Johnston IA (2000) The biomechanics and evolutionary significance of thermal acclimation in the common carp Cyprinus carpio. Am J Phys Regul Integr Comp Phys 279: R657–R665. [DOI] [PubMed] [Google Scholar]
- Walker JA, Ghalambor CK, Griset OL, McKenney D, Reznick DN (2005) Do faster starts increase the probability of evading predators? Funct Ecol 19: 808–815. [Google Scholar]
- Wang G, Dillon ME (2014) Recent geographic convergence in diurnal and annual temperature cycling flattens global thermal profiles. Nat Clim Chang 4: 988. [Google Scholar]
- Wang X, Song L, Chen Y, Ran H, Song J (2017) Impact of ocean acidification on the early development and escape behavior of marine medaka (Oryzias melastigma). Mar Environ Res 131: 10–18. [DOI] [PubMed] [Google Scholar]
- Warren DT, Donelson JM, McCormick MI (2017) Extended exposure to elevated temperature affects escape response behaviour in coral reef fishes. PeerJ 5: e3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S-A, et al. (2018) Ocean warming has a greater effect than acidification on the early life history development and swimming performance of a large circumglobal pelagic fish. Glob Chang Biol 24: 4368–4385. [DOI] [PubMed] [Google Scholar]
- Webb PW. (1978) Temperature effects on acceleration of rainbow-trout, Salmo gairdneri. J Fish Res Board Can 35: 1417–1422. [Google Scholar]
- Webb PW, Zhang HB (1994) The relationship between responsiveness and elusiveness of heat-shocked goldfish (Carrassius-Auratus) to attacks by rainbow-trout (Oncorhynchus-Mykiss). Can J Zool-Rev Can Zool 72: 423–426. [Google Scholar]
- Weihs D. (1973) The mechanism of rapid starting of slender fish. Biorheology 10: 343–350. [DOI] [PubMed] [Google Scholar]
- Welch MJ, Watson S-A, Welsh JQ, McCormick MI, Munday PL (2014) Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat Clim Chang 4: 1086. [Google Scholar]
- Wilmers CC, Post E, Hastings A (2007) The anatomy of predator–prey dynamics in a changing climate. J Anim Ecol 76: 1037–1044. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Lefrançois C, Domenici P, Johnston IA (2010) The effect of environmental factors on the unsteady swimming performance of fish. In Fish Locomotion: An Etho-ecological Perspective, eds. Domenici P and Kapoor BG). Enfield, NH: Science Publishers. [Google Scholar]
- Wintzer AP, Motta PJ (2004) The effects of temperature on prey-capture kinematics of the bluegill (Lepomis macrochirus): implications for feeding studies. Can J Zool 82: 794–799. [Google Scholar]
- Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Chang 3: 995. [Google Scholar]
- Wolf NG, Kramer DL (1987) Use of cover and the need to breathe: the effects of hypoxia on vulnerability of dwarf gouramis to predatory snakeheads. Oecologia 73: 127–132. [DOI] [PubMed] [Google Scholar]
- Wood CM, McDonald DG, SFE B (1997) Global Warming: Implications for Freshwater and Marine Fish. Cambridge, Cambridge University Press. [Google Scholar]
- Zhang A-J, Cao Z-D, Fu S-J (2013) Effects of dissolve oxygen level on fast-start performance of juvenile grass carp (Ctenopharyngodon idellus). Chin J Ecol 32: 927–931. [Google Scholar]


