Abstract
Background:
Decellularization techniques have been widely used in tissue engineering recently. However, applying these methods which are based on removing cells and maintaining the extracellular matrix (ECM) encountered some difficulties for dense tissues such as articular cartilage. Together with chemical agents, using physical methods is suggested to help decellularization of tissues.
Methods:
In this study, to improve decellularization of articular cartilage, the effects of direct and indirect ultrasonic waves as a physical method in addition to sodium dodecyl sulfate (SDS) as chemical agents with 0.1% and 1% (w/v) concentrations were examined. Decellularization process was evaluated by nucleus staining with hematoxylin and eosin (H and E) and by staining glycosaminoglycans (GAG) and collagen.
Results:
The H and E staining indicated that 1% (w/v) SDS in addition to ultrasonic bath for 5 h significantly decreased the cell nucleus residue to lacuna ratio by 66%. Scanning electron microscopy showed that using direct sonication caused formation of micropores on the surface of the sample which results in better penetration of decellularization material and better cell attachment after decellularization. Alcian Blue and Picrosirius Red staining represented GAG and collagen, respectively, which maintained in ECM structure after decellularization by ultrasonic bath and direct sonicator.
Conclusion:
Ultrasonic bath can help better penetration of the decellularization material into the cartilage. This improves the speed of the decellularization process while it has no significant defect on the structure of the tissue.
Keywords: Cartilage, decellularization, extracellular matrix, sonicator, ultrasonic bath
Introduction
Intrinsic repair of cartilage leads to the production of fibrocartilage that does not have mechanical properties of the native tissue. Due to the low chondrocyte and absence of blood vessels in the cartilage tissue, it has a slow self-repair, and if not treated, it can lead to osteoarthritis.[1,2,3] At present, various cartilage treatment methods have been used which include microfracture, osteochondral autograft, and autologous chondrocyte implantation. In these strategies, the limited availability of graft tissue, donor site morbidity, and other problems affect the quality of repair. Recently, the use of natural and synthetic polymers and natural scaffolds produced by tissue engineering has provided promising approaches for cartilage regeneration.[4] Synthetic polymers have good mechanical properties, good biocompatibility, and controlled rate of biodegradation, but their surfaces have low inherent bioactivity and they are hydrophobic. The degradation products of some synthetic polymers are acidic which lead to inflammatory responses. Natural polymers are more bioactive resulting in a better cell attachment, proliferation, and differentiation. However, since extracellular matrix (ECM) as a natural matrix scaffold is a complex material and has difficulties to simulate, using natural acellular ECM has been suggested.[5] Scaffolds derived from acellular ECM are able to supply an organized microenvironment that is very similar to native cartilage. For example, Utomo et al. decellularized the ear cartilage and characterized their biochemical and biomechanical properties.[6] This decellularized ECM maintains tissue structure and bioactive molecules that could help regeneration. Furthermore, it is biodegradable and does not stimulate the immune response.[1] Decellularization of tissues has been done by physical, chemical, and/or enzymatic methods.[4,7] Sonication is a physical method which has been used to improve decellularization of different tissues such as cornea, aorta, small intestine, and meniscus.[8,9,10,11] Cartilage tissue is too dense; so, researchers have used different methods to help the penetration of decellularization materials. For example, cartilage matrix has mechanically shattered, and then, decellularized[3] or circular sheets of cartilage matrix have been decellularized.[12]
In this study, ultrasonic bath and sonicator have been used to improve the penetration of decellularization chemical agents in cartilage tissues for obtaining efficient cell membranes' disruption and cellular debris removal. To evaluate the effects of these decellularization methods on the morphology, scanning electron microscopy (SEM) and histological staining are applied to evaluate cell and ECM components of the osteochondral tissue.
Materials and Methods
Tissue preparation
Knee and hip joint tissues of sheep (1 year old, n = 3) were prepared from slaughterhouse. A trephine was used to excise osteochondral plugs with a diameter of 0.5 cm and a height of 0.5–1 cm. Samples were rinsed an overnight in a falcon tube containing phosphate-buffered saline (PBS) at 4°C and then was applied for experimental evaluations.
Decellularization methods
Decellularization method involves using sodium dodecyl sulfate (SDS) (Sigma-Aldrich, USA) at concentrations of 0.1% and 1% (w/v) with either ultrasonic bath (CD-4820 Codyson, China) with a power of 170 W and a frequency of 42 kHz and direct sonicator (UP400S Hielscher, Germany) with a power of 80 W and a frequency of 12 or 24 kHz.
The samples were divided into seven groups. The first group was the control group immersed in PBS for 55 h to remove blood. The second and third groups were placed in 0.1% and 1% SDS for 5 h. The fourth and fifth groups were placed in the falcon tube containing 0.1% and 1% SDS and put in ultrasonic bath for 5 h. The water temperature of the bath was checked every 8 min, and if the temperature rose above 37°C, the water was changed. The sixth group was placed in a beaker containing 0.1% SDS exposed to sonication with a frequency of 12 kHz and power of 80 W for an hour, and the seventh group was placed in a beaker containing 0.1% SDS under the sonication with a frequency of 24 kHz and power of 80 W for 2 h. Distance of the samples to the tip of the ultrasound probe was set 3 cm. To avoid increasing the sample temperature above 37°C, the beaker containing samples in SDS solution was put in a small container of cold water. Since the ultrasonic bath exerts indirect waves, the exposing time and percentage of SDS were selected higher compared to the direct sonication method. At the end of the procedure, to remove SDS residue, all of the samples were rinsed in PBS for 55 h while the PBS was replaced every 24 h.
Cell removal evaluation
For the preparation of histological sections of osteochondral tissue, the samples were fixed in 10% (v/v)-buffered formalin at room temperature for 24 h and then decalcified using 10% nitric acid for 3 weeks. The samples were dehydrated using graded ethanol and then embedded in paraffin and sectioned at 5 μm thickness, after that stained with hematoxylin (Sigma-Aldrich, USA) and eosin (Sigma-Aldrich, USA) (H and E).
Cell removal was evaluated by counting the cell nucleus of three areas of 400 (μm)2 using ImageJ software (National Institute of Health, Bethesda, USA). Finally, the proportion of nucleus to lacuna in each area was calculated, and for each sample, mean and standard deviation of three areas were calculated. The results were represented as mean ± standard deviation and statistically analyzed by t-test. Results were considered with significant changes at P < 0.05.
Glycosaminoglycans and collagen content in decellularized tissue
Alcian Blue (Sigma, USA) was used for glycosaminoglycans (GAG) staining and Picrosirius Red (Sigma, USA) for collagen staining. GAG and collagen were stained to verify decellularization process effects on destruction or removal of the ECM components.
Cytotoxicity
To evaluate the cytotoxicity of decellularized ECM comparing to control group, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, USA) test was applied. Decellularized sample which was placed in 1% SDS was cut horizontally using a surgical blade. Circular sections with a thickness of about 2 mm and diameter of 5 mm were obtained. The sections were disinfected using 70% ethanol for 2 h and then washed with sterile PBS and dried. After that, each side of the samples was irradiated by ultraviolet waves for half an hour.
To prepare the extract, samples were immersed in 1 ml RPMI-1640 culture medium and incubated for 1 week. Furthermore, 1 ml RPMI-1640 was used as a control group in the same condition. L929 fibroblast cells at passage two were seeded in a 96 well plate (15000 cells per 100 μl suspension).
After 24 h incubation, the culture medium was removed, and 50 μl extract and control medium were added to the different wells. On the third day, extract and control medium were removed, and the 100 μl MTT solution was added to each well.
After that, plates were incubated for 4 h. Then, MTT solution was slowly removed and 100 μl isopropanol was added to each well. After 20 min of additional incubation, the optical density was obtained at 570 nm using ELISA reader (BioTek, USA) to evaluate cell viability.
Scanning electron microscopy
Morphology and cell attachment can be examined using electron microscope images of tissue. The bare specimens and specimens with adherent cells were prepared for SEM. First, the samples were cut horizontally with a thickness of 2 mm. Sections without cell culture were fixed with 4% glutaraldehyde for 90 min and dehydrated using graded ethanol (30%–100%) and then dried at room temperature. To evaluate cell attachment on the samples, sections were disinfected, and then, 10 μl of cell suspension (L929 fibroblast cells at passage 2, 105 per ml) was poured on the samples and incubated for 5 h to let cell attachment. After that, samples were fixed, dehydrated, and dried at room temperature. Finally, all of the samples were vacuumed and sputter coated with gold and observed by SEM (Seron Technology, Korea).
Results
Cell removal
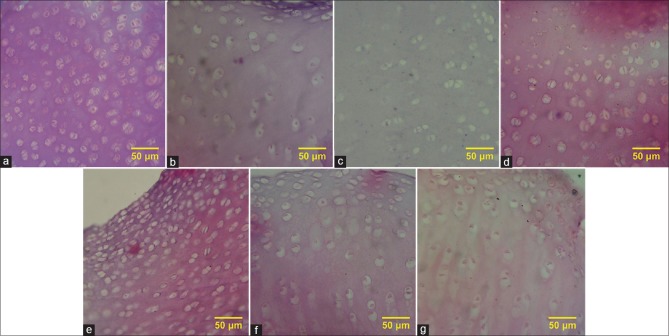
The decellularization was evaluated using H and E staining [Figure 1], and the mean percentage of the remained cells to lacuna was compared to the control [Figure 2]. The greatest effect of decellularization belonged to the sample using SDS at a concentration of 1% together with ultrasonic bath for 5 h, with the mean percentage of 34%.
Figure 1.
(a) H and E staining of native cartilage, (b) H and E staining of cartilage treated with 0.1% sodium dodecyl sulfate, (c) H and E staining of cartilage treated with 1% sodium dodecyl sulfate, (d) H and E staining of cartilage treated with 0.1% sodium dodecyl sulfate in ultrasonic bath, (e) H and E staining of cartilage treated with 1% sodium dodecyl sulfate in ultrasonic bath, (f) H and E staining of cartilage treated with 0.1% sodium dodecyl sulfate in direct sonication (at 12 KHz), (g) H and E staining of cartilage treated with 0.1% sodium dodecyl sulfate in direct sonication (at 24 KHz)
Figure 2.
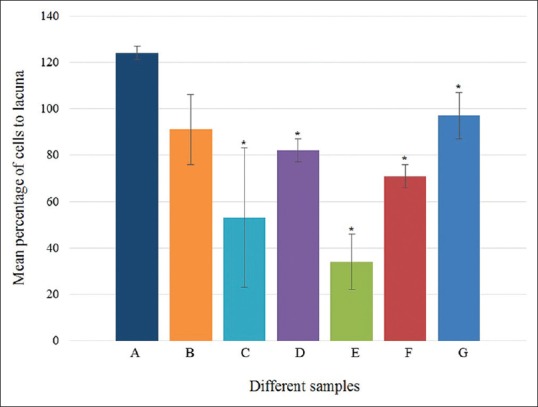
Mean percentage of cells' residue to lacuna of native tissue (A), cartilages treated with 0.1 and 1% sodium dodecyl sulfate (B and C), cartilages treated with 0.1 and 1% sodium dodecyl sulfate in ultrasonic bath (D and E), cartilages treated with 0.1% sodium dodecyl sulfate in direct sonication (at 12 KHz and 24 KHz) (F and G)
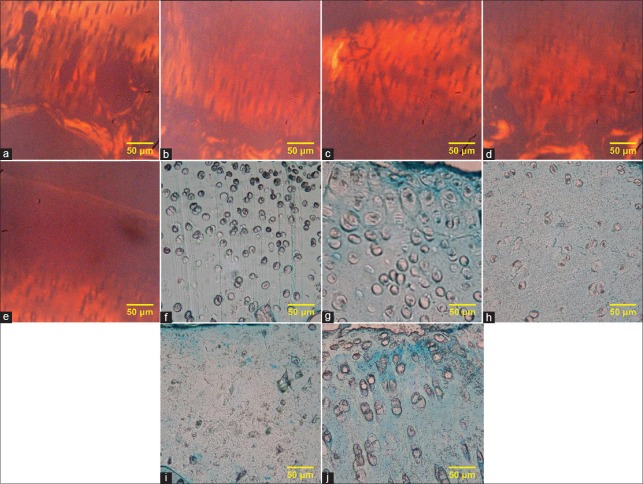
Collagen and glycosaminoglycans morphology after decellularization
According to the obtained images of Picrosirius Red staining [Figure 3a-e], we should say that using SDS, ultrasonic bath or sonicator had no effect on the amount of collagen since samples do not show any significant difference comparing to control. The images of Alcian Blue staining [Figure 3f-j] indicated that the main difference between the samples and control group appeared in the areas around lacuna. In our study, in control sample [Figure 3f], these areas were quite dark, but in decellularized samples with an ultrasonic bath [Figure 3h] and 12 kHz sonication [Figure 3i], color reduction of these areas was clear; however, in the other areas, the GAG content was not changed.
Figure 3.
(a) Picrosirius Red staining of native cartilage, (b) Picrosirius Red staining of cartilage treated with 1% sodium dodecyl sulfate, (c) Picrosirius Red staining of cartilage treated with 1% sodium dodecyl sulfate in ultrasonic bath, (d) Picrosirius Red staining of cartilage treated with 0.1% sodium dodecyl sulfate in direct sonication (at 12 KHz), (e) Picrosirius Red staining of cartilage treated with 0.1% sodium dodecyl sulfate in direct sonication (at 24 KHz), (f) Alcian Blue staining of native cartilage, (g) Alcian Blue staining of cartilage treated with 1% sodium dodecyl sulfate, (h) Alcian Blue staining of cartilage treated with 1% sodium dodecyl sulfate in ultrasonic bath, (i) Alcian Blue staining of cartilage treated with 0.1% sodium dodecyl sulfate in direct sonication (at 12 KHz), (j) Alcian Blue staining of cartilage treated with 0.1% sodium dodecyl sulfate in direct sonication (at 24 KHz)
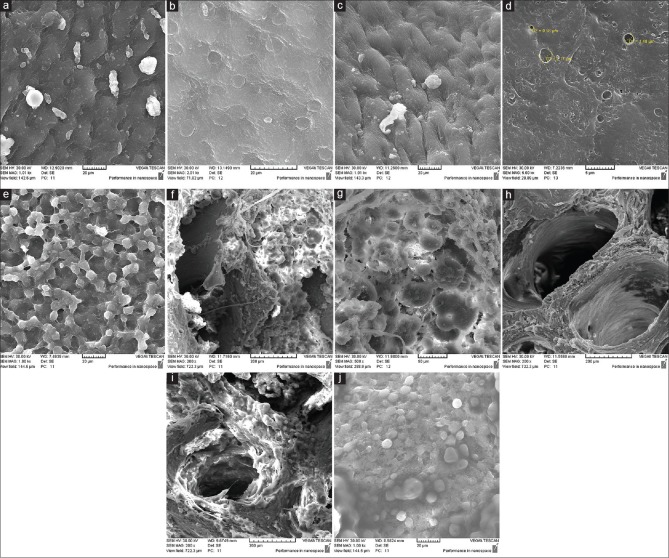
Scanning electron microscopy observation
On the surface of control cartilage which was obtained by SEM images [Figure 4a and b], chondrocyte cells are visible. While in cartilage surface of the decellularized sample using ultrasonic bath, cells are not observed [Figure 4c]. In decellularized sample using direct sonicator, micropores are visible on the surface [Figure 4d]. Micropores can increase surface roughness and cell attachment which can speed up recellularization process. The cell attachment in decellularized scaffold using ultrasound bath is visible [Figure 4e]. Bone marrow cells of the control sample are shown in Figure 4f and g, but such cells in the decellularized samples using ultrasonic bath or sonicator have been greatly reduced [Figure 4h and i]. Furthermore, cell attachment on decellularized scaffold using sonication [Figure 4j] is visible.
Figure 4.
(a) Scanning electron microscopy of cartilage, native tissue, (b) scanning electron microscopy of cartilage, native tissue, (c) scanning electron microscopy of cartilage, decellularized sample by ultrasonic bath, (d) scanning electron microscopy of cartilage, decellularized sample by direct sonication, (e) scanning electron microscopy of cartilage, cell attachment on decellularized extracellular matrix by ultrasound bath, (f) scanning electron microscopy of bone, native tissue, (g) scanning electron microscopy of bone, native tissue, (h) scanning electron microscopy of bone, decellularized sample by ultrasonic bath, (i) scanning electron microscopy of bone, decellularized sample by direct sonication, (j) scanning electron microscopy of bone, cell attachment on extracellular matrix decellularized by direct sonication
Cytotoxicity
MTT test results showed that viability of fibroblast cells which incubated in extract medium obtained from decellularized ECM by 1% SDS was 98.16 ± 5.99; however, this value for RPMI-1640 medium as a control group was 100 ± 1.76. T-test results indicated that there was no significant difference in cell survival between the control and extract medium (P > 0.05). This implies that the decellularized scaffold had no significant cytotoxic effects on fibroblast cells.
Discussion
Previous studies have demonstrated that ionic detergents are more efficient for decellularization of dense tissues than nonionic detergents or zwitterionic. SDS, the most generally used ionic detergent, is effective in eliminating of cellular components from tissues. However, these ionic detergents could damage the protein matrix. Furthermore, the destruction of collagen and reduction of GAG could occur using high amounts of SDS (1%–2% w/v) as an ionic detergent.[7,13,14] SDS with low concentration eliminated chondrocytes completely and also retained the native composition of ECM.[15] For these reasons, in this study, SDS at concentration of 0.1 and 1% was used.
Although the toxic effects of SDS residue are reported,[7] in this study, MTT test results demonstrate the cell viability on ECM extract and so SDS removal after washing the samples with PBS which confirms lack of toxicity of decellularized ECM.
Considering cartilage as a dense tissue, penetration of decellularization chemical agents and removing cellular debris have difficulties.[3,14] To improve the decellularization process for cartilage tissue, ultrasound waves have been recommended.[9] In this study, ultrasonic bath and direct sonication were used to improve the penetration of decellularization materials into the tissue, as well as obtaining better cell membrane disruption and cell debris removal. The results illustrate a better decellularization with ultrasonic bath compared to the sonicated samples. It should be noted that increasing the frequency of the sonication resulted in the reduction of decellularization depth since the frequency of 12 kHz was more effective than 24 kHz. This observation conforms to the fact that lower frequency ultrasounds are more capable of penetration into the tissues.[16]
In this study, staining is used to assess the effects of decellularization on ECM collagen and GAG content, while the results do not indicate any noticeable defect on ECM. Hence, the scaffolds retain large amounts of collagen and GAG. Previous studies have also indicated that using ultrasound waves shows no significant effects on the amount of collagen and GAG.[9]
SEM images of decellularized samples with ultrasound waves have shown micropores with diameter of 10 μm.[9] In this study, SEM images of decellularized samples under sonication demonstrated micropores about 0.5–5 μm in diameter. It seems that by these micropores, surface roughness increases which results in better cell adhesion. SEM images of the cartilage and bone surfaces show suitable cell attachment to the decellularized scaffolds after the cell culture.
Conclusions
Ultrasonic bath is a supportive method for decellularization of cartilage tissue as it simplifies penetration of detergents into the tissue. This method improves decellularization process in comparison with no ultrasound processes while it causes no significant defect on the structure of the ECM. Comparing to the sonicator, ultrasonic bath needs longer period of time for decellularization and assists smoother detergent penetration while has less damages on collagen and GAG content in decellularized tissue.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
BIOGRAPHIES

Farin Forouzesh graduated from Isfahan university with master degree in biomedical engineering in Sep.2019. She got a Master of Business Administration from Shahid Beheshti university in Sep 2018. Her research interests are biomaterials and tissue engineering. She worked on decellularization of cartilage tissue for her master project in Pasteur Institute in Tehran.
Email: farinforouzesh1990@gmail.com

Mohsen Rabbani received the B.Sc. in Mechanical Engineering in 2000 from Sharif University of Technology. He received M.Sc. and Ph.D. in Biomedical Engineering (Biomechanics) from Amirkabir University of Technology (Tehran Polytechnic) in 2004 and 2011, respectively. He is currently working as assistant professor of biomedical engineering department at University of Isfahan. His research focuses are on “Biomechanics role in tissue engineering” and “Tissue Decellularization”. Furthermore, He is working on biosensors based on electrochemical, chemical and biological activities. His main concern in this issue is on designing production of blood glucose sensors and time temperature indicators.
Email: m.rabbani@eng.ui.ac.ir

Shahin Bonakdar received his Ph.D from Amirkabir University of technology in biomaterials engineering. He is currently working as an associate professor at Iran Pasteur Institute- National cell bank department. His research focuses on Cellsurface interactions, the effect of surface topography on stem cell differentiation and cartilage tissue engineering.
Email: Sh_bonakdar@pasteur.ac.ir
References
- 1.Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J, et al. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–76. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Nojoomi A, Tamjid E, Simchi A, Bonakdar S, Stroeve P. Injectable polyethylene glycol-laponite composite hydrogels as articular cartilage scaffolds with superior mechanical and rheological properties. Int J Polym Mater Polym Biomater. 2017;66:105–14. [Google Scholar]
- 3.Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–87. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Yan L, Chen S, Pei M. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater. 2018;74:56–73. doi: 10.1016/j.actbio.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng XF, Lu SB, Zhang WG, Liu SY, Huang JX, Guo QY. Mesenchymal stem cells on a decellularized cartilage matrix for cartilage tissue engineering. Biotech Bioprocess Eng. 2011;16:593–602. [Google Scholar]
- 6.Utomo L, Pleumeekers MM, Nimeskern L, Nürnberger S, Stok KS, Hildner F, et al. Preparation and characterization of a decellularized cartilage scaffold for ear cartilage reconstruction. Biomed Mater. 2015;10:015010. doi: 10.1088/1748-6041/10/1/015010. [DOI] [PubMed] [Google Scholar]
- 7.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azhim A, Yamagami K, Muramatsu K, Morimoto Y, Furukawa KS, Tanaka M, et al. World Congress on Medical Physics and Biomedical Engineering 26-31 May, 2012. Beijing, China: Springer; 2013. The use of sonication treatment to completely decellularize aorta tissue. [Google Scholar]
- 9.Norzarini A, Azhim A, Ushida T. Decellularized Bovine Meniscus in Morphological Assessment Prior to Bioscaffold Preparation, in Control Conference (ASCC), 2015 10th Asian. IEEE; 2015. [Google Scholar]
- 10.Oliveira AC, Garzón I, Ionescu AM, Carriel V, Cardona Jde L, González-Andrades M, et al. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One. 2013;8:e66538. doi: 10.1371/journal.pone.0066538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SL, Sidney LE, Dunphy SE, Rose JB, Hopkinson A. Keeping an eye on decellularized corneas: A review of methods, characterization and applications. J Funct Biomater. 2013;4:114–61. doi: 10.3390/jfb4030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y, et al. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265–73. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 13.Kim BS, Kim H, Gao G, Jang J, Cho DW. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication. 2017;9:034104. doi: 10.1088/1758-5090/aa7e98. [DOI] [PubMed] [Google Scholar]
- 14.Samouillan V, Lamure A, Maurel E, Dandurand J, Lacabanne C, Ballarin F, et al. Characterisation of elastin and collagen in aortic bioprostheses. Med Biol Eng Comput. 2000;38:226–31. doi: 10.1007/BF02344781. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Li Z, Li Z, Wu B, Liu Y, Wu W, et al. Cartilaginous extracellular matrix derived from decellularized chondrocyte sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater. 2018;81:129–45. doi: 10.1016/j.actbio.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Speed C. Therapeutic ultrasound in soft tissue lesions. Rheumatology. 2001;40:1331–6. doi: 10.1093/rheumatology/40.12.1331. [DOI] [PubMed] [Google Scholar]